Академический Документы
Профессиональный Документы
Культура Документы
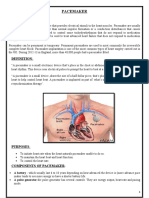
Implantable Pacemaker
Загружено:
Shifa Fauzia Afrianne0 оценок0% нашли этот документ полезным (0 голосов)
156 просмотров33 страницыThe document discusses the history and technology of implantable pacemakers. It provides technical details on how pacemakers work and the key innovations that have occurred in the industry. Specifically:
1) Pacemakers use electrical pulses to regulate heart rate when the heart's natural pacemaker malfunctions. They have saved millions of lives since their invention in 1958.
2) Continuous innovation has driven the multi-billion dollar pacemaker industry. Size reduction and longer battery life have been important areas of development.
3) The document analyzes the innovation strategies of early industry leaders Medtronic and CPI, and how they approached new technology development and market competition differently.
Исходное описание:
Авторское право
© © All Rights Reserved
Доступные форматы
PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документThe document discusses the history and technology of implantable pacemakers. It provides technical details on how pacemakers work and the key innovations that have occurred in the industry. Specifically:
1) Pacemakers use electrical pulses to regulate heart rate when the heart's natural pacemaker malfunctions. They have saved millions of lives since their invention in 1958.
2) Continuous innovation has driven the multi-billion dollar pacemaker industry. Size reduction and longer battery life have been important areas of development.
3) The document analyzes the innovation strategies of early industry leaders Medtronic and CPI, and how they approached new technology development and market competition differently.
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
156 просмотров33 страницыImplantable Pacemaker
Загружено:
Shifa Fauzia AfrianneThe document discusses the history and technology of implantable pacemakers. It provides technical details on how pacemakers work and the key innovations that have occurred in the industry. Specifically:
1) Pacemakers use electrical pulses to regulate heart rate when the heart's natural pacemaker malfunctions. They have saved millions of lives since their invention in 1958.
2) Continuous innovation has driven the multi-billion dollar pacemaker industry. Size reduction and longer battery life have been important areas of development.
3) The document analyzes the innovation strategies of early industry leaders Medtronic and CPI, and how they approached new technology development and market competition differently.
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 33
High Technology Entrepreneurship and Strategy
Prof. Ron Adner
Group Project
IMPLANTABLE PACEMAKER
Balazs Asztalos
Olivier Genelot
Tiago Guerra
Antti Jaaskelainen
Kai Zolleis
Manfred Zurkirch
Fontainebleau, February 2002
1
1. INTRODUCTION........................................................................................... 2
2. PACEMAKER TECHNICAL DESCRIPTION.............................................. 3
2.1. What is the problem with the heart?.................................................................................. 3
2.2. How a pacemaker works.....................................................................................................4
2.3. Key features and technical challenges ............................................................................. 5
2.4. Potential developments ...................................................................................................... 6
3. HISTORY OF THE PACEMAKER INNOVATION......................................... 8
3.1. Overview............................................................................................................................... 8
3.2. The early years..................................................................................................................... 8
3.4. Development of the pacemaker and the emergence of competition............................ 11
4. RELEVANT MARKETS AND VALUE CHAIN DYNAMICS ........................ 14
4.1 Market Size .......................................................................................................................... 14
4.2 Current competitive landscape ......................................................................................... 14
4.3 Market Structure ................................................................................................................. 15
4.4 Market Drivers and Innovation Diffusion ......................................................................... 18
4.5 Substitutes .......................................................................................................................... 19
5. INNOVATION AND TECHNOLOGY MANAGEMENT ................................ 20
5.1. What makes innovation such a challenge in the pacemaker industry? ...................... 20
5.2. Are disruptive or incremental innovations driving the pacemaker industry? ............ 21
5.3. How important are complementary assets?................................................................... 22
5.5 How important is the first-mover advantage? ................................................................. 23
5.6. What uncertainties is the pacemaker industry facing?................................................. 24
6. MEDTRONIC AND CPI: DIFFERENT INNOVATION STRATEGIES.......... 26
6.1. Development of new technologies................................................................................... 27
6.2. Market approaches............................................................................................................ 28
6.3. Defending the market position......................................................................................... 29
7. CONCLUSIONS.......................................................................................... 30
References................................................................................................................................. 32
2
1. Introduction
The pacemaker and especially the implantable pacemaker have saved and
lengthened the lives of millions of people. The implantable pacemaker,
invented by Wilson Greatbatch in 1958 has generated a thriving multibillion-
dollar industry that has been characterised by continuous innovation through
the decades since the original invention. A testimonial to the innovativeness in
this industry is that in 1984 the National Society of Professional Engineers
(USA) cited the implantable heart pacemaker as one of the ten outstanding
engineering achievements of the last fifty years, putting it in the same league
with the electronic computer and the Apollo 11 moon landing.
This dynamic industry is characterized by active innovation and intense
competition. How have some companies been able to make a sustainable
difference by successfully bringing the innovation to the market?
After a technical presentation of the pacemaker, we will describe the history of
the pacemaker industry focusing on the events, persons and companies that
shaped the innovation and the formation of the industry. Then we will analyse
the market structure, the value chain in the industry, the market drivers and the
speed of diffusion of the innovation.
We will then move to analyse the challenges that innovation has represented to
the industry, and we will highlight the key role played by technological and
market related complementary assets. We will focus on two milestones in the
history of the pacing industry and how two companies have managed the
innovation. One of the companies is Medtronic, which was the first mover and
market leader in the early seventies. The second company we analyse is CPI,
which was formed in 1974 as a start-up, established by formed Medtronic
employees to capitalise on the Lithium battery innovation, which at the time
was rejected by Medtronic.
3
2. Pacemaker technical description
2.1. What is the problem with the heart?
1
The hearts ability to pump blood through the body and the lungs depends on
the precise functioning of its electric system. It is the responsibility of this
electric system to co-ordinate the contraction of the chambers of the heart. This
usually happens in a way that a special type of electrical impulse travels
through the heart and sets off contractions in the chambers as it passes
through them.
Normally an area of specialised heart tissue called the SA (sinoatrial) node
(located in the right atrium) generates the electrical impulse. This is the
natural pacemaker of the heart. Each time this tissue fires, the impulse
travels through the chambers in the following sequence: SA node -> left & right
atria -> AV node ->left & right ventricle. The AV (atrioventricular) node locates
between the atriums and ventricles and it conducts the impulse to the
ventricles, which are otherwise completely isolated electrically from the atriums.
