Академический Документы
Профессиональный Документы
Культура Документы
Chapter 9: Manufactured Substances in Industry: Stage 1
Загружено:
malcovishes0 оценок0% нашли этот документ полезным (0 голосов)
25 просмотров12 страницSulphuric acid is a widely used industrial chemical that is manufactured through a four stage contact process. In the first stage, sulphur is burned to produce sulphur dioxide, which is then converted to sulphur trioxide in the second stage. In the third stage, sulphur trioxide is dissolved in concentrated sulphuric acid to form oleum. Finally, oleum is diluted with water to produce concentrated sulphuric acid. Sulphuric acid pollution can cause acid rain and respiratory problems. Ammonia is produced through the Haber process by combining nitrogen and hydrogen under high pressure and temperature. Common alloys include steel, stainless steel, bronze and brass, which are
Исходное описание:
workout
Оригинальное название
Chemistry
Авторское право
© © All Rights Reserved
Доступные форматы
DOCX, PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документSulphuric acid is a widely used industrial chemical that is manufactured through a four stage contact process. In the first stage, sulphur is burned to produce sulphur dioxide, which is then converted to sulphur trioxide in the second stage. In the third stage, sulphur trioxide is dissolved in concentrated sulphuric acid to form oleum. Finally, oleum is diluted with water to produce concentrated sulphuric acid. Sulphuric acid pollution can cause acid rain and respiratory problems. Ammonia is produced through the Haber process by combining nitrogen and hydrogen under high pressure and temperature. Common alloys include steel, stainless steel, bronze and brass, which are
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате DOCX, PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
25 просмотров12 страницChapter 9: Manufactured Substances in Industry: Stage 1
Загружено:
malcovishesSulphuric acid is a widely used industrial chemical that is manufactured through a four stage contact process. In the first stage, sulphur is burned to produce sulphur dioxide, which is then converted to sulphur trioxide in the second stage. In the third stage, sulphur trioxide is dissolved in concentrated sulphuric acid to form oleum. Finally, oleum is diluted with water to produce concentrated sulphuric acid. Sulphuric acid pollution can cause acid rain and respiratory problems. Ammonia is produced through the Haber process by combining nitrogen and hydrogen under high pressure and temperature. Common alloys include steel, stainless steel, bronze and brass, which are
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате DOCX, PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 12
CHAPTER 9: MANUFACTURED SUBSTANCES IN INDUSTRY
9.1 Sulphuric Acid
1. Sulphuric acid, H
2
SO
4
is used to produce:
synthetic fertilizers
o Example: Ammonium sulphate is prepared from the reaction between sulphuric
acid and aqueous ammonia.
o H
2
SO
4
(aq)
+ 2NH
3
(aq)
(NH
4
)
2
SO
4
(aq)
detergents
synthetic fibers (polymers)
electrolyte in car batteries
paints
dyes
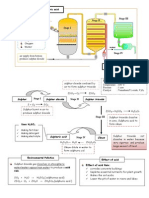
2. Sulphuric acid is manufactures in industry in large scale through the Contact Process. The
Contact process consists of four stages. The raw materials used in the Contact process are
sulphur, air and water.
Stage 1
1. Molten sulphur is burnt in dry air to produce sulphur dioxide.
2. The gas produced is then purified and cooled.
S + O
2
SO
2
3. Sulphur dioxide can also be produced by burning metal sulphide such as lead(II)
sulphide or zinc sulphide in dry air.
2PbS + 3O
2
2PbO + 2SO
2
Stage 2
1. In a converter, sulphur dioxide and excess oxygen are passed through vanadium(V)
oxide.
2. Vanadium(V) oxide act as catalyst to expedite the process.
3. The optimum condition for maximum amount of product are as follow:
i. Temperature: 450 500 C
ii. Pressure: 1 atm
4. About 99.5% of the sulphur dioxide, SO
2
is converted into sulphur trioxide, SO
3
through
this reversible reaction.
Stage 3
Sulphur trioxide is dissolved in concentrated sulphuric acid to form oleum H
2
S
2
O
7
.
SO
3
+ H
2
SO
4
H
2
S
2
O
7
Stage 4
The oleum, H
2
S
2
O
7
is then diluted with water to produce concentrated sulphuric acid, H
2
SO
4
in
large quantities.
H
2
S
2
O
7
+ H
2
O 2H
2
SO
4
Note:
1. The two reactions in the third and fourth stages are equivalent to adding sulphur trioxide,
SO
3
directly to water.
