Академический Документы
Профессиональный Документы
Культура Документы
Method HPLC Ezetimibe
Загружено:
JP López Fraire0 оценок0% нашли этот документ полезным (0 голосов)
40 просмотров6 страницSimple, accurate, precise and economical HPLC method was developed for estimation of ezetimibe in tablet dosage form. Liquid chromatography method was extensively validated for linearity, range, accuracy and precision (intermediate precision, repeatability)
Исходное описание:
Авторское право
© © All Rights Reserved
Доступные форматы
PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документSimple, accurate, precise and economical HPLC method was developed for estimation of ezetimibe in tablet dosage form. Liquid chromatography method was extensively validated for linearity, range, accuracy and precision (intermediate precision, repeatability)
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
40 просмотров6 страницMethod HPLC Ezetimibe
Загружено:
JP López FraireSimple, accurate, precise and economical HPLC method was developed for estimation of ezetimibe in tablet dosage form. Liquid chromatography method was extensively validated for linearity, range, accuracy and precision (intermediate precision, repeatability)
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 6
Prabhat K Shrivastava et al
Int.J.Ph.Sci, May-August 2009; 1(1):174-181
www.ijps.info
176
Original Research Manuscript
Article History: Int.J.Ph.Sci, May-August 2009; 1(1):176-181
Received on: 17-08-09 Available online from: www.ijps.info
Accepted on: 07-09-09 Copyright 2009 www.ijps.info
VALIDATED RP- HPLC METHOD FOR ESTIMATION OF EZETIMIBE IN DIFFERENT
TABLET DOSAGE FORM
PRABHAT K. SHRIVASTAVA
1*
, PAWAN K. BASNIWAL, SUSHANT K. SHRIVASTAVA
1
, DEEPTI JAIN
2
,
1
Department of Pharmaceutics, Institute of Technology, Banaras Hindu University, Varanasi (U.P.) -221 005
2
School of Pharmaceutical Sciences, Rajiv Gandhi Proudyogiki Vishwavidyalaya,
Airport By pass Road, Gandhi Nagar, Bhopal (M.P.) 462 036.
L.B.S. College of Pharmacy, Tilak Nagar, Jaipur 302 004, Rajasthan
ABSTRACT
Simple, accurate, precise and economical HPLC method was developed for estimation of ezetimibe in tablet dosage form. To achieve the
separation, the mixture of solvent acetonitrile and methanol in ratio of 50:50 v/v was selected as mobile phase. This mixture was found to be
appropriate allowing good separation of the ezetimibe at retention time 4.9 minutes at flow rate of 1.0 ml/min and detection wavelength 245.0
nm. The linearity was found in the concentration range 0 50.0 !g/ml. Linearity was determined by the linear regression equation for the drug and
correlation coefficient was found to be 0.998 indicating good linearity. The liquid chromatography method was extensively validated for linearity,
range, accuracy and precision (intermediate precision, repeatability). All these analytical validation parameters were observed and the RSD
was found to be less than one, which indicates the validity of method.
Key-words- Reverse Phase High Performance Liquid Chromatography (RP-HPLC); Ezetimibe; Method Validation; ICH Guidelines
INTRODUCTION
Ezetimibe, 1- (4-fluorophenyl)-3 (R) [3 (4
fluorophenyl) -3 (S) hydroxyl propyl]- 4 (S) (4
hydroxyphenyl)-2- azetidione
1
, a new lipid-lowering
agent, which inhibits the absorption of cholesterol from
* For Correspondence
Department of Pharmaceutics, Institute of Technology,
Banaras Hindu University, Varanasi, U.P., India-221 005
Phone No- 0542-2307049, Fax No- 0542-2368428
E-mail: sushant_itbhu@rediffmail.com
sprabhats@rediffmail.com
intestine
2-3
. Literature survey reveals that few
chromatographic methods are reported for estimation of
ezetimibe in tablet dosage from
4-5
. The objective of
present communication is to developed simple, rapid
(less run time), and economical in case of mobile phase
composition, precise RP-HPLC method for estimation
of ezetimibe in tablet dosage form.
