Академический Документы
Профессиональный Документы
Культура Документы
Porous Hidroxy Apatite
Загружено:
Dwi Yerlis RahmiОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Porous Hidroxy Apatite
Загружено:
Dwi Yerlis RahmiАвторское право:
Доступные форматы
J OURNAL OF MATERI ALS SCI ENCE 36 (2001) 3061 3066
Preparation of porous hydroxyapatite
JI NTAO TI AN, JI EMO TI AN
Beijing Fine Ceramic Laboratory, INET, Tsinghua University,
P. O. Box 1021 Beijing 102201, Peoples Republic of China
E-mail: jttian@263.net
The aim of the paper was to obtain a porous hydroxyapatite (HA) biomaterials relating to
the manufacture of articial implants. The technique of impregnating a body of porous
polyurethane foam with slurry containing HA powder, water and additives was applied and
proved to be more successful. The process of preparations was discussed and the porous
HA with desired properties was obtained at last. C
2001 Kluwer Academic Publishers
1. Introduction
Over the past decades of years hydroxyapatite [Ca
10
(PO
4
)
6
(OH)
2
, HA] and related calcium phosphate ce-
ramic materials have been widely used as implant
materials due to their close similarity in composition
and highly biocompatibility with nature bone [13].
Such bioactive implant materials can be applied both
in the compact and porous forms as well as gran-
ules. In the case of porosint a number of in vivo stud-
ies have demonstrated the occurrence of bone forma-
tion in the pores of the calcium phosphate ceramics
such as hydroxyapatite/-tricalcium phosphate (HA/
-TCP)[4, 5]. However, most of the investigations on
the implantation of the porous calcium phosphate ce-
ramics showed that the degree of inltration of liv-
ing tissue into the pores and formation of new bone
depended greatly on the pore characteristics such as
porosity, pore size, pore size distribution and pore shape
[6, 7]. Hulbert et al. [8] claimed that a minimum pore
size of 100 micrometers is necessary for the porous im-
plant materials to function well and pore size greater
than 200 micrometers is an essential requirement for
osteoconduction.
A lot of methods have been developed to fabricate
the porous HA(or HA/TCP) ceramics with diversi-
ed pore characteristics. There include incorporation
of volatile or combustible burn-outs that are lost during
ring, solid-state sintering, sol-gel process and reticu-
lated open-celled ceramics produced via the replication
of a porous substrate, which is the most common ap-
proach for producing open-cell porous ceramics [9].
The porous ceramics obtained from reticulated poly-
mer substrates have a variety of unique properties such
as controllable pore size, controllable tortuosity and
complex ceramic shapes for different applications.
This paper investigated the technology of obtain-
ingopen-cell macroporous calciumphosphate ceramics
(average pore size larger than 200 micrometers) based
on the replication of a porous substrate. The preparing
process was discussed and the open-cell macroporous
calcium phosphate bioceramics with desirable proper-
ties were obtained at last.
2. Experiment
2.1. Raw materials
The precursor powder of HA was synthesized by wet
chemical method in accordance with:
10Ca(NO
3
)
2
+6(NH
4
)
3
PO
4
+2NH
3
H
2
O
= Ca
10
(PO
4
)
6
(OH)
2
+20NH
4
NO
3
The preparing process of HA powder included the fol-
lowing steps: (i) Preparation of Ca(NO
3
)
2
solution and
(NH
4
)
3
PO
4
solution with appropriate concentrations;
(ii) The Ca(NO
3
)
2
solution was added in drops into the
(NH
4
)
3
PO
4
solution in a stoichiometric proportion and
stirred at the temperature of 90
C; (iii) Meanwhile,
NH
3
H
2
O Solution was added to keep pH value no
less than 9; (iv) The gelatinous sediments were ltered
and washed several times until the pH value equal to 7
approximately; (v)The sediments were dried at 110
C
for 3 hr and calcied at 700
C for 3 hr; (vi) The cal-
cied sediments were dispersed in ethanol solvent and
ground for 24 hr: (vii) The ground powder was dried in
air.
