Академический Документы
Профессиональный Документы
Культура Документы
Mesi art:10.1007/BF00905951
Загружено:
Abhijeet H Thaker0 оценок0% нашли этот документ полезным (0 голосов)
6 просмотров8 страницImportant for acidity reduction
Оригинальное название
Mesi_art%3A10.1007%2FBF00905951
Авторское право
© © All Rights Reserved
Доступные форматы
PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документImportant for acidity reduction
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
6 просмотров8 страницMesi art:10.1007/BF00905951
Загружено:
Abhijeet H ThakerImportant for acidity reduction
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 8
DEALKYLATIONOFMESITYLENE ANDASSOCIATED
ALKYLATIONOF BENZENE ANDTOLUENE INPRESENCE
OF SYNTHETIC ALUMOSILICATES
A. V. T o p c h i e v , G. M. Ma me d a l i e v , a n d L. S. Ko v a l e v a
Institute of Pet rochemi cal Synthesis, Academy of Sciences, USSR
Translated from Izvest i ya Akademi i Nauk SSSR, Otd. I<'himicheskilah
Nauk, 1961, No. 5,
pp. 868-876, May, 1961
Original art i cl e submitted February 8, 1960
Deal kyl at i on of xylenes and the associated al kyl at i on of benzene in presence of al umi num chloride [ 1- 7] , and
also of natural and synthetic al umosi l i cat es [8,9] has been studied in the works of a series of authors. These investi-
gations had as their goal the devel opment of a met hod of industrial production of toluene. Latterly, in connect i on
with the widespread devel opment of the production of a whole series of synthetic mat eri al s, the isomeric xylenes have
achi eved exceedi ngl y i mport ant pract i cal significance. One of the prospective methods for p- xyl ene production, as
has al ready been noted by us [10-12] and subsequently by other authors [13-16], is the isomeric conversion of m- and
o- xylenes into the para- isomer, achi eved compar at i vel y easi l y in presence of synthetic al umosol i cat es under at mos-
pheric pressure and in vacuo.
In our works it has been shown that the xylenes can be synthesized by deal kyl at i on and associated al kyl at i on of
ar omat i c hydrocarbons derived from pol yal kyl benzene sources included in the products of pyrol yt i c coke- gas produc-
tion and of industrial ar omat i zi ng reformat i on of narrow gasoline fractions [17,18]. Thus, on cat al yt i c t r eat ment of
a mi xt ure of toluene and the solvent from coke-gas production (mai nl y a mi xt ure ofpseudocumene, mesi t yl ene and
TABLE 1. Properties of Products of Cat al yt i c Tr eat ment of a Mixture of Benzene and
Mesi t yl ene (t emperat ure 480~ flow r at e 0.5 hour -1)
Fract i onal composition
Commencement of boiling
C. b . - 78
78-- 83
83-- 88
88--t03
103--t08'
108--t t 3
!.t3--118
t i 8--125
1 2 5 - - 1 3 6 9
136--144
t44--149
149--t60
t60--16~
165--i T
End of boiling, ~
Tot al yi el d, wt. %
Residue, Wto %
Losses, wt. %
Mat eri al bal ance, wt. %
Cat al yzat e
Coke -
Gas
Losses
Raw materia~ Expt,. 233, [ Expt. 232, I Expt.421,
mesitylene: , atmospheri~ 10 at m
benzene =1: 2~ 5 at m
79 I 74
Yield of fraction,
- - 4,45
64--69 55,57
0,70
i , 66
0,98
1,77
0,72 0, t8
0,56
t , 42
6,64
0, t 8
0,88
29,90 4,38
t4, 63
165,5 i72, 3
98,77 94,00
3,46 3,04
t , 33 2,96
97,3
0,9
Z } i , 8
I 7 i 7 8
wt. %
t , 83
51,06 49,4i
0,50 0, i 9
0, 7l 0,67
1,05 0,49
i t , 21 t8, 72
0,53 0,24
0,48 0,33
0,81 t , 09
t5, 40 16.2i
0,63 0,63
t , t 6 0,97
t , 87 t , 29
5,64 3,43
167,5 168
92,88 93,6i
4, 8t 4,76
2,3t t , 78
93,4 93,7
2,4 3,6
1,8 i , i
2,4 t , 6
Expt. 286,
15 at m
62
0,34
45,68
0,36
0,85
0,79
22,32
0,35
0,42
3, 2i
i4,01
0,46
0,92
i , 07
3,05
i69,5
93,83
3,20
2,97
9i , 2
