Академический Документы
Профессиональный Документы
Культура Документы
Fatty Material of Different Soap Samples
Загружено:
rohini1997Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Fatty Material of Different Soap Samples
Загружено:
rohini1997Авторское право:
Доступные форматы
FATTY MATERIAL OF DIFFERENT SOAP
SAMPLES
CHEMISTRY PROJECT
CONTENTS
Aim
Procedure
Observation
Conclusion
Bibliography
AIM
To find and calculate the percentage of fatty material in different
soap samples.
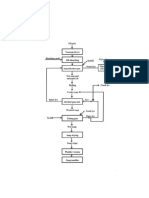
PROCEDURE
Take 10 gm of quantity of each sample in which percentage of fatty
material has to be determined.
Prepare the solution of each soap in water.
3. Add 10 to 12 drops of HCl in each solution and heat the solution
for 5 to 10 min.
Fatty matter float on the soap solution surface by forming upper layer
and how by filter paper are weighed for titration. Now collect the fatty
material from each solution by filtrate ion and again weigh the filter
including filtrate (fatty material) are dissolved in the filterate (fatty
material) in ether for calculating oil materials.
Now take the solution in separating flask on the surface of solution
and remove the solution except oily material.
Now, remaining solution is exposed in sunlight to evaporate ether
from solution.
Now oily matter can be easily weighed by weighing machine.
The percentage of oily materials can be easily calculate by following
observation :
Soap - Soap are the sodium or potassium salt of higher fatty acids.
The fatty acid contains long chain of 16-18 carbon atoms.
Structure Of Soap Soap contains two parts: A long hydrocarbon
chain, which is water repelling called a non polar tail.
Anionic part which is water attracting called hydrophobic. It is called
polar tail.
Soap may be represented as :
CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2-CH2- CH2-CH2-
CH2-CH2-CH2-COONa
Soap are also made from animal fats and vegetable oil. Fats and oils
are ester of higher fatty acids are called Glyceroides. When oils and
fats are heated with a solution of NaOH, they break down to sodium
salt of respective fatty acid soap and glycerol. This process of making
soap by hydrolysis of fats and oil with alkalis is called saponification.
The soap is separated from the solution by a addition of common salt
NaCl. Salts is added in the soap solution to decrease the solubility of
soap due to which soap separates out from the solution in the form of
solid and starts floating on the surface. The crust of soap thus formed
is removed and put it in moulds to get soap cakes. The solution left
behind contains glycerol and NaCl. Limitation Of Soap Soap is not
suitable for washing clothes with hard water because of the following
reasons: Hard water contains salt of Ca and Mg, when soap is added
to hard water, Ca and Mg ions of hard water react with soap forming
insoluble Ca and Mg salt of fatty acids.
2C17H35COONA + MgCl2 - (C17H35COO)2 Mg + 2NaCl
2C17H35COONA + MgCl2 - (C17H35COO)2 Ca + 2NaCl
Therefore a lot of soap is washed if water is hard.
When hard water is used, soap forms insoluble precipitates of Ca and
Mg salt from which sticks of clothes being washed.
Therefore it interfere with the cleansing ability of the soap and makes
the cleansing process difficult.
Cleansing Action Of Soap
The dirt in the cloth is due to presence of dust particle in fat or grease,
which sticks to the cloth. When the dirty cloth is dipped in soap or
detergent solution the soap and dust particle come in contact with
each other the non polar tails of the soap begin to dissolve in non-
polar oil or grease while the polar head part remains directed in water.
As more particle enter the grease each fat or oil surrounde by a
number of negatively charge polar head and the similar charge repel
each other. The oil or grease droplets break off and are still
surrounded by negatively charged polar head of the soap molecule. As
a result the cloth get free from the dirt and the droplets are washed
away with water.
SOAPS DETERGENTS
1) Soap are sodium salt of long
chain carboxylic acids.
1) Synthetic detergents are
sodium salts of long chain
benzene sulphonic acid.
2) Soap are not suitable for
washing purpose when water is
hard.
2) Synthetic detergent can be used
for washing even when water is
hard.
3)Soap have relatively weak
cleansing action.
3 )Synthetic detergent have a
strong cleansing action.