It also slows down the impulse, leaving time for the blood to flow from the
atriums to the ventricles
2
(see figure 1)
Figure 1
Just like any other electrical system, the hearts electrical system can
also malfunction. There are many ways that it can deviate from normal
4
functionality; two common problems that result in the need for pacemaker
implantation are as follows:
Malfunctioning SA node. Normally the SA node generates 60-80 impulses
every minute. In some persons, however, this can slow down to 30-40
(bradycardia). At such slow rates, the heart is unable to pump adequate
amount of blood through the body. A related problem especially at older
age, is when the heart starts missing beats.
Malfunctioning AV node. Just like the SA node, the AV node can also miss
conducting some of the impulses coming from the SA node, causing a drop
in the amount of the blood that the ventricles can deliver. Its most severe
form is fibrillation, which is the uncontrolled beating of the different parts of
the heart.
2.2. How a pacemaker works
3
The above mentioned malfunctioning of the hearts electrical system can be
corrected by delivering timed electrical impulses to the heart. A
pacemaker device is used for this purpose.
A pacemaker consists of a small housing device containing a battery and the
electronic circuit running the pacemaker and one or two long electrical wires
that travel from the pacemaker housing to the heart. The most common way
these wires are implanted is that one is connected to the right atrium and the
other, if used, to the left ventricle. They serve as sensors to detect the electrical
impulses generated by the SA node and also to check whether the AV node
also conducted the impulses to the ventricles.
When it is sensed that the SA or AV node missed to generate an impulse,
these wires are used to send an electric impulse to the heart themselves,
taking over the function of SA and AV nodes. In this manner the pacemaker
can supervise the heart and ensure that it continues to contract at a heart rate
adequate to pump sufficient blood through the body.
5
The implantation of the pacemaker is only a minor surgical procedure,
performed with mild sedation and local anaesthetic and the operation lasts for
about an hour. Usually the device is put just below the collarbone, the wires
inserted into a vein and advanced through that vein into the heart. The skin is
then closed and the patient usually leaves the hospital the following day. In a
week time the patient may resume his/her prior lifestyle without limitation.
2.3. Key features and technical challenges
Size. Pacemaker size influences both the severity of the operation and patient
comfort after returning to normal life. There has been great emphasis on size
reduction which resulted in half sized devices in the 1990s compared to earlier
versions.
Battery lifetime. Present models have lifetime over 10 years, which was
achieved with the introduction of a lithium iodine battery in 1974. This greatly
increases the quality of life for the patient, as the time between two operations
is extended. The devices are checked quarterly for sufficient power, and they
also give off warning signals many months before they actually fail.
Casing. Titanium casing displaced the previously used epoxy resin to enclose
the battery and circuitry. This new casing helps shield the components and
greatly reduces outside electromagnetic interference that could come from
microwave ovens and similar household appliances.
Lead design. Attaching the wires of the pacemaker to the heart was a
challenge; the ones used today work like a screw or like hooks, creating a more
secure attachment to the heart tissue and help prevent the lead from slipping
out of place. The functionality of the leads was extended when sensing was
included. This created the demand pacemaker, which monitors the heart
functionality and paces only when necessary. All models are of this type today.
Programmable devices. By using radio frequency signals it is possible to
reprogram a device when frequency or other functional parameters need to be
6
adjusted. This way no operation needs to be done. Since the devices can
communicate with the outside world, they can provide information also on the
functionality of the heart, making follow-up easier for the physicians.
Intelligent devices. Pacemakers having a small microcomputer inside are able
to change the therapy of the patient also. They are able to recognise an
abnormally fast heart rate and react automatically. Including a small crystal
sensor inside the pacemaker, it can detect body movement, and can adjust the
pacemaker rate up or down according to the wearers activity. This can come
as close as to mimic the natural functionality of the heart.
Reliability. Like any medical device, pacemakers have to be highly reliable,
thoroughly tested and approved before being introduced to healthcare.
Significant portion of the patients are pacemaker dependent, meaning that
without the pacemaker their heart would stop functioning. This creates the need
for high quality in the device and also for reliable signalling methods in case the
device faults, possibly warning well before it actually happens.
2.4. Potential developments
4
Miniaturisation. Pacemakers have got progressively smaller since their first
appearance. The smallest pacemaker just weights 12.8 grams and it is
expected that development eventually will lead to a point where the leads could
be eliminated and the pacemaker itself could be directly implanted into the
heart.
Multiple leads (dual chamber pacing). Inserting more than two wires into the
heart creates the possibility to alter not only the frequency of the electrical
impulses but to alter the electrical activation pattern of the hearts muscle also.
Uses of this include hypertrophic obstructive cardiomyopathy, where overgrown
heart muscles blocks the egress of blood out of the heart or congestive heart
failure, where cardiac muscle deteriorates. With direct activation of different
muscles in the heart it is possible to alleviate these problems.
7
Beat to beat response. One of the most important advances in the
augmentation of pacemaker efficacy has been the development of complex
rate response algorithms. This assures that pacemakers gauge the bodys
physical need for increased cardiac output and that the pacemaker has a way
to interpret those signals and adjust its pulse generation accordingly. In the
future this can possibly include oxygen consumption based rate
responsiveness, patient-triggered rate increase and eventually pacemakers
hardwired into the parasympathetic and sympathetic nervous systems.
Programmers, remote monitoring. The increasing demand for patient
management and follow-up leads to more efficient and informative interface
between patient and physician. These devices are called programmers, and
they more and more encapsulate diagnostic software, trans-telephonic patient
monitoring capabilities (through telephone line, mobile phone)
5
.
Defibrillation. Cardiac diseases are interrelated and often having one cardiac
disease predisposes a patient to having another. Patients with pacemakers
often develop symptoms of ventricular fibrillation or tachycardia (which both
require a device called AICD Automatic Implantable Cardioverter Defibrillator)
and vice versa. In these cases the patients would benefit from a single,
integrated device capable of both functionalities. In the future it is expected that
these devices will eventually be the norm.
Brain pacemaker. As a treatment for depression there is possible treatment
with a pacemaker for the brain system called NeuroCybernetic Prosthesis
System
6
. The system sends 30-second electrical signals every 5 minutes to the
left vagus nerve. Currently approved only for epilepsy, this system could be
used for the treatment of depression, Parkinson disease and possibly other
brain diseases also
7
.