SO
3
+ H
2
O H
2
SO
4
2. However, this is not done in industry because sulphur trioxide, SO
3
reacts too violently
with water.
3. This produces a lot of heat and a large cloud of sulphuric acid, H
2
SO
4
mist. Thus,
sulphuric acid will vaaporises.
4. The mist is also corrosive, pollutes the air and is difficult to condense.
3. Pollution by sulphur dioxide
The sources of sulphur dioxide emission include
(a) Burning of fossil fuels such as petroleum
(b) Volcanic eruption
(c) Burning of products manufactured from sulphuric acid such as rayon
4. Sulphur is a poisonous and acidic gas. Effects of sulphur dioxide pollution:
Acid rain:
Sulphur dioxide reacts with oxygen to form sulphur trioxide.
2 SO
2
+ O
2
2SO
3
Both oxides of sulphur dissolve in rainwater to form sulphurous and sulphuric
acid respectively.
SO
2
+ H
2
O H
2
SO
3
(Sulphurous acid)
SO
2
+ H
2
O H
2
SO
4
(Sulphuric acid)
Effects of acid rain
(i) Corrodes concrete buildings and metal structures
(ii) Destroys trees and plants in forest
(iii) Decreases the pH of the soil which becomes acidic and unsuitable for
growth of plants and destroys the roots of plants
(iv) Reacts with minerals in the soil to produce salts which are leached out
of the top soil; essential nutrients for plants growth are depleted
(v) Acid rain flows into lakes and rivers. This increases the acidity of
water and may kill fish and other aquatic living things.
Respiratory problems
Sulphur dioxide causes respiratory difficulties such as coughing, chest pains,
shortness of breath and bronchitis.
9.2 Ammonia and Its Salt
1. The physical properties of ammonia are:
Colourless and alkaline gas
Strong pungent smell
Very soluble in water
2. Reacts with hydrogen chloride to form white fumes of ammonium chloride (this is used
as a test for ammonia gas).
3. The main uses of ammonia are:
Manufacturing nitrogen fertilizers
Manufacturing nitric acid through Ostwald process.
To make explosives, dyes, household cleaners and nylon
As an alkali to prevent the coagulation of latex
To produce ammonium chloride that is used as electrolyte in dry cells
Manufacturing of synthetic fibers such as nylons.
As a cooling agent.
Making of Ammonia
Ammonia is manufactured by combining nitrogen and hydrogen in an important industrial
process called the Haber process. The reaction is reversible and the production of ammonia is
exothermic.
Nitrogen gas is obtained from the fractional distillation of liquid air.
Hydrogen gas is obtained through the reaction between natural gas and steam.
Nitrogen and hydrogen are mixed in the ratio of 1 : 3
The gas mixture is passed through:
Catalyst: Iron
Temperature: 450 - 550C
Pressure: 200 500atm
9.3 Alloys
1. An alloy is a mixture of two or more elements with a certain composition in which the
major component is metal.
2. Pure metal are weak and soft because the arrangement of atoms in pure metals makes
them ductile and malleable.
Arrangment of atoms in a pure metal
3. (a) Ductility
-Pure metals are soft because of the orderly arrangement of atoms enables the layers of
atoms to slide over each other easily when an external force is applied on them. This makes the
metals ductile and metals can be drawn to form long wires.
(b) Malleability
- There are imperfections in the natural arrangement of metal atoms. Empty space exists
in the structures of pure metals. When pressed together, groups of metal atoms may slide into
new positions. This makes the metal malleable, able to be made into different shapes or pressed
into thin sheets.
4. In making alloys, one or more foreign elements are added to molten metal. When the
alloy hardens, the positions of some of the metal atoms are replaced by atoms of foreign
elements, with sizes bigger or smaller than the original metal atoms. These atoms disrupt
the orderly arrangement of the metal atoms and also fill up any empty spaces in the
metal structure. Hence, layers of metal atoms are prevented from sliding over each
other easily. This makes the alloy harder and stronger than its pure metal.
5. Three aims of alloying a pure metal :
To increase the hardness and strength of a metal
To prevent corrosion or rusting
To improve the appearance of the metal surfaces, with a better finish and luster.