4,6
t , 9
2,3
801
TABLE 2. Properties of Basic Aromat i c Fractions
,,, , , ,
Raw mat eri al
Properties of fractions mesi t yl ene Expt. 233
b e n z e n e =I:
Fraction 78 - 83*
Yield, wt. ~
d ~
Iodine number
Fraction I 0 8 - I 1 3 0
Yield, wt. ~/o
Iodine number
Fraction 136- 144 ~
Yield, wt. %
Iodine number
Fraction 160- 165 ~
Yield, wt. %o
2o
n D
d ~
4
Iodine number
Fraction 165- 175 ~
Yield, wt. %
Iodine number
Expt. 232 Expt. 421
63,69
t,5017
0,8776
0
55,57
1,5000
0,874t
0
5t, 06
t,4984
0,8731
0
49,4t
1,5009
0,8743
0
- - - 7 -
29,90
i,4995
0,8663
0
1,77
t,498~
0
6, 64
1,499t
O, 8659
0,3
4,38
I , 5043
O, 8749
0
t4, 63
t , 505t
0, 8743
0
t l , 21
t,4975
0,8672
0
15,40
t,4990
0,8674
0
t , 87
1,5029
0
5,64
i,5049
0,8745
0
t8, 72
1,4978
0,8668
0
t6,21
1,4990
0,8656
0
1,29
t,5015
3,43
1,5032
0
Expt, 235
45,68
t,5014
0,8779
0
22,32
1,4982
0,8676
0
! 4, 0t
1,4990
0,8684
0
t , 07
1,50t3
3,05
t,5045
0,8754
0
hemi mel l i t ol ) under opt i mmn t emperat ure and pressure conditions, yield of xylenes in one operation amount ed to
28% [19]. This process consists of a complex of a number of reactions occurring together and interdependently:
deal kyl at i on, al kyl at i on, dismutation, and isomeric conversions of initial aromat i c compounds.
In studying these reactions it was expedi ent to carry out an exper i ment al investigation on conversion of indi-
vidual al kyl ar omat i c hydrocarbons. A study had been made previously of demet hyl at i on and al kyl group transfer of
pseudocumene in presence of toluene and benzene over synthetic al umosi l i cat es. It was shown that on cat al yt i c t r eat -
ment of a mi xt ure of pseudocumene and benzene, deal kyl at i on of pseudocumene and associated al kyl at i on of benzene
t ook pl ace mai nl y, with format i on of - 20% toluene and - 17% xylenes. Similar conversions were observed on
50
2 0
c - - . 4 . -
J
I I
5 ~ 75
Pl~SSUre ~ at m
Fig. 1, Relationship between ar omat i c hydrocarbon
yield and pressure during t r eat ment of a benzene -
mesi t yl ene mixture: 1) benzene; 2) toluene; 9b xy-
lenes; 4) t ri met hyl benzenes.
t reat i ng a mixture of pseudocumene and toluene. In the
present communi cat i on resuks are given of an investigation
of deal kyl at i on, transfer of mesi t yl ene met hyl groups and
associated al kyl at i on of benzene and toluene in presence
of synthetic al umosi l i cat es.
EXP ERI MENTAL
As the i ni t i al products the following were used:
mesitylene (boiling range 164.5-164.8~ 1.5002;
0.8668), cryoscopic benzene (78.5-80'~ nnZ~ dZ. ~
0.8790), toluene (109.9-111~ n ~ 1. 4970, -d~ 0 0.8669). 4
Experiments were carried out in ci rcul at ory reactor
equi pment with a stationary cat al yst l ayer, the scheme and
description of which were given in the work [18]. Spectral
investigations of basic products of mesi t yl ene conversion
were carried out in the laboratory of physi cochemi cal
investigation methods by A. N. Kislinskii.
802
Demet hyl at i on of Mesi t yl ene and Associ at ed Met hyl at i on of Benzene. A mi xt ure of mesi t yl ene and benzene
in the r at i o by wei ght 1. 2 was subj ect ed t o cat al yt i c t r eat ment . The product was passed once over the cat al yst at a
t emper at ur e of 480*, flow rat e 0. 5 hour "1 and vari ous pressures. The mat er i al bal ance of the process, propert i es of
t he i ni t i al mi xt ur e and the cat al yzat es obt ai ned from its t r eat ment and t hei r basic ar omat i c fract i ons are gi ven in
Tabl es 1 and 2. Curves showi ng rel at i onshi p bet wenn a r oma t i c hydr ocar bon yi el d and pressure are gi ven in Fig. 1.