Pacemaker for the stomach. Gastroparesis
8
is a weakness of the stomach,
causing the patients digestion not to work properly, resulting in nausea,
vomiting and weight loss. It is caused by the lack of coordination between the
stomach muscles, nerves and hormones. A possible treatment is the
implantation of a pacemaker in the abdominal wall with electrodes sutured to
8
the stomach. The Cleveland Clinic already offers this treatment but it is still to
be generally accepted.
3. History of the Pacemaker innovation
*
3.1. Overview
The timeline below describes key events that shaped the development of the
pacemaker and the evolution of the pacing industry.
1949
Medtronic
founded
1957
Lillehei &
Medtronic
develop
external
PMs
1958
Greatbach
invents
implantable
PM
1960
Medtronic
buys exclusive
rights to
Greatbachs
invention
Implantable
PM published
in scientific
paper
Greatbach
leaves
Taber Inc.
1960 - 1973
Rapid
development of
PM;
complementary
& incremental
innovations
1974
Lithium
battery
powered PM
invented
Medtronic
rejects Li-
battery
Several
Medtronic
employees
leave to form
CPI on the
basis of Li-
powered
pacemaker
1975-1982
CPI success, Medtronic
looses mkt share
In 1978 Eli
Lilly buys
CPI
Competitors emerge: Cordis,
Intermedics, General Electric, CPI
1983 =>
Medtronic
regains
mkt share
Eli Lilly
spins off
medical
equipment
as Guidant
in 1994
Rapid incremental
and complementary
innovation
continues
GE exits in
1976 due to
quality
problems
Medtronic provides
equipment repair
and maintenance
services to hospitals
and has close
relations with
doctors
Medtronic
clear
leader
with
>70% mkt
share
Figure 2 - Technological innovation and companies evolution
3.2. The early years
Physicians had known since 1803 that electricity was effective in stimulating
heart activity. But progress in the development of pacemakers had been slow.
*
This whole chapter relies on information mainly from the following sources: Branbury & Mitchell
(1995), Gobeli & Rudelius (1985), Medtronic corporate website, Short biography of W. Greatbach
website, HBR Medtronic case
9
Pacemakers of the 1950s were bulky, relied on external electrodes, and
had to be plugged into a wall outlet. External electric shocks were frequently
too traumatic for young heart block patients, and the AC-operated pacemaker
could fail during a power blackout.
In most cases, the external pacemaker was used for patients recovering from
open-heart surgery. This market for external pacemakers was relatively small.
However, several physicians were beginning to recognise the value of the
device in treating patients suffering from chronic heart block. Yet, long-term
application presented several problems: an external pacemaker worn 24 hours
a day was inconvenient for the patient, the wires could become dislodged from
the heart, and most important, the passage of wires through the skin to the
heart introduced the possibility of infection.
In the 1950s one of the leading pacemaker researchers was Dr. Lillehei
from the University of Minnesota Medical School. He worked closely with a
small medical service company called Medtronic, which had been founded
in 1949 by Earl Bakken and Palmer Hermundslie
9
. Together they improved the
external pacemaker by decreasing the size of the pacemaker systems and
improving the reliability of the power source.
The implantable pacemaker breakthrough: Wilson Greatbatch, an electrical
engineer from Buffalo, New York, invented the worlds first implantable
cardiac pacemaker in 1958
10
. He worked closely with William Chardack, chief
of surgery at Buffalos Veterans Administration Hospital, who implanted the first
pacemaker to a dog. The device succesfully took control of the dogs heartbeat
but unfortunately the device failed after four hours when the body fluids found
their way to short out the circuit. Nevertheless, the device proved to have
potential for success and Greatbatch, Chardack and another doctor from
Veterans Administration Hospital, Andrew Gage, continued the development of
the pacemaker. Together, they carried out more than two years of experimental
work and testing on dogs before they published a paper in 1960 entitled: A
transistorised, self-contained, implantable pacemaker for the long-term
correction of complete heart block.
10
As the three men advanced in the development of the implantable pacemaker,
they soon reached a stage where an experiment with a human patient was
needed. Greatbatch's then employer, Taber Instrument Corp.
11
, was
unwilling to risk a million-dollar company on a perilous item like the pacemaker
and rejected the idea. We assume that as a materials testing device and
instrumentation company, and having Wilson Greatbatch in their ranks, Taber
Industries would have had the technical capability to continue developing the
pacemaker. However, it was the total lack of knowledge and marketing
capabilities towards the medical device industry that caused Taber to reject this
promising innovation.
Greatbatch left the company and continued the pacemaker development on his
own, relying on his savings. Greatbatch had a clear deadline for himself to get
results: he had calculated that his savings of a little over $2000 would feed his
wife and four children for approximately two years (apparently his wife was very
resourceful and raised a big garden).
The paper published by Greatbatch, Chardack and Gage had not gone
unnoticed by Medtronic. This company, which had a very humble beginning in
a garage in Minneapolis, was established as a service company, providing
medical equipment maintenance and repair services for the hospitals in
the region. On a rainy October evening in 1960, Palmer Hermundslie flew a
private plane to Buffalo, met Dr. Chardack and Greatbatch in the airport, and
signed a contract giving Medtronic exclusive rights to produce and market
the Chardack-Greatbatch implantable pulse generator. Production of the
implantables began in November, and by the end of December 1960,
Medtronic had received orders for 50 of the $375 units. The start was again
modest; Wilson Greatbatch made these 50 first pacemakers himself in his
backyard workshop. But this innovation would launch Medtronic into a growth
path that would bring the company to dominate the pacemaker and medical
electronics industry in the following decades.
While providing their services to the hospitals, the founders became well
acquainted with doctors and nurses throughout the Midwest - among them the
staff members of medical research laboratories
11
Frequently, the medical teams asked Medtronic engineers to modify equipment
or design and produce new devices needed for special tests. The company
responded by building single-use, custom-made products, and thereby entered
the manufacturing business. Due to these close relationships with doctors
and the customer demand, what had started as a service company was in
the late fifties transforming into a product development intensive
innovator.
In fact, the very close relationships of Medtronic with the medical
community not only changed to companys focus from being a service
company into product development but it also laid out the groundwork for the
companys future success. It was the special relationships and active
collaboration with leading cardiologists that gave credibility to the companys
products in the early years. In the later years the close relationships with
doctors, hospitals and patients enabled Medtronic to identify unmet needs and
create incremental innovations that expanded the pacing market significantly.