6. Comparison between alloy and pure metal
Properties Pure metal Alloy
Hardness Soft Hard
Corrosion Less resistant to corrosion More resistant to corrosion
Appearance Dull Shiny
The Composition, Properties and Uses of Some Common Alloys
Alloy Composition Properties Uses
Steel 99% Iron
1% Carbom
Hard and Strong Construction of building and
bridges
Stainless steel 74% Iron
8% Carbom
18% Chromium
Shiny
Strong
Non-rusty
Making cutlery
Making surgery instruments
Bronze 90% Copper
10% Tin
Hard and strong
Has Shiny surface
Making of medals, swords.
Building statues or
monuments
Brass 70% Copper
30% Zinc
Harder than copper Making of kitchenware.
Making of electrical
connectors and musical
instruments.
Duralumin 93% Aluminium
3% Copper
3% Magnesium
1% Manganese
Light
Strong
Hard
Building of body of
aeroplanes and bullet trains.
Magnalium 70% Aluminium
30% Magnesium
Light
Hard
Strong
Building aeriplanes and
bullet trains.
Making rims of racing car
tyres.
Pewter 96% Tin
3% Copper
1% Antimony
Lustre
Shiny
Strong
Making of souvenirs and
decorative ornaments.
Cupro-nickel Copper, nickel
(% according to
colour)
Hard
Shiny
Resists corrosion
To make coins of 10 cents,
20 cents and 50 cents.
9.4 Synthetic Polymers
1. Polymers are large molecules consisting of monomers which are joined together by covalent
bond.
2. Monomers are joined together by a process called polymerization.
3. There are two types of polymerization reaction.
a. Addition polymerization
i. a process of joining many monomers to form a large molecules without
any loss of small molecules
b. Condensation
i. a process of joining many monomers to form a large molecule with the
loss of small molecules such as water.
4. There are two types of polymers:
(a) Natural polymers
- polymers that occur naturally in plants and animals
Examples: Starch, cellulose, wool, protein, and natural rubber
Protein is a natural polymer consisting of many amino acids.
Starch is a natural polymer consisting of many glucose molecules.
Natural rubbers are elastomers with elastic properties able to regain original shape
when unstretched. The monomer for natural rubber is isoprene.
(b) Synthetic polymers
-manufacture polymers, made using chemicals derived mostly from fractions of petroleum or
crude oil.
-mainly classified into plastics, synthetic rubbers and synthetic fibres.
-Some of the various types of synthetic polymers and their uses are shown in table below.
Synthetic Polymer Monomer Uses
Polythene
Ethene Shopping bags, plastic bags, and
insulation for electrical wiring
Polypropene
Propene Plastic containers, bottles and
automobile battery casings.
Polyvinyl chloride,
PVC
Chloroethene / vinyl chloride Artificial leather, Waterproof and
insulating materials such as
raincoats, water pipes, insulator for
electrical wires and cables.
Polystyrene Styrene Food containers, wrappers and
packaging materials
Perspex Methylmethacrylate Safety glass
Terylene Ethan-1,2-diol
Benzene-1,4-dicarboxylic acid
Clothing, ropes
Teflon Tetrafluoroethene Coating for surface of non-stick pans
Nylon Hexane-1,6-diamine
Hexane-1,6-dioic acid
Clothing, ropes
Effects of improper disposal of Synthetic Polymers
1. Most synthetic polymers are stable and resistant to oxidation, chemicals and
microorganisms. They cannot be broken down easily by microorganisms and therefore, they
are classified as non-biodegradable.
2. They may cause pollution, blockage of drainage systems, suffocation of aquatic animals and
flash floods.
3. Burning synthetic polymers release toxic gases such as hydrogen cyanide, hydrogen
chloride, and carbon monoxide.
Ways to control the disposal problems of Synthetic Polymers
1. The use of synthetic polymers depleted natural resources such as petroleum (Main source of
raw materials for the production of synthetic polymers) which is a non-renewable resource.
The wastes pollute the environment.
2. Use synthetic polymers in a wiser manner
(a) Use of biodegradable synthetic polymers
(b) Recycling of synthetic polymers
(c) Replace synthetic polymers with other materials (eg. Recycled paper)
(d) Pyrolysis Process of heating the plastic in the absence of air to break it down
9.5 Glass and Ceramics
1. Glass is often regarded as supercooled liquid. The particles in glass are not arrange
orderly like in a liquid but their movement is restricted to vibrations about a fixed