Вам также может понравиться
- Kia Picanto II Gen. (TA) EWD - Engine Control System (General Head Lamp Type)Документ9 страницKia Picanto II Gen. (TA) EWD - Engine Control System (General Head Lamp Type)mutaz ahmed100% (4)
- Totse Knowledge Thread 4Документ14 страницTotse Knowledge Thread 4negzОценок пока нет
- Soap-Making Manual: A Practical Handbook on the Raw Materials, Their Manipulation, Analysis and Control in the Modern Soap PlantОт EverandSoap-Making Manual: A Practical Handbook on the Raw Materials, Their Manipulation, Analysis and Control in the Modern Soap PlantОценок пока нет
- Foaming Capacity of SoapsДокумент11 страницFoaming Capacity of SoapsNeetika Mishra100% (1)
- Investigation of Foaming Capacity of Different Washing SoapДокумент5 страницInvestigation of Foaming Capacity of Different Washing SoapAshwin Brahmane40% (15)
- Fatty Material of Different Soap SamplesДокумент8 страницFatty Material of Different Soap SamplesSoumendu KonaeОценок пока нет
- Soap ManufacturingДокумент34 страницыSoap ManufacturingPatrick Chin68% (19)
- Soap PreparationДокумент40 страницSoap PreparationDesiana Anggraeni100% (1)
- Effect of Temperature and Pressure On The DensityДокумент7 страницEffect of Temperature and Pressure On The DensityDrSaurabh TewariОценок пока нет
- Sebp4195 76 01 Allcd - 003 PDFДокумент965 страницSebp4195 76 01 Allcd - 003 PDFFacturas hidrodieselОценок пока нет
- Cutting Torch InstructionДокумент2 страницыCutting Torch Instructionfoxtrot mikeОценок пока нет
- Surge TankДокумент2 страницыSurge TankBilel MarkosОценок пока нет
- Soap-Making Manual. A practical Handbook on the RControl in the modern Soap PlantОт EverandSoap-Making Manual. A practical Handbook on the RControl in the modern Soap PlantРейтинг: 5 из 5 звезд5/5 (2)
- TOPIC 12 Soaps and DetergentsДокумент14 страницTOPIC 12 Soaps and DetergentsKaynine Kiko50% (2)
- Alkanes: Structure, Naming, Properties and ReactivityДокумент73 страницыAlkanes: Structure, Naming, Properties and ReactivityChona TuyОценок пока нет
- Soap Action and PHДокумент16 страницSoap Action and PHKuldeep SharmaОценок пока нет
- Soap Making Guide With Recipes: DIY Homemade Soapmaking Made Easy: DIY Homemade Soapmaking Made EasyОт EverandSoap Making Guide With Recipes: DIY Homemade Soapmaking Made Easy: DIY Homemade Soapmaking Made EasyРейтинг: 4.5 из 5 звезд4.5/5 (17)
- The Soap Manufacturing ProcessДокумент8 страницThe Soap Manufacturing ProcessSohinee Tinks100% (2)
- Oil & Gas Financial JourneyДокумент85 страницOil & Gas Financial Journeyabsolutvacio82Оценок пока нет
- Building Services AssignmentДокумент19 страницBuilding Services AssignmentRoushell KhanОценок пока нет
- DSM Carbon Footprint StudyДокумент12 страницDSM Carbon Footprint StudyAbdul Rahman100% (1)
- Chemistry Project Class 12th - Foaming Capacity of Soaps and Effect of Addition in It.Документ16 страницChemistry Project Class 12th - Foaming Capacity of Soaps and Effect of Addition in It.nitin sankhla100% (3)
- Effect of Sodium Carbonate On Foaming Capacity of A SoapДокумент6 страницEffect of Sodium Carbonate On Foaming Capacity of A SoapAvi AОценок пока нет
- Understanding the Chemistry of Soaps and DetergentsДокумент33 страницыUnderstanding the Chemistry of Soaps and DetergentsDimas Dwisardi PutraОценок пока нет
- Chemistry ProjectДокумент26 страницChemistry ProjectMaster PrateekОценок пока нет
- To Find Fatty Material of Different Soap SamplesДокумент17 страницTo Find Fatty Material of Different Soap SamplesRohan Singh0% (2)
- Soap and Detergents PDFДокумент33 страницыSoap and Detergents PDFsab_franc5286100% (4)
- Sree Narayana Guru Central SchoolДокумент14 страницSree Narayana Guru Central SchoolgreeshmaОценок пока нет