3.4. Development of the pacemaker and the emergence of competition
After the initial development of the implantable pacemaker, significant
advancements in all areas of pacing occurred rapidly. Medtronic was not left
alone into the industry, several other companies soon entered: Cordis and
General Electric were among the first to enter and later entrants included
Intermedics and CPI. Still, as a first entrant in cardiac pacing, Medtronic had a
strong technological lead and the company enjoyed over 70% market
share through the 1960s.
After 1960, due to the visibility of these first attempts, many academic and
commercial laboratories entered in the research race. In an effort to enter the
industry, the rights and results of these researches were bought by existing
firms in the medical and electronic areas or by new firms usually headed by
researchers themselves.
12
However, all this activity didnt change much the dominant design that
Medtronic had already brought to the market. The dominant design was
characterized by the following:
Synchronous (fixed rate) or asynchronous (variable rate) options
Single chamber of the heart (atrium or ventricle) pacing
Mercury-Zinc batteries
Epoxy-Silicon casing
Between 1960 and 1990, 32 firms sold external pacemakers and 86 firms sold
implantable pacemakers, of which 44 shutdown and 23 sold out (Branbury and
Mitchell 1995).
12
The number of new entrants could be explained simply by the
attractiveness of the high margins in the pacing industry probably all
medical equipment companies at least considered entering the market.
However, the number of exits (including the much publicised GE sell out) and
consolidation suggest that pacing industry has not been an easy industry for
the new entrants. We assume that in most cases the exits have resulted from
companies either not being able to keep up with the fast incremental
innovations or from problems related to product quality. An example of the
first exit reason, inability to innovate, could be Intermedics (bought by Guidant
in 1999), which in the 1990 for example failed to develop an implantable
defibrillator. The extreme importance of product quality aspects can be
exemplified by two cases: most notably, the 1976 exit of GE (sold out to
Teletronics) after product quality problems and the later exit of Teletronics
(bought by St. Jude) after the companys product failures caused two deaths
and a recall of 36,500 devices. We will analyse the success factors of the
industry more in chapter 5.
Even though the fast pace of innovation probably was very challenging for
many entrants, on the other hand fast development also provided
opportunities to successfully enter the industry. A case in point is CPI,
which was established by people who left Medtronic to capitalise on a new
related innovation, the Lithium battery.
13
Li-battery was developed outside the pacemaker-industry and was available to
all firms. The battery was specifically adapted to pacemakers by Wilson
Greatbatch. However, Medtronic initially rejected it, although Li-battery
provided significant opportunities to improve pacemakers with longer battery
life and with better certainty of avoiding leakages. We can only speculate the
reasons behind this initial rejection but probably Medtronics dominant design
and safety record was an inhibitor to take this kind of bigger step and integrate
this radical change. In addition, the fact that a longer device lifetime would
shrink the replacement market was most likely part of the decision. With about
60% market share and a heavy influence on the market, Medtronic had few
incentives to rock the boat and cut its revenues.
CPI became very successful and gained significant market share in the
seventies, mainly at the expense of Medtronic. CPI was later bought by Eli
Lilly (1978), which later in 1994 spun the unit off under the name Guidant.
14
4. Relevant markets and value chain dynamics
4.1 Market Size
In 1999, the bradycardia pacing market represented 2.5 bn$ sales worldwide
with a growth rate around 10%. Around 540 000 pacemakers are implanted
each year, mainly in the US and in Europe (see repartition chart)
13
.
1999 Bradycardia pacemaker market repartition
(% total units)
US
38%
Europe
40%
Japan
6%
Other
16%
4.2 Current competitive landscape
Consecutive to a long history of entries, exits and consolidations, the market is
now in the hand of three global players, all US corporations: Medtronic, St Jude
and Guidant CPI. Other manufacturers are local manufacturers (Biotronik in
Germany, ELA in France, Sorin in Italy).
Global Market Share 1999
(% $ sales)
Medtronic
51%
Guidant
22%
St Jude
21%
Others
6%
15
4.3 Market Structure
The market is composed of several actors: suppliers, manufacturers, regulatory
bodies like FDA in the US, doctors, hospitals and insurance companies. The
relationships are complex, as they do not take place along a linear process that
would end in the end customer, the patient.
Suppliers: they provide critical elements (battery, sensors), both in terms of
reliability and increased performances (battery lifetime, functionalities). In
particular, batteries are quite specific to the application and 90% (60% directly
and 30% through licensing)
14
of the battery pacemaker market is held by one
company, founded by Greatbatch himself. This position probably enables
Wilson Greatbatch Technologies Inc. to capture a part of the pacemaker
industry profit (in 2000, Greatbatch EBITDA totaled 26% of sales!
15
). Since
almost all sales of these specific batteries depend on pacemaker market,
they can be viewed as a co-specialized asset to the pacemaker.
Cardiac surgeons, general cardiologists: pacemaker represents the bread
and butter operations for cardiac surgeons and cardiologist
16
. After having had
Manufacturers Suppliers
FDA
or equivalent
Patients
EP,
Cardiologists
Hospitals
Health
Insurance
Manufacturers Suppliers
FDA
or equivalent
Patients
EP,
Cardiologists
Hospitals
Health
Insurance
16
at least six years residency training, pacemaker implantation represents a low
complexity operation and correspond to high volume - low risk part of their
business. The primary functions the doctors will look for are an adequate
treatment of the medical problem and a high level of reliability of the
device. Their reputation is somehow attached to the device, as they are the
ones who select the brand and model. Furthermore, doctors have no
incentive to spend part of their time to get an in-depth knowledge of the
technology that would enable them to assess a new manufacturer offer. Thus,
they have little incentive to switch from one manufacturer to another. In
contrast, a relation of trust is progressively established between the doctor
and the manufacturer through the sales representative.
The doctors value the ease of implantation, the ease of programming, the
existence of standard programming interfaces across models, the availability of
service, training and support, as well as automatic functions that can help
increase productivity in follow-up activities. Once the main market players are
at parity in terms of adequate treatment and reliability, these additional features
play a distinctive role and can further increase switching costs (eg. acquisition
of a programming device and training). As far as technical innovation is
concerned, manufacturers will generally target thought leaders among doctors
to promote a new product introduction.