position. The major component of glass is silica, SiO
2
2. The types of glass, properties and their uses.
Type of glass Composition Properties Uses
Fused glass
(quartz glass)
Silicon dioxide, SiO
2
High melting point
Ultraviolet light able to pass
through
Resistant to chemical attack
Laboratory apparatus,
optical lenses
Soda lime glass Silicon dioxide
Calcium oxide
Sodium oxide
Low melting point
Mouldable into shapes
Good chemical durability
Glass containers,
Plates and bowls,
Windows, bulbs, and
lamps, bottles
Borosilicate
glass (Pyrex)
Silicon dioxide,
Boron dioxide
Resistant to heat due to low
expansion coefficient
High melting point
Laboratory apparatus,
cooking utensils
Lead glass
(Crystal glass)
Silicon dioxide,
Lead(II) oxide
High refractive index
High density
Soft and easy to melt
Prisms, lenses, crystal
glassware, ornaments
3. Ceramics are made from clay. The main elements contained in ceramics are silicon,
oxygen and aluminium.
4. The common clay used is the china white clay or kaolinite.
5. Kaolinite contains hydrated alumino-silicate Al
2
O
3
.2SiO
2
.2H
2
O.
6. The process of forming ceramic objects involves the adding of water to make the clay
mouldable. The clay is moulded and then heated in a furnace at about 1300C. Once it is
dried, the ceramic object hardens.
7. Ceramic has the following properties :
Strong and hard but brittle
Inert towards chemical
Can withstand compression
Resistant to corrosion
Good electrical insulator
8. The uses of ceramics:
Construction materials (eg. Bricks, tiles, cement and pipes)
Ornamental articles (eg. Bowls, cups, plates, vase and porcelain)
Electrical insulator (eg. Spark plugs, fuses, insulators in electric iron and ovens)
Refractory materials for lining of furnace, nuclear reactors and space shuttles tp
withstand high temperature
9.6 Composite Materials
1. Modern technologies require materials with unusual combinations of properties.
2. The new structural materials must have stronger properties, more durable, lighter, and
resistance to heat and corrosion.
3. Composite materials are formed by combining two or more different substances for specific
application.
4. Table below shows the comparison between various composite materials.
Composite Content Properies Uses
Superconductor Ceramic,
sodium oxide,
barium oxide,
copper (II) oxide
Zero electrical
resistance
Elevated trains,
Electromagnets
(magnets which are
light but thousands of
times stronger than
the normal magnet),
MRI
Photochromic glass Glass, Silver chloride Transparent and
darkens in the
presence of light
Sunglasses, windows
Reinforced concrete Cement, steel Stronger, harder, and
able to withstand
stronger weight or
force
Concrete pillars for
buildings, bridges and
highways
Fibre glass Glass, polyester resin Light, resistant to
corrosion, stronger,
inflammable
Water tanks, boats,
electrical appliances,
racquets
Fibre optic Glass, Copper,
Aluminium
Transmit data, sounds
and images in a digital
format
Telecommunication
cables, endoscopes
Ceramic glass Glass that contains
certain amounts of
metal to UV rays and
heating at high T
Strong, translucent,
heat resistant
Cooking utensils,
Rocket heads
Plastic strengthened
with glass fibres
Plastic, glass Very strong
Light
Easily formed
Withstands corrosion
Helmets, body of cars
and aeroplanes
Вам также может понравиться
- Objective: Concept MapДокумент17 страницObjective: Concept MapNenek Tak Guna100% (1)
- MRSM BETONG CHEMISTRY FORM 4 CHAPTER 9 KEY CONCEPTSДокумент12 страницMRSM BETONG CHEMISTRY FORM 4 CHAPTER 9 KEY CONCEPTSQisthina Azmina AbdullahОценок пока нет
- Chemistry Folio Manufacture Substance in IndustryДокумент23 страницыChemistry Folio Manufacture Substance in Industryseela gunalanОценок пока нет
- Extractive Metallurgy 2: Metallurgical Reaction ProcessesОт EverandExtractive Metallurgy 2: Metallurgical Reaction ProcessesРейтинг: 5 из 5 звезд5/5 (1)