Prabhat K Shrivastava et al
Int.J.Ph.Sci, May-August 2009; 1(1):174-181
www.ijps.info
177
EXPERIMENTAL
Materials and method
Ezetimibe working standard was obtained as generous
gift from Torrent Pharmaceuticals, Baroda. Ezetimibe
tablets were procured from market [Zetia Torrent
Pharmaceutical, Ezta Zydus Cadila and Ezedoc
Pinnacle Lupin]. HPLC grade acetonitrile and methanol
were obtained from Merck (India) Limited.
Instrumentation and chromatograph
The HPLC system consisted of a solvent delivery
module LC-10ATvp Shimadzu Liquid chromatograph
pump equipped with 20 !l loop and model SPD
M10Avp Shimadzu UV/VIS diode array detector.
Integration was achieved by using the software LC-10
7
. Separation was carried out on a Phenomenex,
(250x4.6mm) Luna 5! C-18 (2) 100A column. The
chromatographic analysis was performed at ambient
temperature. The mobile phase consisted of the mixture
of solvents, acetonitrile: methanol in the ratio 50:50;
v/v. The prepared mobile phase was filtered through
ultipore N
66 nylon, 6,6 membrane (0.45!m) filter and
ultrasonically degassed prior to use. The detection
wavelength was set 245.0 nm and the peak area was
recorded using chromatographic data system. The flow
rate and runtime was set to 1.0 ml/min and 10.0 minute
respectively.
Preparation of solution
All the system suitability parameters capacity factor,
plate number, tailing factor and retention time was
optimized by freshly prepared standard solution of 10.0
!g/ml. To develop a suitable method accurately
weighed 10.0 mg of ezetimibe working standard was
transferred into 50 ml volumetric flask, dissolved in 5.0
ml of mobile phase than volume was made up to 50.0
ml with mobile phase to get concentration of solution
200 !g/ml (Stock-A). The 12.5 ml volume of stock-A
was taken and diluted up to 25.0 ml to get concentration
of 100 !g/ml (Stock-B). Further dilution were made
from stock-B to get a series of concentration 10, 20, 30,
40, and 50 !g/ml and before the injection the
solution was filter through 0.45 micron HPLC
filter and subjected for analysis. Peak area under
the curve of mixed standard were observed and plotted
against respective concentration. The representative
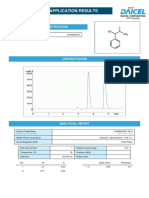
chromatogram of ezetimibe was given in figure no. 1.
Prabhat K Shrivastava et al
Int.J.Ph.Sci, May-August 2009; 1(1):174-181
www.ijps.info
178
Figure-1 HPLC chromatogram of Working Standard of Ezetimibe
Method validation
The LC Method for estimation of ezetimibe was
extensively validated as per ICH guideline for
"analytical method validation", for linearity, range,
stability, accuracy and precision (repeatability and
intermediate precision)
8
. Different system suitability
parameters were observed is retention times, capacity
factor, tailing factor and plate number. For linearity,
series of dilutions were prepared and response ratios
were determined respectively. The range was
determined by preparing a series of dilutions from 80 %
to 120 % of test concentration in six replicate. For the
stability of the drugs, solutions were stored at room
temperature and analysed on HPLC at different time
intervals. Accuracy of methods was determined by
recovery studies in which known amounts of standard
drug were added to the previously analysed tablet
sample and mixture were analysed by the proposed
method. Precision was studied for repeatability and
intermediate precision (days, and analysts).