The obtained powder was analyzed by SA-CP3 parti-
cle size analyzer andthe result was giveninFig. 1. Fig. 1
shows that the powder particles have an average size of
about one micrometer. The scanning electron micro-
scope (SEM) micrograph of the precursor powder was
shown in Fig. 2. The XRDanalysis of the powder shows
a crystallographic structure closely resembling that of
hydroxyapatite (JCPDS#9-432), as illustrated in Fig. 3.
Asequential X-ray uorescence spectrometer (Made in
Shimadzu, Japan) was used to obtain the powders cal-
cium to phosphate ratio (Ca/P ratio). Table I shows a
value of 1.677, the same thing to hydroxyapatite. Fig. 3
and Table I demonstrate that the precursor powder syn-
thesized above was pure hydroxyapatite.
In order to improve the sintering performance and
mechanical properties of porous HA some nano-scale
ceramics such as silicon carbide and magnesia were
used in the experiments. Other organic agents used as
additives were analytically pure.
00222461 C
2001 Kluwer Academic Publishers 3061
TABLE I Ca/P ratio of HA powder and porous HA
Ca P Ca/P
HA powder 34.24 20.42 1.677
Porous HA 31.07 18.52 1.678
Figure 1 Particle size distribution of HA powder.
Figure 2 SEM micrograph of HA powder.
Figure 3 XRD pattern of HA powder.
2.2. Experimental procedure
The experimental procedure is showed schematically
in Fig. 4. In order to obtain an open-cell macroporous
HA, a suitable reticulated substrate should be selected
at rst. Then, a ceramic slurry was prepared and coated
Figure 4 Preparing procedure of porous HA.
onto the substrate. After that, the porous samples were
dried and sintered carefully. The sintered samples were
machined, washed and sterilized. The details will be
described in the following.
2.2.1. Selection of reticulated substrate
In order to obtain an open-cell macroporous HA based
on the replication of a porous substrate, a body of
organic foam should be adopted as substrate, which
was impregnated with ceramic slurry and burned out
during the sintering. The organic foam should have an
open-cell porous structure. Moreover, the foam should
also be:
1 able to recover their original shape successfully
after impregnation;
2 easily and thoroughly eliminated at a temperature
of less than 1200
C which is the sintering temperature
of HA;
3 able to vary its pore size upon requirements of the
products.
There are several types of organic foam which
could match the requirements described above very
well, such as polyurethane and polyvinyl chloride. The
polyurethane used in our experiments has enough re-
covering ability and could be thoroughly burned out
during sintering. Fig. 5 shows the porous structure of
the polyurethane substrate. In the case shown in Fig. 5
the polyurethane substrate is composed of a large num-
ber of interconnecting pores. The pores are of char-
acteristics of open cell, well-distributed pore size and
homogeneous pore distribution that determine the nal
structures of porous ceramics.
The polyurethane substrate was formed into desired
shape depending on the particular applications.
Figure 5 Porous structure of polyurethane substrate.
3062
TABLE I I Composition of slurry (wt%)
Component Content (wt%)
Hydroxyapatite 40%
Silicon carbide 2.5%
Magnesia 2.5%
Binder 1 5%
Antifoaming agent 1 3
Distilled water 40 50
2.2.2. Preparation of coating slurry
The slurry used in the experiments was made up to the
recipe given in the Table II.
In Table II, the additions of magnesia and silicon
carbide conduce to the improvements of the sintering
performance and mechanical properties of porous HA.
The binder agent can become the slurry adherent to
the substrate while the antifoaming agent can reduce
the tendency of formation of bridge and cell walls
[10]. All the ingredients were put in a jar with alumina
grinding media and milled for an hour. The resulting
slurry was suitable for coating.
2.2.3. Coating the slurry onto the substrate
In this step, the organic foam was dipped into the ce-
ramic slurry and withdrawn. Surplus slurry was re-
moved by centrifuging and the foam was dried in air.
The dried foam was then dipped into the slurry again,
centrifuged and dried. Similar operations could be re-
peated till a desirable ceramic coating was obtained at
last.
The difculty encountered during the impregnation
was the tendency of the ceramic slurry to drain almost
completely from parts of the foam structure due to in-
terfacial phenomena between the slurry and the sub-
strate. As a result, the deposit of ceramic slurry was
thinner than was desirable and its thickness was also
extremely variable. Additionally in reticulated foamthe
slurry tended to accumulate at the joint points between
rods of polymer rather than along the length of the rods
themselves.