Cardiac surgeons and cardiologists enable the diffusion of the product and can
be considered as being an extension of the distribution network. In this respect,
the relationship and trust that is built with them is a critical
complementary asset. To the extent that these physicians derive a large
part of their revenue from implants, we could even consider them as co-
specialized.
FDA approval process
17
: medical devices are assigned to one of three
regulatory classes based on the level of control necessary to assure the safety
and effectiveness of the device. Classification is risk based, that is, the risk the
device poses to the patient and/or the user is a major factor in the class it is
assigned. Class I includes devices with the lowest risk and Class III includes
those with the greatest risk. Pacemakers belong to class III, which requires
17
pre-market approval, post-market surveillance, follow up of safety issues
and alerts through MedWatch
18
as well as the implementation of a tracking
system. Tracking system provides information that may be used to facilitate
notifications and recalls ordered by FDA in the case of serious risks to health
presented by the devices.
Good relationship with FDA may help speed the processes of approval and
new product introduction, as these procedures are naturally a heavy burden on
the medical equipment companies. In this respect, this relationship can be
viewed as a complementary asset, though preventing its access to
competition may be difficult.
Hospitals: they represent the purchasing entity. It seems that the current
trend is to a limitation of the number of vendors through long-term contract, but
with an increased bargaining power given by a pooling of purchases among
different hospitals
19
.
Sales Channel: Medtronic and Guidant are using direct sales, leveraging the
product line breadth (use pacemaker as an entry point to sell ICD for Medtronic
and vice versa for Guidant). St Jude uses exclusive distributors. The direct
contact with sales representative is a key element of the relationship
between manufacturers and doctors. Sales representatives are involved
with the implant process, and in some instances the sales representative will
assist to the operation in the operating room.
Patients: they want their problem solved with a reliable device, increased
comfort through less operations (increased battery lifetime) and easier follow
up activities. Price can be an issue if the pacemaker is not entirely covered by
insurance. Manufacturers target the patients not by promoting heavily their
product and technology, but rather by taking the patient and its illness as an
entry point, providing clear explanations and eventually redirecting the patient
to a cardiologist
20
.
Insurance companies: they look for a lower overall cost (device, installation,
replacement). In some case like Medicare managed care programs,
18
reimbursement is a fixed amount of dollars per year for patient therapy, which
is a driver for longer life devices.
4.4 Market Drivers and Innovation Diffusion
In the early days of the discovery, the market size that was envisioned was
about 10000 devices per year. Today, close to 3 million people wear a
pacemaker and more than 500000 implant operations are performed each
year. Several factors can explain this important progression.
Reliability: an increased reliability has reduced the level of seriousness that
makes the risk return trade-off favor the pacemaker. More reliable devices
can be used to treat less serious cases or even prevention and comfort
type of indications.
Awareness and acceptance: this driver has been progressively
consolidated through an overall good track record.
Demographics: an aging population more prone to heart condition
problems has been the main growth driver in the past decade.
Replacement: though this driver impact has been reduced by the
introduction of Lithium batteries with longer lifetime, the replacement market
(25% of the implants) is now driven by attractive products improvements on
newer generations.
Increased scope: pacemakers widen their scope and target new indication
(antitachycardia, defibrillation, treatment of atrial fibrillation by ablation that
can require subsequent use of pacemaker, congestive heart failure,
cardiomyopathy, Parkinson). The potential customer base gets larger. In
many of these new indications, pacemakers substitute for inefficient drugs.
Increased scope (new devices targeting new indications) brings economies
of scale in marketing and distribution and leverages the
complementary assets represented by hospital and doctors contacts.
19
Growing pool of trained doctors: with a large awareness, good reliability
track record and an increased ease of implantation, in addition to
cardiologists, an increasing number of sub-specialists like electro-
physiologists perform pacemaker implants. Currently, about 35% of the
implants are performed by electro-physiologists, and this proportion is
increasing. Thus the distribution channel that doctors represent grows and
increases the market penetration.
4.5 Substitutes
Although there is no general substitute for a pacemaker there are certain
diseases where substitutes exist.
Treatment of atrial fibrillation by ablation. Atrial fibrillation is a
dysfunctionality in the heart that is accompanied by abnormal electrical
New Indication Scope Extension
Bradycardia
Congestive Heart Failure
Parkinson Disease
Antitachycardia
From severe to comfort application
1958 2001
2001
Vasovagal Syncope
M
a
r
k
e
t
G
r
o
w
t
h
New Indication Scope Extension
Bradycardia
Congestive Heart Failure
Parkinson Disease
Antitachycardia
From severe to comfort application
1958 2001
2001
Vasovagal Syncope
M
a
r
k
e
t
G
r
o
w
t
h
20
impulses. The physician can identify the precise area in the heart that starts the
abnormal signal, and then this tissue can be eliminated by ablation.
Drugs. Certain types of tachycardia (fast pacing of the heart) or atrial fibrillation
can be treated by drugs slowing heart pacing. Drugs include beta-blockers,
calcium-channel blockers and digoxin (slowing the AV node).
It seems that pacemaker is not being threatened by drug substitutes. On the
contrary, new pacemakers are actually replacing some inefficient drugs, which
have problematic side effects especially when the treatment has to be
permanent.
5. Innovation and technology management
5.1. What makes innovation such a challenge in the pacemaker industry?
The pacemaker industry is remarkable because its innovation process blends
contradictory forces:
Western Society requires the best pacemakers possible leading to a
demand for steady technological advancements.
There is a tension in the attitudes of cardiologist and EPs. On one hand
they dislike to introduce novel devices because of the potential risk for
their patients. On the other hand, they like to have the latest technology
to work with.
All the players along the value chain - suppliers, regulators, insurance
companies, doctors and patients - have to be involved in the introduction
of a new version. There are many interdependencies as already
highlighted in chapter 4.
21
5.2. Are disruptive or incremental innovations driving the pacemaker
industry?
Among the several innovations that occurred in the pacing industry, some bear
incremental characteristics while two innovations bear characteristics of
disruptive innovation. The table below provides a selection of the important
innovations in the pacemaker industry. From the 11 innovations displayed, we
consider two as disruptive: the invention of the implantable pacemaker in
1958 and the development of the Lithium battery for pacemakers in 1974. The
other nine innovations we deem incremental.
The two disruptive innovations gave the innovators a clear competitive
advantage. The production of implantable pacemakers in 1958 enabled
Medtronic to gain and keep market shares around 70 % over several years.