- Chemistry Folio Form 4 2013Документ17 страницChemistry Folio Form 4 2013Megat AshmannОценок пока нет
- Solutions Manual to accompany Engineering Materials ScienceОт EverandSolutions Manual to accompany Engineering Materials ScienceРейтинг: 4 из 5 звезд4/5 (1)
- 9.1: Sulphuric AcidДокумент20 страниц9.1: Sulphuric AcidSyaza JeaОценок пока нет
- Chemistry Folio Chapter 9 Form 4Документ27 страницChemistry Folio Chapter 9 Form 4Suhaila MohamedОценок пока нет
- Manufactured Substances in Industry2Документ20 страницManufactured Substances in Industry2Sam ZeeОценок пока нет
- Form 4 Manufactured Substances in Industry Chapter 9Документ43 страницыForm 4 Manufactured Substances in Industry Chapter 9Zafirah IdrisОценок пока нет
- Manufacture D Substances in IndustryДокумент53 страницыManufacture D Substances in IndustrySuriana ShamsuddinОценок пока нет
- H2SO4 Uses and ManufactureДокумент12 страницH2SO4 Uses and ManufactureFaakir GhazaliОценок пока нет
- Folio ChemistryДокумент16 страницFolio Chemistryamirah_dayanaОценок пока нет
- Folio KimiaДокумент12 страницFolio KimiaAishiteru RamenОценок пока нет
- Chemistr Y: Manufactured Substances in IndustryДокумент26 страницChemistr Y: Manufactured Substances in IndustryMuhd AmirudDinОценок пока нет
- FolioДокумент24 страницыFolioHeng CzОценок пока нет
- Folio Chemis AyiepДокумент15 страницFolio Chemis AyiepAniece AmirahОценок пока нет
- Chemistry Form 4 Solihin Full 1Документ24 страницыChemistry Form 4 Solihin Full 1Aiman ShuhaimiОценок пока нет
- Mind MapДокумент16 страницMind MapJr SparkОценок пока нет
- Sekolah Menengah Sains Seri Puteri Kuala Lumpur: Manufactured Substances in IndustryДокумент21 страницаSekolah Menengah Sains Seri Puteri Kuala Lumpur: Manufactured Substances in IndustryNur Wani Amat SujangiОценок пока нет
- Manufactured substances in industryДокумент9 страницManufactured substances in industryeyeda2000Оценок пока нет
- Sulphuric Acid, H SOДокумент9 страницSulphuric Acid, H SOcommen100% (3)
- ConclusionДокумент2 страницыConclusionnurulsyamim0% (1)
- KkimiaДокумент27 страницKkimiaNoralyz LyzcatОценок пока нет
- Chemistry: Manufactured Subtances in IndustryДокумент29 страницChemistry: Manufactured Subtances in IndustrySaber DateОценок пока нет
- Manufactured substances in industry and their environmental impactsДокумент15 страницManufactured substances in industry and their environmental impactsAsiah AsОценок пока нет
- Understand Industrial MaterialsДокумент32 страницыUnderstand Industrial MaterialsSurinjeet KaurОценок пока нет
- Chemist Chapter 9 Form 4Документ55 страницChemist Chapter 9 Form 4Muhamad ArifОценок пока нет
- ChemistryДокумент6 страницChemistryshaz_suryaОценок пока нет
- The 9 uses of sulphuric acid and properties of alloysДокумент47 страницThe 9 uses of sulphuric acid and properties of alloysWan AkramОценок пока нет
- Manufactures in Industry and Chemical For ConsumerДокумент22 страницыManufactures in Industry and Chemical For ConsumerMuhammad DanishОценок пока нет
- Chemistry Extra ClassДокумент7 страницChemistry Extra Classjuan barrettОценок пока нет
- Uses of Sulphuric AcidДокумент18 страницUses of Sulphuric AcidJian Jet LeeОценок пока нет
- Kimia Chapter 9Документ35 страницKimia Chapter 9Mohammad AmirОценок пока нет
- Manufacture of Substance in IndustryДокумент17 страницManufacture of Substance in IndustryAniff NajibОценок пока нет
- Chemistry Form 4 (Manufactured Substances in Industries)Документ24 страницыChemistry Form 4 (Manufactured Substances in Industries)Fariezuan HamidОценок пока нет
- Folio ChemistryДокумент16 страницFolio Chemistryaisyah_94Оценок пока нет
- ChemistryДокумент10 страницChemistryfi3mnorОценок пока нет
- Chemistry Form 4 Chapter 9Документ23 страницыChemistry Form 4 Chapter 9Ng Wan LinОценок пока нет
- Manufactured Substances in IndustryДокумент16 страницManufactured Substances in IndustryAhda Sabila Yusuf100% (1)
- Folio Kimia Bab 9 Tingkatan 4Документ27 страницFolio Kimia Bab 9 Tingkatan 4Muhammad Ikhlas100% (8)