In order to solve these problems mentioned above
and form a satisfactory coating of ceramic particles on
the foam, a kind of occulating agent was employed
during the coating process. The occulating agent was
prepared in accordance with:
2AlCl
3
+5NH
3
H
2
O Al
2
(OH)
5
Cl +5NH
4
Cl
Fig. 6 shows the preparing procedure. The prepared
occulating agent has a pH value of 5 6.
Before impregnation the substrate was pretreated
with the occulating agent. When the so-treated sub-
strate was dipped into the ceramic slurry having a
pH value of 9 10, a local gelatinous precipitate of
Al(OH)
3
would be generated on the interface between
the slurry and the substrate. The formed precipitate
of Al(OH)
3
did not then readily redisperse into the
slurry and occluded suspended ceramics particles to
drain from substrate. The treated/coated circle was re-
peated as many time as necessary and the desirable
Figure 6 Preparing procedure of the occulating agent
coating with a continuous and uniformthickness would
be formed at last.
2.2.4. Drying and sintering
After coated the samples were dried in air. The time for
air-drying was always no less than 24 hr.
The porous samples were sintering at the temperature
of 1200
C. During sintering, the organic substrate be-
gan to decompose at the temperature of 200
C300
C
and lost its supporting function. The samples without
substrate supporting have a weak strength and easily to
be destroyed before sintered. The porous samples ob-
tained in the experiments have a large sintering shrink-
age of about 30%. If the porous samples couldnt shrink
freely during sintering, large cracks always generated
during sintering.
In order to solve the problem of shrinking freely dur-
ing sintering and obtain porous HA without cracks,
a special sintering process was carefully planned,
schematically showed in Fig. 7. In the case shown
in Fig. 7, a compact sheet of HA having a sintering
shrinkage of about 15% was placed in the oven and
could shrink freely during sintering. On the compact
sheet, a porous cushion was placed. The porous sample
of HA was nally placed on the cushion. The cush-
ion has a shrinkage of about 30%, similar with that
of the porous sample. During sintering, the compact
sheet fully shrank and contributed to the shrinkage of
the bottomof the cushion, where small cracks would be
formedbecause of partiallyshrinkage. However, the top
surface of the cushion hasnt any resistance and shrink
easily. As a result, the porous sample of HA placed on
the cushion could shrink freely. The preparations and
Figure 7 Placements of porous HA in the oven during sintering.
3063
TABLE I I I A special sintering process for porous HA
Shrinkage
Method of content in Character of
Sintering preparation the level shrinking
Porous samples Impregnating
on the top a foam with 30% Fully
ceramic slurry Fully on the
Porous cushion Impregnating a 19 30% top surface
in the middle foam with Partially at the
ceramic slurry bottom surface
Compact sheet Pressed in a model 15% Fully
at the bottom
Figure 8 The heating speed of porous HA during sintering.
relevant shrinkage of the sheet and the cushion were
given in Table III.
Another attention during sintering was the heating
speed. The temperature should be increased slowly so
as to make the substrate decompose gradually and avoid
cracks formation, especially in the low temperature
stage. The heating curve adopted in the experiments
was showed in Fig. 8.
2.2.5. Machining, washing and sterilizing
The porous HA could be little machined because the
shapes of the samples have already been obtained via
the shapes of the foam. After machined, the samples
were washed by ultrasonic and sterilized by Co60 radial
(25 kGry).
3. Results and discussion
3.1. Phase composition and macrostructure
of porous HA
Fig. 9 shows the XRD analysis result. The porous sam-
ples sintered in air almost lost their carboxyl and con-
verted into -tricalcium phosphate (-TCP). However,
the porous samples sintered in vapor have a HA con-
tent of about 80%. In the case of sintering in vapor, the
conversion of HA into -TCP is obstructed strongly.
The porous samples have a Ca/P ratio of 1.678, shown
in Table I.
The macrostructure of the porous HAwas controlled
by the porous structure of the polymer substrate. Cor-
responding to the structure of the polymer substrate
selected in the experiments (Fig. 5), Figs 10 and 11
illustrate the resultingmacrostructure of the porous HA.
Figure 9 XRD pattern of porous HA.
Figure 10 Porous structure of HA from foam of YG-40(5x).