Though it could not initially match the external pacemaker performances on
several dimensions (except wearability!), technological trajectory rapidly
improved performances to a level that was meeting the patients needs.
The invention of the long-lasting Lithium battery has disruptive characteristics
as it initially introduced risks and established incumbents had no incentive to
pursue it. The inventor CPI (today Guidant) managed to capture a market share
of above 20%. Today both players are considered to be the most promising in
the industry.
Incremental innovations have a less drastic impact on the prosperity of the
different competitors but are nonetheless essential. There are two major factors
Selection of important pacemaker innovations
1958 First implantable pacemaker
1965 Demand standby pacer
1971 Programmable pacer
1973 Pacer programmer
1974 Lithium battery
1976 Telemetry
1979 Multiprogrammable pacer
1979 Programmable dual-chamber pacer
1983 Antitachycardia pacer
1985 Rate-responsive pacer
1985 Antitachycardia plus defibrillator pacer
1986 Defibrillator pacer
22
that distinguish them from the disruptive technologies in the pacemaker
industry. First, medical doctors are less reluctant to adopt devices with minor
changes because the potential risk of failure is relatively small. When an
incremental improvement is made, obtaining FDA approval is a much simpler
and faster process. Secondly, a company does not need to be always the first
to market such an incrementally improved product to remain competitive in the
industry.
5.3. How important are complementary assets?
In order to be successful in the pacemaker industry, it is critical to have access
to complementary assets and to exploit them optimally. Medtronic as well as
CPI have excelled in this exploitation. In the pacemaker industry we consider
the following complementary assets to be key:
Good relationships to cardiologists/EPs and hospitals, in other words to the
distribution channel
Effective access to the regulators that licence the use of a particular
pacemaker type
Close collaboration with suppliers of critical components
In our opinion, the most important complementary asset is the good
relationship with medical doctors and hospitals. They act not only as the
main distribution channel but also provide valuable information about the
market requirements. The decision of which pacemaker to implant is usually
based on trust and experience from the past. Not surprisingly, the patient
leaves the decision up to the expert. As a pacemaker is a highly critical device
for the life of the patient, physicians are very reluctant to switch suppliers
(conservatism). For a new entrant, it is difficult to overcome this barrier due
to trust based tacit contracts between the incumbent and the medical
institution. Therefore, incumbents have a clear first-mover advantage reflected
in a high-switching cost for customers.
23
It is also crucial to have effective access to the governmental regulators, who
are in charge of approving new types of pacemakers. However, this asset not
as critical as the first one because an entrant has to overcome the same
hurdles as the incumbent.
Last but not least, excellent relationships to suppliers of critical components are
a key complementary asset as well. This applies for example to sensors and
micro-devices but especially to the battery producer (virtual monopoly). If the
pacemaker producer is able to develop its product in cooperation with the
supplier, time-to-market and product quality improve. Additionally, this
collaboration leads to lower production costs and better supply-chain
performance. Again, all competitors should basically have equal access to
these suppliers. That is, criticality is much lower than for the distribution
channels.
5.5 How important is the first-mover advantage?
Both for large incumbents, as Medtronic, and for new entrants like CPI, it is
crucial to understand when and why the first mover has an advantage. We
have to distinguish between first movers in the industry and first movers in the
new technical developments in pacing systems.
What comes to the first type, first mover in the industry, we claim that a
significant first mover advantage exists. This advantage is tightly linked to
the extreme importance of safety, product quality and trust. A first mover has
the advantage of leveraging the cumulative experience that doctors and
patients have from the first movers products. This related to both the safety
record and the switching barriers that result from the in-depth product
knowledge of doctors (invested learning).
The same reasons that create an advantage for industry first movers naturally
make the conditions difficult for new entrants, even though the entrants product
would be technically superior. Only in specific cases where the technological
improvement has been very significant and the incumbents have been very
24
slow to react, a new entrant has been able to successfully gain market share
(CPI with the Li-battery powered pacemaker being an example)
5.6. What uncertainties is the pacemaker industry facing?
The pacemaker industry is in many aspects a predictable environment, mainly
due to the criticality of the product. However, disruptive technologies and big
market changes (e.g., regulatory issues) are a significant risk for the market
players.
Technological uncertainty. As outlined above, both disruptive and
incremental innovations can have a big impact on companies future
performances. Incremental uncertainties can be largely overcome if the market
player makes sure that it is on the technological forefront. In product
improvements, it is not necessary to be the first mover in this industry, as long
as a company is fairly quick to respond to competitor actions. At the current
stage of development for the standard bradycardia treatment, the marginal
value brought by technological improvements may be decreasing (in other
words, we may be reaching the end of the S curve). Hence, disruptive
innovations are more critical and can be a risk for the incumbents. Companies
are therefore well advised to devote enough attention to watch out for
potential technological changes.
Market uncertainty. All customers along the value chain - regulators, insurers,
hospitals, doctors, patients - are contributing to the market uncertainty. For
example, national regulations change, insurance companies have different cost
coverage policies or doctors might simply dislike a certain product. The market
players can reduce some of the risk by making sure that they have continuous
fast access to information about potential changes. This applies to the
interface to the most critical players of the value chain (see also
complementary assets). Nevertheless, current pressure to lower health care
bills could encourage less refined disruptive innovation. In such a context,
advanced secondary functions or comfort features may lose pre-eminence in
the face of a less expensive and more basic technology.
25
Competitive uncertainty. While this industry is seemingly attractive for
incumbents and new entrants alike, it is full of traps as we have already
discussed above. There are complementary assets that cannot be accessed
easily, disruptive technologies that take the incumbents by surprise or failed
innovations that immediately backfire to the innovator. Another interesting
story tells that Eli Lilly has attempted to preclude Medtronic from entering the
defibrillator market segment, claiming that Medtronic violated a CPI patent. In
order to settle the case, the two companies cross-licensed technology, giving
CPI a rate responsive pacemaker in an OEM arrangement with Medtronic.
Without the responsive unit, CPI would have been at a serious competitive
disadvantage.
21
This kind of moves further locks the market, raises entry
barriers and stabilizes the competitive picture.
Operational uncertainty. The degree of operational uncertainty is considered
to be low. Market players hold a stable market share with limited fluctuations.