TABLE 1:
RESULTS OF SYSTEM SUITABILITY PARAMETER*
Retention time
(min)
Capacity Factor Plate Number Tailing Factor
4.959 0.86 8913 1.24
*Mean values of six replicates
Prabhat K Shrivastava et al
Int.J.Ph.Sci, May-August 2009; 1(1):174-181
www.ijps.info
179
Tablet analysis
For analysis of tablet sample, twenty tablets were
accurately weighed and than average weight of the
tablet were determined. The tablets were powdered and
powder equivalent to 10.0 mg of ezetimibe accurately
weighed and dissolved in mobile phase and volume was
made-up to 50 ml. Resulted solution was sonicated for
10 min and filtered through Whatman filter paper no.
42 and diluted to get the concentration in the range of
linearity. Before the injection solution was filter
through 0.45 micron HPLC filter and subjected for
analysis. The peak area under the curve of tablet
solution was observed and plotted against respective
concentration.
RESULTS AND DISCUSSION:
All the system suitability parameters plate number,
tailing factor and retention time was optimized before
starting the experiment and this parameter were found
with in the limit that shows the stability of method
(table 1). For standard drug, linearity was observed by
linear regression equation method. Equation is AUC =
0.423 x 10
6
X 0.0111 x 10
6
and correlation coefficient
for drug was found to be more than 0.998, indicating
good linearity (table 2).
TABLE 2:
RESULTS OF LINEAR REGRESSION ANALYSIS
Regression Parameter Observation
Regression equation
Slope of curve (m)
Intercept (C)
Correlation Coefficient (r
2
)
Linearity range (!g/ml)
Absorption maxima (nm)
AUC = mx + C
0.423 x 10
6
0.0111 x 10
6
0.998
0-50
245.0
The LC Method has been extensively validated for
estimation of ezetimibe using linearity, range, accuracy
and precision. All these analytical validation parameters
were observed and the RSD was found to be less than
one, which indicates the validity of method (table 3).
The concentration of ezetimibe was estimated in the
three different branded tablet dosage from. Sample
were found to be in the range 99.21-101.58 %, while
the % relative standard deviation values obtained from
replicate analysis of tablet were found to be in the
range of 0.135-0.269, which indicates satisfactory
applicability and reproducibility of the method (table
4).
Prabhat K Shrivastava et al
Int.J.Ph.Sci, May-August 2009; 1(1):174-181
www.ijps.info
180
TABLE 3:
RESULTS OF VALIDATION PARAMETER
Validation
Parameter
Mean
a
SD
a
%RSD
a
Linearity
Accuracy
Precision-
Repeatability
Intermediate
precision
- Days
- Analyst
99.50
100.82
100.23
99.08
99.72
0.121
0.448
0.065
0.084
0.182
0.216
0.563
0.272
0.328
0.463
a
Maximum values of SD and RSD of six replicates, SD = Standard deviation
RSD = Relative standard deviation
TABLE 4:
RESULTS OF TABLET ANALYSIS FOR EZETIMIBE
Tablet
formulation
Label Claim
(mg/tab)
Percentage of label
estimated* mg/tab
SD RSD SE!
A
B
C
10
10
10
99.21-100.35
99.54-101.23
100.56-101.58
0.204
0.228
0.134
0.269
0.234
0.135
0.091
0.053
0.054
Maximum and minimum values of three different
concentration level in three replicates, A Zetia, 10mg
tablet of Torrent Pharmaceutical, B- Ezta, 10mg tablets
of Zydus Cadila, and C- Ezedoc, 10mg tablets of
Pinnacle Lupin.
This method was found to be simple, economical, rapid,
accurate and precise for estimation of ezetimibe in
different tablet dosage form and as the method was
extensively validated therefore has routine industrial
application.
ACKNOWLEDGEMENTS
The authors wish to thanks, Head of the Department,
School of Pharmaceutical Sciences, Rajiv Gandhi
Proudyogiki Vishwavidyalaya, Bhopal, for providing
necessary facilities and Torrent Pharmaceuticals,
Baroda, for supplying ezetimibe as a gift sample. The
financial assistance received from AICTE in the form
of fellowship for postgraduate studies in Engineering
and Technology is being gratefully acknowledged by
one of the author.