At at mospher i c pressure yi el d of r eact i on product s amount ed t o 97. 3 %, gas - f or mat i on was pr act i cal l y unob-
served, coke deposits on cat al ys t amount ed t o 0.901o, Even under these condi t i ons a cer t ai n amount of mesi t yl ene
deal kyl at i on and associ at ed benzene al kyl at i on was observed. Yi el d of t ol uene f r act i on amount ed to 1. 7%, of xyl ene~-
- 7%. Increase of pressure to 5 at m. not i ceabl y i nt ensi fi ed the r eact i on di r ect i on i nt ended. As a resul t of i nt ensi fi -
cat i on of t ransfer of the me t hyl group from t he mesi t yl ene mol ecul e t o the benzene mol ecul e, cont ent of t ol uene
and xyl ene f r act i on in the cat al yzat e i ncreased r espect i vel y t o 11. 2 and 15. 4%. Yi el d of c a t a l yz a t e amount ed t o
- 93%, of g a s - 1. 8%, of coke- 2. 401o. At 10 at m. pressure yi el d of t ol uene and xyl ene f r act i on i ncreased and a-
mount ed t o 18.7 and 17% r espect i vel y. Amount of benzene f r act i on decr eased from - 64% in the i ni t i al mi xt ure
to ~ 49% in the c a t a l yz a t e , t r i met hyl benzene f r act i on from ~ 30% t o 4. 5%. At 15 at m. pressure the process was
char act er i zed by c ompa r a t i ve l y l arge coke - and gas- f or mat i on (4.6 and 1.9% r espect i vel y) . Yi el d and qual i t y of
basi c ar omat i c fract i ons did not di ffer subst ant i al l y f r om the dat a of the exper i ment car r i ed out at 10 at m.
At a t emper at ur e of 480 ~ and 5- 10 at m. pressure cat al yt i c t r eat ment of a mi xt ur e of mesi t yl ene and benzene
over al umosi l i cat es resul t ed ma i nl y in mesi t yl ene deal kyl at i on and associ at ed benzene al kyl at i on
I t
_ / / \ _
%/
/ / \
\ /
In addi t i on, under the exper i ment al condi t i ons i somer i c conversi ons of mesi t yl ene t ook pl ace, as a resul t of
whi ch t he t r i met hyl benzene fract i ons of t he c a t a l yz a t e were char act er i zed by a hi gh pseudocumene cont ent .
Demet hyl at i on of Mesi t yl ene and Associ at ed Met hyl at i on of Tol uene. A mi xt ur e of t ol uene and mesi t yl enr in
the r at i o by wei ght 2: 1 was subj ect ed t o c a t a l yt i c t r eat ment . Anal yt i c dat a on t he cat al yzat es obt ai ned and t hei r
basi c a r oma t i c fract i ons are gi ven in Tabl es 3 and 4. Experi ment s were car r i ed out at a t emper at ur e of 480 ~ rat e of
raw ma t e r i a l i nfl ow 0.5 hour -1 and vari ous pressures. Curves showi ng r el at i onshi p bet ween yi el d of t ol uene, xyl enes,
t r i met hybenzenes , and pressure are gi ven in Fi g. 2.
At amospher i c pressure yi el d of c a t a l yz a t e yi el d amount ed t o 99. 4%, gas - f or mat i on was not observed, and
yi el d of c oke - l i ke r eact i on product s did not e xc e e d 0.4~ As a resul t of mesi t yl ene deal kyl at i on and associ at ed t o-
l uene al kyl at i on -~ 14% of xyl enes was f or med, as a resul t of whi ch cont ent of t ol uene and t r i met hyl benzene f r ac-
tions decr eased r es pect i vel y from 65. 3 and 31. 1% in t he i ni t i al mi xt ur e t o 56.7 and 20.101o in t he c a t a l yz a t e . I n-
crease in pressure t o 3- 5 at m. l ed t o i ncrease in yi el d of xyl ene f r act i on t o 26. 7 and 32%r es pect i vel y. At t he same
t i me, as a resul t of par t i al hydr ocr acki ng of side chai ns of i ni t i al and resul t i ng ar omat i c hydrocarbons, f or mat i on of
- 2-301o benzene was observed.