Complementary innovations, unless disruptive, can be dealt with. Still, although
supply seems much less uncertain than in other high-tech industries there are
some potential uncertainties to be considered. It is interesting to note, for
instance, that some suppliers terminated sales of certain materials to
manufacturers of implantable devices to reduce potential product liability
exposure. Or take the supplier of Lithium batteries, Wilson Greatbatch Inc.,
which acts as the single supplier for the whole industry. Naturally, it may be
risky to rely on a single source of supply.
Organizational uncertainty. The organizational uncertainty is fairly low.
Investments are done on a mainly incremental basis and extreme resource
constraints are rare. Some organizational uncertainty can originate from the
fear of product cannibalisation. However, C. M. Branbury and W. Mitchell
(1995) demonstrated that the effect of cannibalisation is less dangerous than
the effect of delayed innovation.
26
6. Medtronic and CPI: different innovation strategies
Even though the pacemaking industry had to some extent a dominant design
and even the means of competition in the value chain appear to be fairly
similar, there are still significant differences in how companies approached
innovation management and strategy. These differences are somewhat
reflected in the market share development of these two companies (see picture
on the next page). We have already described many of the differences between
Medtronic and CPI earlier in this document. In this chapter we aim to highlight
the differences in the strategies of the two companies and the possible reasons
behind these different approaches. As the companies we concentrate on,
Medtronic and CPI, were established at different points in time, our
comparisons are not totally overlapping in time.
Figure 3 - Market share development and the timing of innovations in the pacemaker industry
(Gobeli & Rudelius 1985) .
22
27
As expected, firms in the same industry have a lot of similarities in their
approach however, we think there are also significant differences. Our
comparisons are focused on three aspects: how new technologies are
developed, how companies conquer the market with new products and how
companies defend their position.
6.1. Development of new technologies
In this comparison aspect we distinguish between Medtronics characteristics in
the early years (1950s) and at the time when CPI was established (1970s).
In the early years Medtronic was a small and very agile company that was
very much influenced by the companys founders. Medtronic also had close
relationships with the medical community. These factors contributed to the
focus and very fast decision making which was exemplified in the decision
to buy exclusive rights to the initial implantable pacemaker innovation. Being a
start-up, CPI was in the 1970s similarly flexible and focused. The company
was able to capitalise on one big leap in the pacemaker development, the Li-
battery. In both companies, it was not the organisational structure but the
important individuals that made the difference in both development and
decision making.
In addition to our comparison of Medtronic and CPI approaches, it is important
to note that initial implantable pacemaker innovation was rejected by the
inventors employer Taber Inc. Even though this instrumentation company
probably had the technical capabilities to further develop the pacemaker, they
had no knowledge of the medical market. We consider this rejection a further
proof of the crucial importance of customer and market understanding in
combination with the technical capabilities.
This entrepreneurial context is in contrast to the Medtronic of the
seventies. As they grew, the company apparently lost some of their
flexibility, which was exemplified by the slow product development cycle that
28
Medtronic had in the 1970s. Due to the companys leading position, and its
excellent contacts to the medical community Medtronic had a vast inflow of new
development ideas from their customer base. At the same time Medtronics
product development organisation appears to have been very functional and
specialised. As a combination of these issues, Medtronic had difficulties in
both deciding where to focus their development efforts and then
delivering fast enough. These problems were exemplified by the Li-battery
invention, which was first overlooked by Medtronic and later developed with
great time lags (4 years after CPI had entered the market with their Li-battery
powered pacemaker).
As a consequence of the problems Medtronic faced in the seventies, the
company later improved the efficiency of their product development process
significantly, for example by establishing cross-functional approaches to new
product development.
6.2. Market approaches
The approaches to marketing and customer relationships have actually been
surprisingly similar in both Medtronic during the 1950s and CPI in the
seventies. Both companies started with a brilliant breakthrough product and
were able to leverage existing relationships and knowledge of the medical
community, especially the key users. In the case of Medtronic, the deep
customer understanding was due to the approximately ten years of pre-
pacemaker service and maintenance business experience with hospitals in the
Midwest. On the other hand, CPI was able to leverage the experience and
contacts of former Medtronic employees who founded CPI. Both companies
had the remarkable benefit of combining the advantages of a start-up on
the technology side (flexibility, no legacy constraints) and the advantages of
an established player with brilliant customer understanding. Similar
advantages to customer understanding were present also in the dealings with
the regulators.
29
In contrast, in the beginning of the 1970s Medtronic was the clear market
leader and the company had established a first class distribution network and a
broad and deep customer base. Medtronic invested significantly in
customer education such as providing seminars to doctors and sponsoring
medical conferences and publications in medical journals.
Even though Medtronic was the market leader, they were not always the
technological leader, especially in the crucial case of Li-battery pacemaker,
as already mentioned before. At the time, Medtronic was an increasingly
bureaucratic organisation, slowing down innovation and improvements. Also,
introducing a Li-battery powered pacemaker would have been a threat to
Medtronics replacement business in the short-run. Another issue was the
conservative environment, which led to slow reaction: conservative
customers continued to use Medtronics conventional pacemakers for a long
time after the initial Li-battery pacemakers launch. Medtronics sales were
stable for years, but the companys market share eroded. These issues explain
why it took them so many years to react (CPI came to market in 1974,
Medtronic in 1978). In contrast, CPI had no legacy of replacement business
to protect and their means of reaching the wide market were initially limited.
CPI had to break through with a significantly improved product and rely
on the acceptance of lead users.
6.3. Defending the market position
As Medtronics market share declined during the 1970s the company started
to react: they improved their internal processes and had a stronger focus on
technological innovation than before. Also, they focused on cross licensing
and widening their product range. The latter meant that hospitals and
cardiologists were able to purchase the entire range of pacing and cardiology
products from Medtronic. This created a clear switching barrier, as the
relationship with cardiologists became so comprehensive. Medtronics sales
and market share started to increase again
30
CPI on the other hand, developed very differently: they were bought by Eli
Lilly, which later spun this division off as Guidant. Being part of Eli Lilly
probably helped CPI to get access to a world-class distribution network, so
crucial in this industry. In the long run, sustained innovation helped Guidant
take the lead over the rest of competition and resulted in the acquisition of
Intermedics.
We speculate that CPI might have had a very different fate if they had
managed to acquire William Greatbatch Inc in the mid-seventies (which is still
the quasi-monopoly producer of pacemaker Li-batteries today). This vertical
integration of a critical complementary asset would have left Medtronic in a
competitively very bad position, and CPI might have become market leader.