- Manufactured Subtances IN IndustryДокумент49 страницManufactured Subtances IN IndustryAhmad AzriОценок пока нет
- HKDSE Chemistry Bridging Programe 1CДокумент76 страницHKDSE Chemistry Bridging Programe 1Cthe222Оценок пока нет
- 9 Manufacture Substances in IndustryДокумент35 страниц9 Manufacture Substances in IndustryazharsarahОценок пока нет
- Chemistry Holidays Assignment: Form 4Документ39 страницChemistry Holidays Assignment: Form 4Hafiz HakimiОценок пока нет
- Folio Chemistry F4 (Manufactured Substances in Industry)Документ31 страницаFolio Chemistry F4 (Manufactured Substances in Industry)JackOss93Оценок пока нет
- Electroplating Plastic ProcessДокумент7 страницElectroplating Plastic ProcessImpian SiberОценок пока нет
- 4 UtmДокумент32 страницы4 UtmKilau WatiОценок пока нет
- Research Proposal IsdbДокумент19 страницResearch Proposal IsdbBashir AhmadОценок пока нет
- Unit 4 - Reading Material IДокумент9 страницUnit 4 - Reading Material IYishakОценок пока нет
- Chemistry New NotesДокумент5 страницChemistry New NotesDaniel CannywoodОценок пока нет
- Stage II Stage III: Sulphur Sulphur Dioxide Sulphur TrioxideДокумент6 страницStage II Stage III: Sulphur Sulphur Dioxide Sulphur TrioxideSulaiman Mohamad100% (1)
- Chapter 8 Form 4 ScinceДокумент5 страницChapter 8 Form 4 ScincePurnima KadarasenОценок пока нет
- Chemistry For Engineers: Metallurgy of TungstenДокумент22 страницыChemistry For Engineers: Metallurgy of TungstenJustine joy cruzОценок пока нет
- Chemistry Form 4 Chapter 9Документ6 страницChemistry Form 4 Chapter 9Suriati Bt A Rashid100% (1)
- Uses of Sulphuric Acid and Manufacturing ProcessДокумент15 страницUses of Sulphuric Acid and Manufacturing ProcessKia LisaОценок пока нет
- Discussion and Conclusion ChemistДокумент10 страницDiscussion and Conclusion Chemistnaniekorean100% (2)
- Music BookДокумент1 страницаMusic BookmalcovishesОценок пока нет
- Album For Violinist Vol. 3 Violin PartДокумент16 страницAlbum For Violinist Vol. 3 Violin PartmalcovishesОценок пока нет
- CRCT Prep: Grade 5 Reading ComprehensionДокумент14 страницCRCT Prep: Grade 5 Reading ComprehensionmalcovishesОценок пока нет
- Present Simple and Free Time ActivitiesДокумент2 страницыPresent Simple and Free Time ActivitiesMujib Sadeque ChowdhuryОценок пока нет
- The PrincipalДокумент1 страницаThe PrincipalmalcovishesОценок пока нет
- Helping Your Child To Practice - Speech by MyselfДокумент1 страницаHelping Your Child To Practice - Speech by MyselfmalcovishesОценок пока нет
- Angry Bird InvoiceДокумент1 страницаAngry Bird InvoiceJacqueline TiongОценок пока нет
- Terms and Symbols Flash CardsДокумент16 страницTerms and Symbols Flash Cardsmalcovishes100% (4)
- Violin Grade 1Документ4 страницыViolin Grade 1malcovishes100% (1)
- Long Term Timetable ChangeДокумент5 страницLong Term Timetable ChangemalcovishesОценок пока нет
- Diploma Violin Song List AbrsmДокумент1 страницаDiploma Violin Song List AbrsmmalcovishesОценок пока нет
- SPM Chemistry Formula List Form5Документ15 страницSPM Chemistry Formula List Form5Jia Hui JoanaОценок пока нет
- Diploma Violin Song List AbrsmДокумент1 страницаDiploma Violin Song List AbrsmmalcovishesОценок пока нет
- Music BookДокумент1 страницаMusic BookmalcovishesОценок пока нет
- Music Grades Syllabus LCM StuffДокумент32 страницыMusic Grades Syllabus LCM StuffOtto Von TrappОценок пока нет
- Biology Plants 1Документ16 страницBiology Plants 1malcovishesОценок пока нет
- Sonatensatz in C MinorДокумент1 страницаSonatensatz in C MinormalcovishesОценок пока нет
- DrepreciationДокумент4 страницыDrepreciationmalcovishesОценок пока нет
- Yoga Violin MusicianДокумент122 страницыYoga Violin Musicianmalcovishes100% (1)
- Violinists Popular Repertoire 1918 VN PDFДокумент18 страницViolinists Popular Repertoire 1918 VN PDFjoorloОценок пока нет