Furt her i ncrease in pressure t o 10 at m. had no subst ant i al ef f ect on yi el d of basi c ar omat i c hydrocarbons,
Sever al exper i ment s were car r i ed out at 5 a t m. pressure over t he t emper at ur e range 425- 480". In Fig. 3 is
shown the r el at i onshi p bet ween yi el d of basic ar omat i c cat al yzat e fract i ons and t emper at ur es. At 425* xyl ene f r ac-
t i on yi el d amount s t o 96% as agai nst 30% at 480*~ Further decr ease in t emper at ur e depresses me t hyl group transfer,
and t he c a t a l yz a t e is char act er i zed by compar at i vel y l ow xyl ene cont ent .
803
TABLE 3. Properties of Products of Cat al yt i c Tr eat ment of a Mixture of Tol uene
. . . . . . . . . . . Raw ma -
Fractional composi t i on t i al , me = Temper at ur e 480"
and properties of product fi t yl ene: atmospheric [ 3 at m 5 at m
toluene =
= 1 : 2 expt . 219 I expt . 221 expt . 220
Commencement of boiling, *C
C. b. -78
78-- 83
83-- 88
88--103
10"3--]08
108--t t 3
t t 3 - - t t 8
1t 8--i 25
t25--136
136--144
144--149
149--i60
160--i65
165--175
i75--185
End of boi l i ng, ~
Tot al yi el d, wt. %
Residue
Losses
Mat eri al bal ance, wt. %
Ca t al yzat e
or e
Gas
L o s s e s
t06
0,26
65,32
0,I0
0,17
0,25
0,13
0,16
0,68
3 t , t t
164,8
98,18
1,70
0, t2
75 i 53
Yield of f r act i on, wt.
0,18 0,23
0,33 2,69
0,20 0,24
0,56 0,78
0,90 0,50
56,70 50,73
0,24 0, t 8
0,25 0,72
0,67 0,55
13,93 26,69
0,48 0,88
0,93 0,96
3,86 2,75
t7, 33 8,95
{73 i 7i
96,56 96, 46
t , 72 2,71
1,72 0,80
99,4i 95,7
0,38 2,3
0,2f } 2,0
75
0,27
3,03
0,13
0,65
1,14
43,04
0,56
0,71
2,21
30,01
0,63
t , 06
t , 62
10,36
0,96
176
96,38
3,00
0,62
94,2
2,6
1,8
t , 4
) )
%/ %/
% /
% /
-L 11_
% /
50
4 0 ! I ) .
IO
2 ~-- I ' L T 1
495 r
i
) ,
!
os
Fig. 3. Relationship between yield of basic aromat i c
fractions and t emperat ure: 1) benzene~ 2) toluene~
3) xylenes; 4) trimethylbertzenes.
! , ) "
0 t 2 3 e 5
Pressure, at m
Fig. 2. Relationship between yield of ar omat i c hydro-
carbons and pressure in t reat ment of a t ol uene- mesf -
t yl ene m/ xt ure: 1) benzene; 2) t ol uene; 3) xyl enes;
4) t ri met hyl benzenes.
Results of investigations carried out showed t hat under
opt i mum conditions (480*; 5 at m. pressure; flow rat e 0.Shr "1)
t r eat ment of a mi xt ure of toluene and mesi t yl ene over al umo-
si l i cat e was charact eri zed mai nl y by occurrence of mesi t y-
lone demet hyl at i on and associated toluene met hyl at i on with
f o r ma t i o n of 30-82% of a mi xt ure of xylenes. According to
spect ral anal yt i cal data, the cat al yzat e xylene fraction con-
sisted of a mi xt ure of ~ 50% m- xyl ene, - 20% p- xyl ene
and ~ 30% o-xylCne. As a result of isomeric mesi t yl ene
804
TABLE 4.