REFERENCES
1. Scheen AJ. Ezetimibe (Ezetrol), Rev. Med. Liege.
2004; 59 (4): 246 250.
Prabhat K Shrivastava et al
Int.J.Ph.Sci, May-August 2009; 1(1):174-181
www.ijps.info
181
2. Davidson MH. Ezetimibe. A novel option for lowering
cholesterol. Expert Rev. Cardiovasc. Ther. 2003; 1 (1):
11-21.
3. Darkes MJ, Poole RM, Goa KL. Ezetimibe. Am. J.
Cardiovasc. Drugs, 2003; 3 (1): 67-76.
4. Sistla R, Tata VSSK, Kashyap YV, Chandrasekar D,
Diwan PV. Development and validation of a reversed-
phase HPLC method for the determination of ezetimibe in
pharmaceutical dosage forms. J Pharm Bio Ana. 2005;
39: 517522.
5. Singh S, Singh B, Bahuguna R, Wadhwa L, Saxena R.
Stress degradation studies on ezetimibe and development
of a validated stability-indicating HPLC assay. J Pharm
Bio Anal. 2006; 41: 10371040.
6. Davidson AG. The Ultra violetvisible absorption
spectrophotometry practical pharmaceutical chemistry,
4th Ed.; Beckett AH, Stenlake JB, Eds, CBS Publishers
and Distributors, New Delhi, 1997; Vol. 2: 296-300.
7. Shimadzu HPLC User's Manual, LC-10 ATVP,
Shimadzu CorporationAnalytical and Measuring
instrumentals Division, Kyoto, Japan; 2-4.
8. Code Q 2B validation analytical procedure step. 4,
consensus guidelines ICH harmonized tripartite
guidelines, 1994.
******************************************
Source of Support: NIL
Conflict of Interest: NONE
Вам также может понравиться
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Amphetamine ChiralДокумент19 страницAmphetamine ChiralJP López Fraire100% (1)
- Norefedrina Columna AD HДокумент1 страницаNorefedrina Columna AD HJP López FraireОценок пока нет
- Columns HPLC EPДокумент4 страницыColumns HPLC EPJP López FraireОценок пока нет
- Chromatography 2008011462Документ1 страницаChromatography 2008011462JP López FraireОценок пока нет
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- Arguing A PositionДокумент17 страницArguing A PositionmsdrawbondОценок пока нет
- Leanhminh Btec d01 k12 BKC12089Документ23 страницыLeanhminh Btec d01 k12 BKC12089Minh Lê AnhОценок пока нет
- Full Thesis Elyan Rizky Fix PrintДокумент78 страницFull Thesis Elyan Rizky Fix PrintResiОценок пока нет
- Project 1 - Multi-Modal Literacy Narrative - Part 1 Literacy Experience AccountДокумент1 страницаProject 1 - Multi-Modal Literacy Narrative - Part 1 Literacy Experience AccountscoelongОценок пока нет
- The Diagnostic ProcessДокумент58 страницThe Diagnostic ProcessberitahrОценок пока нет
- Dangerous & Difficult Bulk Cargoes - Best Practice and The IMSBC CodeДокумент12 страницDangerous & Difficult Bulk Cargoes - Best Practice and The IMSBC CodeRadu GeorgeОценок пока нет
- Living in The Time of Tok Hang 2018Документ21 страницаLiving in The Time of Tok Hang 2018reynald_ecijaОценок пока нет
- DRRM Training MatrixДокумент2 страницыDRRM Training MatrixMhoty Fortney100% (3)
- MCQ's On Attitude in OB - SpeakHRДокумент15 страницMCQ's On Attitude in OB - SpeakHRNilesh VarshneyОценок пока нет
- Wahl Participative Political Institutions in Pre-Modern EuropeДокумент14 страницWahl Participative Political Institutions in Pre-Modern EuropeMauro FazziniОценок пока нет
- Teradyne CaseДокумент2 страницыTeradyne Casekwathom150% (2)