Figure 11 A single pore of HA from foam of YG-70.
They had circular open-cell pores and the pore structure
resembling that of the original polymer substrate.
3.2. Inuence of content of the slurry
Based on the coating process mentioned above,
Tables IV and V show some experimental data for the
content of slurry and pore size of the foam.
In the case shown in Table IV, a body of porous poly-
mer foam of pore size YG-70 was selected as impreg-
nating substrate. Three different contents of the slurry,
1, 0.75, and0.65, were adopted. The values of the poros-
ity from 58% to 80% showed an increasing tendency
with the decrease of the content of the slip, while the
content of 0.75 and porosity of 75% were preferable.
3064
TABLE I V Experimental data for different slurry content
Content Weight of Weight Volume of
of slurry foam after of porous HA after
per one cm
3
of impregnating HA after sintering
foam (g/cm
3
) (g) sintering (g) (cm
3
) Porosity
1 20.9 9 8.9 58%
0.75 15.9 6.7 9.5 75%
0.65 13.9 5.9 9.9 80%
TABLE V Experimental data for different pore size foam
Content
Pore size of slurry Pore size of
Sorts of foam per one cm
3
HA produces
of foam (mm) of foam (g/cm
3
) (mm) Porosity
YG-40 0.7 0.75 0.4 80.1%
YG-70 0.4 0.75 0.25 78%
YG-100 0.25 0.75 0.15 75%
3.3. Inuence of pore size of substrate
Three different polymer substrates with different pore
size of YG-40, YG-70, and YG-100 were selected in
Table V. Figs 12 and 13 showthe microstructures of the
obtained porous HA. The differences between Figs 12
and 13 were not only the pore size which was resulted
from the pore size of substrate but also the former pos-
sessed a lot of cracks. However. adopting the porous
substrate of small pore size (Fig. 13) could easily reduce
these cracks. This was due to the fact that the cracks
were caused by the pyrolysis of the polymer substrate
Figure 12 SEM morphology of porous HA from foam of YG-40.
Figure 13 SEM morphology of porous HA from foam of YG-70.
while the polymer substrate having small pore size pos-
sessed thinner struts and were easily pyrolysied than
that of large pore size. Moreover, drying and sintering
carefully were conducive to the cracks reducing.
3.4. Mechanical properties
Porous samples with dimensions of 20 20 20 mm
3
were tested in compression on an servo tester (Model
MTS810. USA) at a crosshead feed rate of l mm/min.
For porous materials, the properties related directly
to the porosity and their relationship could be described
by D. M. Liu [11] as Equation 1:
(Property) = (property)
0
exp
b p
(1)
Porous HA with different porosity obtained in the ex-
periments were tested in compression and the results
were showed in Fig. 14. In the case shown in Fig. 14,
the compressive strength decreased markedly with the
porosity increasing. The tting curve has an exponen-
tial tendency and is showed in Equation 2:
Y = 14.6 exp
(0.36x)/0.15
= 161 exp
6.7 x
(2)
WithEquation2the porous HAwithdesirable compres-
sive strength and suitable porosity could be designed
and prepared according to the clinical requirements.
Figure 14 Compressive strength of porous HA.
Figure 15 Specimen from porous HA, thirty days after implantation
(P: pore in the porous HA; S: skeleton of the porous HA; B: bone tissue).
3065
3.5. Animal experiments
The porous HA with marrow cell fostered after steril-
ization was implanted into dorsal muscular tissues of rat
for animal experiments. The implanted sites of the rats
did not show any infection and inammation. Fig. 15
shows the experimental results. From Fig. 15 we know
that the bone tissue, bone cells, and blood vessels have
been growing into the pores of the porous HA and the
osteoblast is converged onto the skeleton surface of the
porous HA to form new bone.
4. Conclusions
(1) With suitable slurry, porous HAbioceramics having
interconnectingpores andhighporosity(upto80%) can
be obtained by the method of the replication of porous
polymeric substrates.
(2) The porosity of HA can be varied easily with dif-
ferent content of the slurry.
(3) The pore size of HAcan also be varied freely from
selecting different substrates while the largest pore size
of substrates was no more than 2 mm.
(4) The compressive strength of porous HA has a ex-
ponential tendency with porosity.
(5) The animal experiments showthat porous HApos-
sess growing bone ability.