Properties of fractions
Properties of Basle Ar omat i c Fractions
Raw ma t e r i a l
mesi t yl ene : Expt. 2!9
toluene =l: 2
Fraction 78--83*
Yield, wt. %
Iodine number
Fraction 108- 113 ~
Yield, wt. %
d~ 0
Iodine number
Fraction 136-144"
Yi el d, wt. %
n~
dZ0
4
Iodine number
Fraction 160-165 ~
Yield, wt. %
ng
Iodine number
Fraction 165-175"
Yi el d, wt. '%
n D
Iodine number
m
65;32
1,4977
0,8660
0
31,1t
1 , 5 0 0 4
0,8756
0
m
m
0,33
1 , 4 9 0 4
56,70
1,4977
0,8678
0
t3,93
t,4998
0,8675
0
3,86
1,50t3
0,8700
0
17,33
t,5058
0,8764
0,8
Expt. 221
2, (9
t,4990
0,874i
0
50,37
1,4980
0,8656
0
26,69
t,4999
0,8660
0
2,75
t , 50t 8
0,8686
0
8,95
t,5054
0,8757
0,7
Expt. 220
3,03
1 , 4 9 9 0
2 . 2
4 3 , 0 4
~,4978
0,8652
0
3 0 , 0 i
1 , 4 9 9 0
0,8666
0
1,62
t,5017
0
t0, 36
1,5048
0,8731
0
,3
93o
/0
I , I i i
0 1 g .~
Pressure, at m
Fig. 4. Relationship between yield of ar omat i c hydro-
carbons and pressure during t r eat ment of mesi ryl ene:
1) t ol uene; 2) xylenes; 3) t ri met hl benzenes; 4) t et r a-
met hyl benzenes.
conversion the resulting t ri met hyl benzene fraction of the
cat al yzat e cont ai ned a considerable amount of pseudo-
cumene. In this case, mesi t yl ene dismutation was pract i -
cal l y unobserved.
Met hyl Group Transfer and Isomeric Mesitylene Con-
version. Cat al yt i c treatnaent of mesi t yl ene in presence of
benzene and toluene, as has been shown, was charact eri zed
by occurrence of demet hyl at i on and associated met hyl at i on
of i ni t i al aromat i c hydrocarbons. Presence of benzene and
toluene depressed mesi t yl ene dismutation and appreci abl e
format i on of t et ramet hyl benzenes was not observed.
A series of experi ment s was carried out on cat al yt i c
t r eat ment of mesi t yl ene. The mat er i al bal ance of the
process and results of analysis of resulting cat al yzat es and
their aromat i c fractions are given in Tables 5 and 6.
Curves showing relationship between yield of basic con-
version products and pressure are given in Fig. 4.
At at mospheri c pressure - 40% of the initial mesi t yl ene underwent dismutation with format i on of 16% xylenes
and 24% t et ramet hyl benzenes. Yield of liquid react i on products amount ed to 94%, of g a s - 1 . 4 %, of coke-1. 6~
Increase in pressure not i ceabl y intensified disproportionation of mesi t yl ene met hyl groups. In addition, part i al mesi -
805
TABLE 5. Properties of Products of Cat al yt i c Mesitylene Conversion (flow rat e 0.5 hour -1)
Fractional composition
and product properties
a t m o s -
p h e r i c
expt. 223
Temperature 480*
Commencement of boiling, *C t09,5
" - 7 "
0,30
0,33
0,42
0,56
t 5, 90
0,88
2,38
2t , 31
30,74
2,88
t80
75,70
24,05
0,25
94,4
t , 6
t , 4
2,6
__2ai m 8 ai m I 5 ai m
expt. 231 expt. 228 [ expt. 226
90 I 84,5
Yield of fraction, wt . %
C.b. - - 88
88--103
103--108
t08--t13
t t 3- - 1t 8
t t 8- - t 25
t25--t36
136--t44
t 44--t 49
t 49--t 62
t62--167
t 67--t 72
t72--180
End of boiling
Tot al y i e l d , wt. %
R e s i d u e
L o s s e s
Mat eri al bal ance, wt. @o
Cat al yzat e
Coke
Gas
Loses
0,52
0,35
3,77
0, t 6
0,39
0,86
25,52
0, t 3
1,26
1t,94
26,59
4,09
t80
75,58
23,47
0,95
90,8
4,8
4,2
0,2
0, t 4
0,50
0,29
5,11
0,31
0,37
t , 27
.)6, 52
0,83
1,61
L2,75
).3,00
3, 40
t ~0
'6, t0
',0,72
3, t 8
;3,6
7,3
6,4
2,7
Temperature
400*
3 at m
expt. 236
83,5 80,5
0, t 7 0, t t
0, t 5 0,33
0,30 0,23
2,50 0,61
0,22 0,22
0, 49 0,34
t , 04 0,77
23,68 15,63
0,33 0,63
4, 3t 1,61
26,86 15,72
i2,76 35,2t
2,79 3,16
t80 t80
75,60 74,57
22,6t 24,98
t , 79 0,45
84,7 97,0
9,9 0,7
5, t
0,3 2,3
tylene cracking occurred with formation of 4-5% toluene. At 2 ai m. yield of xylene fraction amounted to 25%, of
t et ramet hyl benzene to - 28%. With increase in pressure, a noticeable increase was observed in yield of coke-l i ke
and gaseous reaction products.