- Malaysia LCM Examination Fees 2013: Music GradesДокумент2 страницыMalaysia LCM Examination Fees 2013: Music GradesmalcovishesОценок пока нет
- Sonatensatz in C MinorДокумент3 страницыSonatensatz in C MinormalcovishesОценок пока нет
- Brahms Scherzo in C Minor ViolinДокумент4 страницыBrahms Scherzo in C Minor ViolinmalcovishesОценок пока нет
- I Still Believe Mariah Cary#eyДокумент1 страницаI Still Believe Mariah Cary#eymalcovishesОценок пока нет
- Gr4 Wk1 Where On Earth Are YouДокумент2 страницыGr4 Wk1 Where On Earth Are YoumalcovishesОценок пока нет
- Yoga Violin MusicianДокумент122 страницыYoga Violin Musicianmalcovishes100% (1)
- Rhythm Worksheet #1: Name: - ClassДокумент1 страницаRhythm Worksheet #1: Name: - ClassmalcovishesОценок пока нет
- What Is A Detoxification Program?Документ6 страницWhat Is A Detoxification Program?malcovishesОценок пока нет
- Order Form Template for Business OrdersДокумент3 страницыOrder Form Template for Business OrdersmalcovishesОценок пока нет
- SMJK PHOR TAY PAPER 1 SCIENCE EXAMДокумент12 страницSMJK PHOR TAY PAPER 1 SCIENCE EXAMshng84Оценок пока нет
- SPECTROSPIN NMR Magnet System Safety NotesДокумент12 страницSPECTROSPIN NMR Magnet System Safety NotesSamuel AguiarОценок пока нет
- Parr Instrument CompanyДокумент12 страницParr Instrument CompanyAhmad RidjalОценок пока нет
- Monoethylene MSDSДокумент8 страницMonoethylene MSDSJustine Kei Lim-OrtegaОценок пока нет
- Pimag Optimizer: Drink Magnetically Charged, Superoxygenated Pi Water "The Water of Life"Документ5 страницPimag Optimizer: Drink Magnetically Charged, Superoxygenated Pi Water "The Water of Life"surintanОценок пока нет
- Elements and Compounds: Learner's Module in Science 7 First Quarter - Module 3Документ23 страницыElements and Compounds: Learner's Module in Science 7 First Quarter - Module 3Sarah DarriguezОценок пока нет
- Pyrophoric Chemicals GuideДокумент21 страницаPyrophoric Chemicals Guidedhavalesh1Оценок пока нет
- Cambridge IGCSE: Chemistry 0620/23Документ16 страницCambridge IGCSE: Chemistry 0620/23Yau Yee LeungОценок пока нет
- User Manual of Oxygen Generator PDFДокумент25 страницUser Manual of Oxygen Generator PDFBernard KoffiОценок пока нет
- 0620 - 42 - May - 16 SolvedДокумент11 страниц0620 - 42 - May - 16 SolvedMuhammad HassaanОценок пока нет
- Lung alveolus and blood vessel diagramДокумент22 страницыLung alveolus and blood vessel diagramZoonieFRОценок пока нет
- Air Pollution ChemistryДокумент25 страницAir Pollution Chemistryjanice omadtoОценок пока нет
- Exam Weekly Exam5Документ4 страницыExam Weekly Exam5Gab LibetarioОценок пока нет
- Everyday Science MCQS 2023 For Css Pcs PPSC KPPSC BPSC SPSCДокумент578 страницEveryday Science MCQS 2023 For Css Pcs PPSC KPPSC BPSC SPSCSharif JamaliОценок пока нет
- Gas Purging Optimizes Root WeldsДокумент3 страницыGas Purging Optimizes Root Weldsta_binhaaaОценок пока нет
- WQ-FDO Global WaterДокумент1 страницаWQ-FDO Global WaterleonardoОценок пока нет
- Gen Chem 1 Module 1 2nd Edition 2021Документ24 страницыGen Chem 1 Module 1 2nd Edition 2021Florenz Jay DingdingОценок пока нет
- Design of A Methanol-To-Olefinprocess Using Aspen Hysys: Material and Energy BalancesДокумент9 страницDesign of A Methanol-To-Olefinprocess Using Aspen Hysys: Material and Energy BalancesAbdulwahab GIWAОценок пока нет
- ScienceBook 9Документ341 страницаScienceBook 9Ismaks73% (11)
- Material Safety Data SheetДокумент5 страницMaterial Safety Data SheettranhungОценок пока нет
- THE KACHCHA THEEVU?... (A New Theory On " Islands")Документ11 страницTHE KACHCHA THEEVU?... (A New Theory On " Islands")AJER JOURNALОценок пока нет
- Latihan Kimia Ting 4Документ7 страницLatihan Kimia Ting 4nna80Оценок пока нет
- UV Spectroscopy Structure DeterminationДокумент5 страницUV Spectroscopy Structure DeterminationMuhammad UsmanОценок пока нет
- Oxygen DebtДокумент3 страницыOxygen DebtrajeevkeyarОценок пока нет
- Orsat Gas AnalysisДокумент3 страницыOrsat Gas AnalysisSandeep MishraОценок пока нет