Acknowledgments
We are very grateful to Beijing Fine Ceramic Labo-
ratory, INET, Tsinghua University, for supporting this
work.
References
1. K. DE GROOT, Biomaterials 1 (1980) 47.
2. M. J ARCHO, Clin. Orthop. Rel. Res.157 (1981) 259.
3. J . W. FRAME and C. L. BRADY, Brit. J. Oral Maxillofac.
Surg. 25 (1987) 452.
4. H. GHGUSHI , Y. DOHI , S. TAMAI and S. TABATA,
J. Biomed. Mater. Res. 27 (1993) 1401.
5. R. E. HOLMES, V. MOONEY, R. BUCHOLZ and
A. TENCER, Clin. Orthop. Rel. Res. 188 (1984) 252.
6. S. F. HULBERT, R. S. YOUNG and J . J . KLAWI TTER.
J. Biomed. Mater. Res. 4 (1970).
7. J . J . KLAWI TTER and S. F. HULBERT, ibid. 5 (1971).
8. S. F. HULBERT, S. J . MORRI SON and J . J .
KLAWI TTER, J. Biomed. Mater. Res. Symp. 2 (1) (1970) 269.
9. P. SEPULVEDA, Amer. Ceram. Soc. Bull. 76 (10) (1997) 62.
10. RAVAULT, US. Patent no. 3 907 579, Sept. 23, 1975.
11. R. W. RI CE, in Treatice in Material Science and Technology,
Vol. 11 edited by R. K. MacCrone (Academic Press, New York,
1977) p. 200.
Received 2 April 1999
and accepted 11 April 2000
3066
Вам также может понравиться
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- 10 1016@j Eti 2019 100369 PDFДокумент39 страниц10 1016@j Eti 2019 100369 PDFDwi Yerlis RahmiОценок пока нет
- Wide LL 1984Документ9 страницWide LL 1984Dwi Yerlis RahmiОценок пока нет
- Yu 2008Документ7 страницYu 2008Dwi Yerlis RahmiОценок пока нет
- Microcarrier Culture: Applications in Biologicals Production and Cell BiologyДокумент17 страницMicrocarrier Culture: Applications in Biologicals Production and Cell BiologyDwi Yerlis RahmiОценок пока нет
- Chapter 4Документ10 страницChapter 4Dwi Yerlis RahmiОценок пока нет
- Nurul - Afifah (Bio1)Документ6 страницNurul - Afifah (Bio1)Dwi Yerlis RahmiОценок пока нет
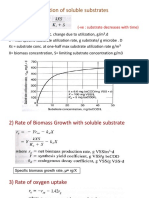
- 1) Rate of Utilization of Soluble SubstratesДокумент5 страниц1) Rate of Utilization of Soluble SubstratesDwi Yerlis RahmiОценок пока нет
- Benzene Specification: Properties Test Method Unit SpecificationsДокумент1 страницаBenzene Specification: Properties Test Method Unit SpecificationsDwi Yerlis RahmiОценок пока нет
- Chapter 12 - Surface Water TreatmentДокумент36 страницChapter 12 - Surface Water TreatmentDwi Yerlis RahmiОценок пока нет
- MF7134 MSDSДокумент5 страницMF7134 MSDSDwi Yerlis RahmiОценок пока нет
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- TB Mag ManualДокумент60 страницTB Mag Manualyanina25Оценок пока нет
- Carbon Black Fundamentals 041206Документ8 страницCarbon Black Fundamentals 041206gems_gce074325Оценок пока нет
- Hot Workability and Corrosion Behavior of EN31 Grade Steel-CompressedДокумент7 страницHot Workability and Corrosion Behavior of EN31 Grade Steel-CompressedJaggu TitlerОценок пока нет
- Frost Growth RateДокумент12 страницFrost Growth Ratechemsac2Оценок пока нет
- Ceramic Laminate Veneers-Materials Advances and SelectionДокумент12 страницCeramic Laminate Veneers-Materials Advances and Selectiondentace1Оценок пока нет
- Desalination and Water Treatment: Click For UpdatesДокумент6 страницDesalination and Water Treatment: Click For UpdatesMALIK ZARYABBABARОценок пока нет
- Materials Research Bulletin: Xiaolian Chao, Zhongming Wang, Ye Tian, Yanzhao Zhou, Zupei YangДокумент10 страницMaterials Research Bulletin: Xiaolian Chao, Zhongming Wang, Ye Tian, Yanzhao Zhou, Zupei YangSamah SamahОценок пока нет