At 3 at m. decrease in temperature to 400* depressed mesitylene hydrocracking, and in addition lowered the
dismutation ef f ect somewhat. Yield of xylene and t et ramet hyl benzcnes amounted to - 17% and ~ 25%. Cat ai yzat e
composition in this experi ment was pract i cal l y i dent i cal to the composition of the cat al yzat e obtained at atmospheric
pressure and 480*.
According to spectral anal yt i cal data, the xylene consisted of a mixture of - 45% m- xyl ene, - 25% o-xyl ene,
Thus, cat al yt i c mesitylene conversion over alumosilicates at 480* and 2-3 at m. pressure was charact eri zed
mai nl y by disproportionation of met hyl groups with formation of xylenes and tetramethylbenzenes:.
I
//\
- i
%/
%/
%/
i
I
%/
//\
%/
%/
//\
-I L
%/
806
TABLE 6. Properties of Basic Ar omat i c Fractions of Products of Mesi t yl ene Conversion
Properties of fract i on
Fraction 108- 113 ~
Yield, wt. %
Iodine number
Fraction 136- 144 ~
Y i e l d , wt. %
d ~ ~
~ o d i n e n u m b e r
Fraction 1 6 2 - 1 6 7 "
Yield, wt. %
G ~
Iodine n u m b e r
Fraction 1 6 7 - 1 7 2 ~
Yield, wt. ,~o
G ~
Iodine n u m b e r
Fraction 1 7 2 - 1 8 0 ~
Expt. 223
0,30
i,4942
1 5 , 9 0
i , 4 9 9 0
0 , 8 6 9 2
0
2 1 , 3 i
1 , 5 0 1 8
0 , 8 7 0 4
0,3
30,74
t,5052
0,8769
0,2
Expt. 281
3,77
t,4952
0,8659
0,28
2 5 , 5 2
1 , 4 9 9 9
0 , 8 6 7 8
0 , 2 0
i l , 94
i,5040
0,8728
0
26,59
1,5058
0,876i
0
Expt. 228
5, i i
1,496i
0,8636
0
26,52
1 , 4 9 9 8
0,8662
0
12,75
1,5020
0,8689
0
23,00
1 , 5 0 5 i
0,8755
0
Expt. 226 } Expt. 236
2,50
t,4959
0,8666
1,2
23,68
i , 4 9 9 0
O, 8662
1,5
26,86
t,5040
O, 8727
0,3
i2, 76
l , 5055
O, 8754
0,8
Yield, wt. %
d ~ ~
I o d i n e n u m b e r
R e s i d u e
Y i e l d , w t .
n ~
I o d i n e n u m b e r
S u l f o n a b i l i t y , v o l , %
2,88
1,5t05
O, 8868
0
24,05
1,5t75
O, 8960
0,2
lO0
4,09
i , 5092
O, 8820
0
23,47
O, 9014
1,8
lO0
3,40
i,5119
O, 8881
4,1
20,72
t , 5233
O, 9032
5,5
t00
2,79
i , , 5 0 7 3
2 2 , 6 i
t , 5f 34
O, 9006
1,5
iO0
0 , 6 1
i,4880
15,63
1,4982
O, 8628
0,5
15,72
1,5006
0,8677
0
35,21
t,5035
0,8753
0
3,16
1,5t03
0,8872
24,98
1,5174
0
LO0
In the course of the process a cert ai n amount of mesi t yt ene i someri zed with format i on of pseudocumene and
part i al l y of semi met l i t ene
% / % / % /
I
Besides the conversions indicated, hydrocracking of al kyl benzenes with format i on of a cert ai n amount of toluene,
met hane and its homologs occurred to a compar at i vel y smal l ext ent ,
807
S UMMAR Y
1. Cat al yt i c conversi on of mesi t yl ene mi xed with benzene was studied. Under condi t i ons of an el evat ed pressure
of 10- 15 at m. and a t emper at ur e of 480", deal kyl at i on of mesi t yl ene and associ at ed al kyl at i on of benzene occurs
mai nl y, with f or mat i on of 18- 22% t ol uene and - 17% xyl enes.