- Preparation of SoapДокумент13 страницPreparation of Soapeyasu milkiasОценок пока нет
- Practicals InvestagatoryДокумент3 страницыPracticals InvestagatorysanyamskismnОценок пока нет
- Foaming Capacity of SoapsДокумент18 страницFoaming Capacity of SoapsAnkit KushwahaОценок пока нет
- EXP6 Soap and DetergentheheДокумент19 страницEXP6 Soap and DetergenthehesamengОценок пока нет
- Project SoapsДокумент14 страницProject Soapskeru9718Оценок пока нет
- SoapДокумент6 страницSoapkaram13Оценок пока нет
- Investigatory Project On Foaming Capacity of SoapsДокумент5 страницInvestigatory Project On Foaming Capacity of SoapsMuthu ManickamОценок пока нет
- SaponificationДокумент4 страницыSaponificationtedy yidegОценок пока нет
- Name - Vaibhav Tiwari Class & Section - Xi-A Roll No-40 Chemistry Project FileДокумент21 страницаName - Vaibhav Tiwari Class & Section - Xi-A Roll No-40 Chemistry Project FilebaijiОценок пока нет
- Anish ChemДокумент15 страницAnish ChemVishalОценок пока нет
- Preparation of Toilet SoapsДокумент4 страницыPreparation of Toilet Soapspradyumansharmaj333Оценок пока нет
- Class 12th Chemistry Project On Cleaning Action of Soaps 1Документ17 страницClass 12th Chemistry Project On Cleaning Action of Soaps 1ankur bharali60% (5)
- Experiment 15 It's A Soap Opera!: OutcomesДокумент8 страницExperiment 15 It's A Soap Opera!: OutcomesKrystel Monica ManaloОценок пока нет
- Foaming Capacity of SoapДокумент11 страницFoaming Capacity of SoapAditya Sethi100% (1)
- Bche Fa19 018Документ8 страницBche Fa19 018Muhammad AliОценок пока нет
- Chemical Engineering Laboratory-1 (CHE F312) Lab Report Engineering Chemistry Lab E-6 SaponificationДокумент12 страницChemical Engineering Laboratory-1 (CHE F312) Lab Report Engineering Chemistry Lab E-6 SaponificationHritik LalОценок пока нет
- Chemistry - Investigation of Foaming Capacity of Different Washing SoapДокумент8 страницChemistry - Investigation of Foaming Capacity of Different Washing SoapSanjeeth SОценок пока нет
- Fatty Material of Diff Soap SamplesДокумент13 страницFatty Material of Diff Soap SamplesRohitОценок пока нет
- Presentation 1Документ22 страницыPresentation 1B59 Diganshu JaiswalОценок пока нет
- Tanu Chem ProjectДокумент3 страницыTanu Chem ProjectTanu TomarОценок пока нет
- Foaming Capacity of SoapsДокумент8 страницFoaming Capacity of SoapsHariomОценок пока нет
- ChemistryДокумент23 страницыChemistryelagovanautoОценок пока нет
- Lab 6 Uitm. Soap Preparation. Comparison Soap and Detergent Properties.Документ16 страницLab 6 Uitm. Soap Preparation. Comparison Soap and Detergent Properties.niniwani59Оценок пока нет
- Soap Formation FlowchartДокумент5 страницSoap Formation FlowchartTarus BrianОценок пока нет
- Foaming Capacity Comparison of SoapsДокумент5 страницFoaming Capacity Comparison of SoapsBhavesh Pratap Singh0% (1)
- 7 SoapДокумент5 страниц7 Soapnamthuy8384Оценок пока нет
- Chemistry InvestigatoryДокумент15 страницChemistry InvestigatoryTushar JaiswalОценок пока нет
- Chapter IV Soap and DetergenДокумент6 страницChapter IV Soap and Detergenpuguh diknaОценок пока нет
- Investigation of The Foaming Capacity of Different Washing Soaps and The Effect of Addition of Sodium Carbonate On ItДокумент10 страницInvestigation of The Foaming Capacity of Different Washing Soaps and The Effect of Addition of Sodium Carbonate On ItPrasanna kudale100% (1)
- Soap and DtergenetsДокумент13 страницSoap and DtergenetsAkanksha PanigrahyОценок пока нет
- Cleansing Action of Soap and Detergent (Investigatory Project - Class 12)Документ22 страницыCleansing Action of Soap and Detergent (Investigatory Project - Class 12)Yati JainОценок пока нет
- TFM ProjectДокумент22 страницыTFM Projectsai anju100% (1)
- Chem Proj 1Документ10 страницChem Proj 1Mrinaal RoshanОценок пока нет