- Pall Gas Solid Separation SystemsДокумент16 страницPall Gas Solid Separation SystemsAndrew HillОценок пока нет
- Binder 1Документ42 страницыBinder 1Anindya RoyОценок пока нет
- Silicate Ceramics: Based Ceramic Such As Porcelain, Earthenware, Stoneware and Bricks System KДокумент3 страницыSilicate Ceramics: Based Ceramic Such As Porcelain, Earthenware, Stoneware and Bricks System KNisar Ahmed AnsariОценок пока нет
- Powder Metallurgy (ISE)Документ107 страницPowder Metallurgy (ISE)likydo100% (1)
- A Review On Binderless Tungsten Carbide DevelopmenДокумент37 страницA Review On Binderless Tungsten Carbide DevelopmenRubens RochaОценок пока нет
- The Preparation and Characterization of Porous Alumina Ceramics Using An Eco Friendly Pore (2305843009215216609)Документ37 страницThe Preparation and Characterization of Porous Alumina Ceramics Using An Eco Friendly Pore (2305843009215216609)Marin MedvedОценок пока нет
- Evaluation of Mechanical Properties of Aluminum Alloy-Alumina-Boron Carbide Metal Matrix CompositesДокумент8 страницEvaluation of Mechanical Properties of Aluminum Alloy-Alumina-Boron Carbide Metal Matrix CompositesPremier PublishersОценок пока нет
- Skim 63/93: Reliability and Performance Increase Due To Advanced TechnologyДокумент4 страницыSkim 63/93: Reliability and Performance Increase Due To Advanced Technologyraza239Оценок пока нет
- Bryers1996 PDFДокумент92 страницыBryers1996 PDFBayu LukmanaОценок пока нет
- Lec29 PDFДокумент32 страницыLec29 PDFVignesh Raja PОценок пока нет
- Classifying Dental CeramicsДокумент7 страницClassifying Dental CeramicsERIKA BLANQUETОценок пока нет
- Metallized Surfaces On Ceramic: Standard Specification ForДокумент3 страницыMetallized Surfaces On Ceramic: Standard Specification Forvuqar0979Оценок пока нет
- Korner - Mesoscopic Simulation o Selective Beam Melting ProcessesДокумент27 страницKorner - Mesoscopic Simulation o Selective Beam Melting ProcessesHelen FuentesОценок пока нет
- A General View of Graphene Reinforcements On Metal Matrix Composites (GR-MMC)Документ12 страницA General View of Graphene Reinforcements On Metal Matrix Composites (GR-MMC)International Journal of Innovative Science and Research TechnologyОценок пока нет
- Synthesis and Consolidation of Boron Carbide A ReviewДокумент37 страницSynthesis and Consolidation of Boron Carbide A ReviewAlexandru PrisecaruОценок пока нет
- Material Iii To Viii PDFДокумент66 страницMaterial Iii To Viii PDFRaja PrabhuОценок пока нет
- Friction in OrthodonticsДокумент71 страницаFriction in OrthodonticsNimisha SharmaОценок пока нет
- Metal Powder Processing TechniquesДокумент26 страницMetal Powder Processing TechniquesAzhar Ali0% (1)
- Powder Processing Science and Technology For Increased Reliability PDFДокумент13 страницPowder Processing Science and Technology For Increased Reliability PDFfishvalОценок пока нет
- Traditional Machining Processes Research AdvancesДокумент242 страницыTraditional Machining Processes Research AdvancesGema Rodriguez DelgadoОценок пока нет
- Cat FlowAids CFAEN 0120 Rev09 PDFДокумент36 страницCat FlowAids CFAEN 0120 Rev09 PDFthanh nguyenОценок пока нет
- Oil and Gas Filter: ApplicationsДокумент3 страницыOil and Gas Filter: ApplicationschandussctОценок пока нет
- Continuous Dew Point Monitoring SystemДокумент2 страницыContinuous Dew Point Monitoring SystemSatyendra PandeyОценок пока нет