2. As a result of si ngl e- st age t r eat ment of a mi xt ure of mesi t yl ene and t ol uene over an al umosf l i cat e, 30- 32%
of a xyl ene f r act i on is f or med, consi st i ng of 45- 50% m- xyl e ne , ~ 20- 25% p- xyl ene, and - 30% o- xyl ene. Presence
of t ol uene and benzene depresses mesi t yl ene di smut at i on pr act i cal l y ent i r el y.
3. On cat al yt i c t r eat ment of mesi t yl ene in presence of al umosi l i cat es, di sproport i onat i on of mot hy1 groups occurs
mai nl y, with f or mat i on of xyl enes and t et r amet hyl benzenes. In this case, part i al hydr ocr acki ng of mesi t yl ene me t hyl
groups occurs, with f or mat i on of a smal l amount of t ol uene, met hane and its homol ogs.
L I T E R AT UR E C I T E D
1. R. Anschut z, and H. I mmendor f , Bet. 18, 657 (1885).
2. R. Heise and A. To11, Liebigs Ann. Chem. 270, 155 (1892).
3. O. Jacobsen, Ber. 18, 338 (1885).
4. L. I . Smi t h a nd O. W. Gass, J. Amer . Chem. See. 54, 1603- 1621 (1932).
5. I . F . Norris and J. N. I ngr aham, I . Amer . Che m. Soc. 6_22, 1298 (1940).
6. J . F. Norris and G. T. Vaal a, J. Amer . Chem. Soc. 61, 2131 (1939).
7. Ch. Fri edel and I. M. Craft s, Compt . rend. 109, 692 (1885).
8. B. L. Mol davski i and L. S. Bezdel ' , Zhur. Obshchei Khi m. , 16, 1633 (1946).
9. R. C. Hansford, C. G. Mej ers, and A. N. Sachanen. , Industr. and Engng. Chem. 37, 671 (1945).
10. Yu. G. Mamedal i ev, A. V. Topchi ev, G. M. Mamedal i ev, and G. N. Sul ei manov, Dokl ady Akad. Nauk SSSR,
106, No. 6, 1027 (1956).
11. A. V. Topchi ev, G. M. Mamedal i ev, and Yu. G. Mamedal i ev, Izvest . Akad. Nauk SSSR, Ot del . Khi m. Nauk,
No. 11, 1390 (1956).
12. G. M. Mamedal i ev, Yu. G. Mamedal i ov, and A. V. Topchi ev, Izvest . Akad. Nauk SSSR, Ot del . Tekhn. Nauk,
No. 6, 91 (1958).
13. E. F. Boedeker and W. E. Erner, J. Amer . Chem. Soc. 76, 5, 3591 (1954).
14. A. D. Sul i mov, V. I. Karisev, T. V. Zhakhovskaya, V. M. Ol evski i , E. G. Wendel st ei n, E. I. s i r c h e n k o , N. V.
Shavol i na, and A. A. Voi t ekov, Khi mi ya/ i t ekhnol ogi ya t opl i va 1, 33 (1956).
15. N. I . Shuikin and Dashzhamt s Batyn, No. 3247 (1957).
16. N. I . Shuikin, E. D. Tul upova, and Z. P. Pol yakova, Izvest . Akad. Nauk SSSR, Ot del . Khi m. Nauk, 1958, 1476.
17. A. V. Topchi ev, G. M. Mamedal i ev, A. N. Kislinskii, and G. N. Ani ki na, Dokl ady Akad. Nauk SSSR, 112,
1071 (1957).
18. A. V. Topchi ev, G. M. Mamedal i ev, and S. M. Al i ev, Izvest . Akad. Nauk SSSR, Ot del . Khi m. Nauk, 1959, 861.
19. A. V. Topchi ev and G. M. Mamedal i ev, Dokl ady Akad. Nauk SSSR, 117, No. 6, 1007 (1956).
All abbreviations of periodicals in the 'above bibliography are l et t er-by-l et t er transliter-
at i ons of the abbrevi at i ons as given in the original Russi an journal. Some or all of t hi s peri-
odi cal l i t erat ure may wel l be avai l abl e in Engl i sh t ransl at i on. A complete l i st of the cover-t o-
cover Engl i sh t ransl at i ons appears at the back of this issue.