- Soap ReportДокумент16 страницSoap ReportAddison JuttieОценок пока нет
- Chem Investigatory ProjectДокумент13 страницChem Investigatory Projectdamm itОценок пока нет
- Lab 6 (Soaps & Detergents)Документ21 страницаLab 6 (Soaps & Detergents)AmeerRashidОценок пока нет
- Compare Properties of Homemade Soap and DetergentsДокумент16 страницCompare Properties of Homemade Soap and DetergentsNurul AinОценок пока нет
- Frothing Power of SoapДокумент3 страницыFrothing Power of SoapT sidharthОценок пока нет
- Acknowledgement Preface Commercial Preparation Introduction To Experiment Objective and Theory Procedure Observation Table Result BibliographyДокумент20 страницAcknowledgement Preface Commercial Preparation Introduction To Experiment Objective and Theory Procedure Observation Table Result BibliographyNilamya PrakashОценок пока нет
- Soap and DetergentДокумент19 страницSoap and DetergentNahid HasanОценок пока нет
- Chemistry Project 11-2Документ6 страницChemistry Project 11-2SCHWARTZ. A cseОценок пока нет
- Quantum TheoryДокумент16 страницQuantum Theoryrohini1997Оценок пока нет
- Method of MultiplicationДокумент7 страницMethod of Multiplicationrohini1997Оценок пока нет
- Atomic StructureДокумент7 страницAtomic Structurerohini1997Оценок пока нет
- Alpha ParticlesДокумент5 страницAlpha Particlesrohini1997Оценок пока нет
- Atomic MassДокумент4 страницыAtomic Massrohini1997Оценок пока нет
- Hypercell 1994 Engl by Hans HassДокумент126 страницHypercell 1994 Engl by Hans HassClaimDestinyОценок пока нет
- Heat Exchangers of PolypropyleneДокумент2 страницыHeat Exchangers of PolypropyleneTan So100% (1)
- Wave-Particle Duality FundamentalsДокумент7 страницWave-Particle Duality FundamentalsRahmatullahОценок пока нет
- Installation: Residential/Light Commercial Generator SetsДокумент64 страницыInstallation: Residential/Light Commercial Generator SetsJackОценок пока нет
- Hybrid Safety and Service Procedures: Automotive Technology, Fifth EditionДокумент40 страницHybrid Safety and Service Procedures: Automotive Technology, Fifth EditionSkyAnimal ChannelОценок пока нет
- Cast Resin Transformers for Distribution, Rectification, Traction and Special SolutionsДокумент80 страницCast Resin Transformers for Distribution, Rectification, Traction and Special SolutionsQUANGОценок пока нет
- Solar Electric Tiffin Box (1) - 2Документ36 страницSolar Electric Tiffin Box (1) - 2imrashi18kОценок пока нет
- Thermal Power Plant: " " NTPC (Dadri)Документ17 страницThermal Power Plant: " " NTPC (Dadri)Kulvinder SinghОценок пока нет
- Catalase TestДокумент2 страницыCatalase TestsekaralingamОценок пока нет
- MSBTETE Thermal Engineering Model Answer KeyДокумент15 страницMSBTETE Thermal Engineering Model Answer KeyMohit D MoreОценок пока нет
- Biofuel For Sri LankaДокумент0 страницBiofuel For Sri LankaJaanu SanthiranОценок пока нет
- Turbo CompressorДокумент6 страницTurbo Compressorrajanrane420Оценок пока нет
- Sliit L Epdc 15Документ108 страницSliit L Epdc 15sulochana priyashanОценок пока нет
- 3.electromagnetic Theory NET-JRF VKSДокумент31 страница3.electromagnetic Theory NET-JRF VKSSijil SalimОценок пока нет
- LevyДокумент4 страницыLevyRamon FerreiraОценок пока нет
- Cyclotron Booklet Black Final PrintДокумент10 страницCyclotron Booklet Black Final PrintNawaf BamasoudОценок пока нет
- Rod BaffelsДокумент2 страницыRod BaffelsVenkatesh SivarchanaОценок пока нет
- Miroljub Todorović - ApeironДокумент25 страницMiroljub Todorović - Apeiron"Mycelium" samizdat publishersОценок пока нет
- MMTДокумент36 страницMMTAvoyОценок пока нет
- Rotational Motion Engineering Mechanics IIT KanpurДокумент67 страницRotational Motion Engineering Mechanics IIT KanpurNitin SharmaОценок пока нет