- 9700 w16 QP 23Документ16 страниц9700 w16 QP 23DevalОценок пока нет
- Gore Gwiazda Jezusowi Nuty Na FletДокумент3 страницыGore Gwiazda Jezusowi Nuty Na FletJesusОценок пока нет
- Physical Geography A Landscape Appreciation 9Th Edition Mcknight Test Bank Full Chapter PDFДокумент57 страницPhysical Geography A Landscape Appreciation 9Th Edition Mcknight Test Bank Full Chapter PDFlegacycuttinglkhd100% (5)
- Traces of Volatile Chlorides in Butane-Butene Mixtures: Standard Test Methods ForДокумент8 страницTraces of Volatile Chlorides in Butane-Butene Mixtures: Standard Test Methods ForDaniel HernándezОценок пока нет
- Q1. Write A Brief Note On OxygenДокумент3 страницыQ1. Write A Brief Note On OxygenRonnith NandyОценок пока нет
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeОт EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeРейтинг: 4.5 из 5 звезд4.5/5 (3)
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeОт EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeРейтинг: 5 из 5 звезд5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeОт EverandChemistry for Breakfast: The Amazing Science of Everyday LifeРейтинг: 4.5 из 5 звезд4.5/5 (14)
- Napoleon's Buttons: 17 Molecules That Changed HistoryОт EverandNapoleon's Buttons: 17 Molecules That Changed HistoryРейтинг: 4 из 5 звезд4/5 (25)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableОт EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableРейтинг: 3.5 из 5 звезд3.5/5 (22)
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsОт EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsРейтинг: 4 из 5 звезд4/5 (146)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolОт EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolОценок пока нет
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsОт EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsРейтинг: 5 из 5 звезд5/5 (3)
- Chemistry: a QuickStudy Laminated Reference GuideОт EverandChemistry: a QuickStudy Laminated Reference GuideРейтинг: 5 из 5 звезд5/5 (1)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilОт EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilРейтинг: 5 из 5 звезд5/5 (1)
- The Periodic Table: A Very Short IntroductionОт EverandThe Periodic Table: A Very Short IntroductionРейтинг: 4.5 из 5 звезд4.5/5 (3)
- Stuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldОт EverandStuff Matters: Exploring the Marvelous Materials That Shape Our Man-Made WorldРейтинг: 4 из 5 звезд4/5 (289)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeОт EverandChemistry for Breakfast: The Amazing Science of Everyday LifeРейтинг: 4.5 из 5 звезд4.5/5 (90)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeОт EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeРейтинг: 4 из 5 звезд4/5 (9)
- Guidelines for Asset Integrity ManagementОт EverandGuidelines for Asset Integrity ManagementРейтинг: 5 из 5 звезд5/5 (1)
- An Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksОт EverandAn Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksРейтинг: 5 из 5 звезд5/5 (1)
- Chemical Elements Pocket Guide: Detailed Summary of the Periodic TableОт EverandChemical Elements Pocket Guide: Detailed Summary of the Periodic TableОценок пока нет
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeОт EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeРейтинг: 4 из 5 звезд4/5 (1)
- Perfume Engineering: Design, Performance and ClassificationОт EverandPerfume Engineering: Design, Performance and ClassificationРейтинг: 4 из 5 звезд4/5 (5)
- Introduction to Biological and Small Molecule Drug Research and Development: Theory and Case StudiesОт EverandIntroduction to Biological and Small Molecule Drug Research and Development: Theory and Case StudiesC. Robin GanellinРейтинг: 5 из 5 звезд5/5 (1)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincОт EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincРейтинг: 3.5 из 5 звезд3.5/5 (150)