808
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Formula PerfumesДокумент5 страницFormula PerfumesArantxa YarzaОценок пока нет
- MMSA Methanol World Supply and Demand Summary Jan 2020Документ2 страницыMMSA Methanol World Supply and Demand Summary Jan 2020rifqi98Оценок пока нет
- Macromolecules PRACTICE Test 2012 2013Документ8 страницMacromolecules PRACTICE Test 2012 2013edeceОценок пока нет
- Pengaruh Penambahan Ekstrak Bawang Putih Terhadap Kadar Kolestrol Daging Kambing Peranakan Boer KastrasiДокумент5 страницPengaruh Penambahan Ekstrak Bawang Putih Terhadap Kadar Kolestrol Daging Kambing Peranakan Boer KastrasiBryan AswОценок пока нет
- Consuelo National High School: Bio-Plastic Experiment Video ScriptДокумент2 страницыConsuelo National High School: Bio-Plastic Experiment Video ScriptMarlon S. BarangganОценок пока нет
- Chemistry of COMPOUND Lipids.Документ60 страницChemistry of COMPOUND Lipids.QueenОценок пока нет
- Functional GroupДокумент20 страницFunctional GroupCatherine R. FelipeОценок пока нет
- Contoh SURVEY GIZIДокумент12 страницContoh SURVEY GIZINandya AgustinaОценок пока нет
- Introduction To Biopharmaceutics and PharmacokineticsДокумент40 страницIntroduction To Biopharmaceutics and PharmacokineticsSyeda Eshaal JavaidОценок пока нет
- On Lesson 14 Nucleic Acids and LipidsДокумент40 страницOn Lesson 14 Nucleic Acids and LipidsKamto EzenwamaduОценок пока нет
- Plastics Identification Flow ChartДокумент1 страницаPlastics Identification Flow ChartchiralicОценок пока нет
- IUBAC Naming Organic CompoundsДокумент28 страницIUBAC Naming Organic CompoundsLakshОценок пока нет
- Protein Synthesis Drag & Drop Activity 2021Документ6 страницProtein Synthesis Drag & Drop Activity 2021Gloria LaneОценок пока нет
- Product Catalogue-Henan Ouber Technology Co., Ltd.Документ8 страницProduct Catalogue-Henan Ouber Technology Co., Ltd.anna.zhangОценок пока нет
- Carbohydrates 2Документ81 страницаCarbohydrates 2smcm11Оценок пока нет
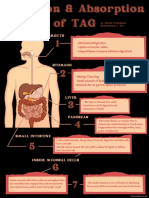
- Digestion & Absorption of TAGДокумент1 страницаDigestion & Absorption of TAGJanine Franchesca SuministradoОценок пока нет
- Protein MCQ Final RevisionДокумент11 страницProtein MCQ Final RevisionMohamed KhalelОценок пока нет
- Urea CycleДокумент13 страницUrea CycleShampa SenОценок пока нет
- GR 10 Unit Exam 1 (EM)Документ2 страницыGR 10 Unit Exam 1 (EM)chaminda dayarathneОценок пока нет
- 12~19 생화학 테뱅 정리Документ117 страниц12~19 생화학 테뱅 정리조주상Оценок пока нет
- Registered Medicine List 07-04-2014 Sse Frequently UpdatedДокумент116 страницRegistered Medicine List 07-04-2014 Sse Frequently Updatedjema; belihuОценок пока нет
- AQA Biology Topic 8.3 Structures of Ribonucleic AcidДокумент4 страницыAQA Biology Topic 8.3 Structures of Ribonucleic AcidfОценок пока нет
- BIS 102 MT 1 Hilt F10 BlankДокумент6 страницBIS 102 MT 1 Hilt F10 BlankKimОценок пока нет
- BCH101 - L1 - The Chemical Basis of LifeДокумент52 страницыBCH101 - L1 - The Chemical Basis of Lifesrabonty.siddikyОценок пока нет
- Update Stok 22 Juni 2023Документ8 страницUpdate Stok 22 Juni 2023Lulut Hening PrasetyoОценок пока нет
- Vitamins and MineralsДокумент4 страницыVitamins and MineralsNoreen Orro BernalОценок пока нет
- Bansal Chemistry 1Документ424 страницыBansal Chemistry 1Waseem0% (1)
- Methods of Synthesis of Deuterium-Labelled LipidsДокумент13 страницMethods of Synthesis of Deuterium-Labelled LipidsDavid SweedlerОценок пока нет
- Akema Fine ChemicalsДокумент1 страницаAkema Fine ChemicalsJulia BottiniОценок пока нет
- Addition Polymers (4.8.1)Документ4 страницыAddition Polymers (4.8.1)KeerthikaОценок пока нет