- Chapter 9a - Risk - Management & Accident PreventionДокумент31 страницаChapter 9a - Risk - Management & Accident PreventionEng. Ibrahim Abdullah AlruhmiОценок пока нет
- Flow Measurement HandbookДокумент9 страницFlow Measurement HandbookIzhamKhairi0% (1)
- Investigating The Impact of Marketing Mix Elements On Consumer Loyalty: An Emprical Study On Nigerian Breweries Plc.Документ12 страницInvestigating The Impact of Marketing Mix Elements On Consumer Loyalty: An Emprical Study On Nigerian Breweries Plc.Kumar SwamyОценок пока нет
- Sustainability Innovators and Anchor Draggers: A Global Expert Study On Sustainable FashionДокумент13 страницSustainability Innovators and Anchor Draggers: A Global Expert Study On Sustainable FashionKsОценок пока нет
- Sti Thesis FormatДокумент6 страницSti Thesis Formatbkrj0a1k100% (2)
- OrganizationalBehavior OP PDFДокумент703 страницыOrganizationalBehavior OP PDFSamuel Aina100% (3)
- The Role of Patients' Psychological Comfort in Optimizing Indoor Healing Environments A Case Study of The Indoor Environments of Recently Built Hospitals in Sulaimani City, Kurdistan, IraqДокумент16 страницThe Role of Patients' Psychological Comfort in Optimizing Indoor Healing Environments A Case Study of The Indoor Environments of Recently Built Hospitals in Sulaimani City, Kurdistan, IraqAndika AhsanaОценок пока нет
- CV Sjschmidt Sept 2016Документ5 страницCV Sjschmidt Sept 2016api-277536406Оценок пока нет
- Diagnostic Test-EAPPДокумент8 страницDiagnostic Test-EAPPJAHYRAH BARTOLOMEОценок пока нет
- Feelings of Blameworthiness and Their Associations With The Grieving Process in Suicide MourningДокумент9 страницFeelings of Blameworthiness and Their Associations With The Grieving Process in Suicide MourningYleis Vanessa Hernández SilvaОценок пока нет
- Looking at Movies An Introduction To Film PDFДокумент68 страницLooking at Movies An Introduction To Film PDFFabian FlorezОценок пока нет
- IIC Paper On Modelling The Intra Day Activity in Stock MarketДокумент32 страницыIIC Paper On Modelling The Intra Day Activity in Stock MarketabhimailsОценок пока нет
- Writing Effective Summaries and Avoiding Plagiarism: What Is A Summary?Документ3 страницыWriting Effective Summaries and Avoiding Plagiarism: What Is A Summary?tgrlОценок пока нет
- Focalizacion SensorialДокумент14 страницFocalizacion SensorialCarlos Contreras SalasОценок пока нет
- Research in Daily Life: Understanding Data and Wa Ys To Systematically Collect Dat AДокумент24 страницыResearch in Daily Life: Understanding Data and Wa Ys To Systematically Collect Dat AOkaayОценок пока нет
- Brand Love ScalesДокумент24 страницыBrand Love ScalesVishal AgarwalОценок пока нет
- University of Eswatini Dlamini Kuhle L. DEM 102: Fertility Assignment 202002378Документ6 страницUniversity of Eswatini Dlamini Kuhle L. DEM 102: Fertility Assignment 202002378KuhleОценок пока нет
- 7basic Econometrics Chapter ViДокумент16 страниц7basic Econometrics Chapter ViAfsheena MeccaОценок пока нет
- Inua Jamii Senior Citizens' Scheme: Main Lessons LearnedДокумент6 страницInua Jamii Senior Citizens' Scheme: Main Lessons LearnedreaganОценок пока нет