7. Conclusions
Through the study of the demanding pacing industry, we have been able to
assess how some of the companies were able to successfully bring innovation
to the market. This gives good insights on the relative importance of access to
technology and access to market as well as on the impact of disruptive
innovation.
In the first instance, when the implantable pacemaker was invented, Taber Inc.
had a clear technological advantage. However, this company either did not
recognize the market potential or acknowledged that they were not in a position
to market the product. Medtronic, on the other hand, had no particular
technological advantage but had excellent market understanding and was quick
to realize the opportunity.
This goes against the popular view that technological leadership is the main
driver of success in innovation management. Clearly, thorough market
knowledge and an efficient access to the distribution channel are key success
factors to recognize the potential value and commercialise a technological
innovation.
31
The second milestone in the pacemaker development is a good example of
how a small player can take advantage of a disruptive technology. At the time
of Lithium battery introduction, Medtronic was a successful incumbent holding a
very large share of a profitable market that they could influence. Medtronic had
no particular pull from its customer base and saw no incentive to introduce the
new technology and bear the risks of it. In addition, the new innovation might
have decreased their profitable replacement business.
In this industry, successful innovation requires both technical innovation and
market intimacy. Patients and physicians expect continuous product
improvement, but the market is highly unforgiving, and a product failure can
cost dearly in terms of market share or even company survival. That tension
makes innovation particularly risky. This risk, coupled with high entry barriers
mainly in the form of a tacit trust contract with physicians, may be part of the
explanation why only few companies prosper in this industry.
32
References
Catherine M. Branbury, Will Mitchell, The Effect of Introducing Important Incremental
Innovations on Market Share and Business Survival, Strategic Management Journal, Vol. 16
(1995), p. 161-182.
C. M. Christensen, Weve Got Rhythm! Medtronic Corporations Cardiac Pacemaker Business,
Harvard Business School, 9-698-004 (1997).
1
The heart
http://www.bae.ncsu.edu/bae/courses/bae465/1995_projects/scho/htmls/heartfunc.html
2
http://a1977.g.akamai.net/f/1977/1448/1d/webmd.download.akamai.com/1448/Anatom-E-
Tools/Heart/Heart_Tool.swf
3
Pacemaker Cardiology Channel http://www.cardiologychannel.com/pacemaker/, Pacemaker
Implantation http://www.healthyhearts.com/pacemaker.htm
4
The near future of pacemaker technology
http://biomed.brown.edu/Courses/BI108/BI108_1999_Groups/Cardiapacing_Team/nearfutur
5
Medical Industry Today, 25 oct 2001
6
Brain pacemaker to beat depression
http://news.bbc.co.uk/hi/english/newsid_1418000/1418091.stm, New depression treatments in
development http://depression.about.com/library/weekly/aa071000.htm
7
Saint Paul Pioneer Press, 15 jan 2002
8
Gastroparesis http://www.clevelandclinic.org/gastro/motility/paresis.htm
9
Medtronic corporate information and pacemaker history: http://www.medtronic.com/corporate
10
Short biography of Wilson
Greatbach:http://www.si.edu/lemelson/centerpieces/ilives/lecture09.html
11
www.taberindustries.com
12
Catherine M. Branbury, Will Mitchell, The Effect of Introducing Important Incremental
Innovations on Market Share and Business Survival, Strategic Management Journal, Vol. 16
(1995), p. 161-182.
13
Morgan Stanley Dean Witter, Investor Guide, Medical Technology
14
The Buffalo News, 27 sep. 2000
15
www.greatbatch.com
16
Interview with Phil Downer, Orthopedic Surgeon
17
www.fda.gov
18
www.fda.gov/medwatch
19
Medtronic form 10K
20
http://www.naspe.org/find_heart_rhythm_specialist/
21
MarkIntel, Industry and Market Intelligence, Cardiac Pacemakers, Inc. Company Report.
22
David H. Gobeli, William Rudelius, Managing Innovation: Lessons from the Cardiac Pacing
Industry, Sloan Management Review, Summer 1985, p. 29-43
Вам также может понравиться
- Case Assignment 1 - BUSI 472Документ7 страницCase Assignment 1 - BUSI 472ShannonОценок пока нет
- Final ProjectДокумент26 страницFinal Projectapi-309427868Оценок пока нет
- DIRECTIONS: Please Read Down The Columns and Check The Words You Believe Describe YourselfДокумент8 страницDIRECTIONS: Please Read Down The Columns and Check The Words You Believe Describe YourselfSachin TomarОценок пока нет
- Sonos ITC Complaint Against GoogleДокумент62 страницыSonos ITC Complaint Against GoogleRussell Brandom100% (2)
- Chemtrail Essay U of U WritingДокумент6 страницChemtrail Essay U of U Writingapi-286303526Оценок пока нет
- Real Crime or Crime DramaДокумент109 страницReal Crime or Crime DramaChris Corbet100% (1)
- Loretto Hospital AuditДокумент18 страницLoretto Hospital AuditKelly BauerОценок пока нет
- Who Killed The Music Industry Pt1Документ17 страницWho Killed The Music Industry Pt1scribdsvenОценок пока нет
- Fats That Heal Fats That Kill - Udo ErasmusДокумент954 страницыFats That Heal Fats That Kill - Udo ErasmusjavalassieОценок пока нет
- Basic Counselor's Attending Skills & ResponsesДокумент20 страницBasic Counselor's Attending Skills & ResponsesDARRO6 FOIОценок пока нет
- 4 Day Power Muscle Burn Workout SplitДокумент11 страниц4 Day Power Muscle Burn Workout SplitWahyu Yuki RplОценок пока нет
- Catalogo Mavic 2009Документ26 страницCatalogo Mavic 2009Muhammad Syafi' MubarakОценок пока нет
- LWS Operational GuidelinesДокумент94 страницыLWS Operational GuidelinesNidhi DubeyОценок пока нет
- The Evolution of Synthetic Thought: Takshashila EssayДокумент42 страницыThe Evolution of Synthetic Thought: Takshashila EssayLINDYLL PONO100% (1)
- Trust Case StudiesДокумент40 страницTrust Case Studiesvikash_2410100% (1)
- Mankiw - Chapter 1. "Ten Principles of Economics," 3-18Документ21 страницаMankiw - Chapter 1. "Ten Principles of Economics," 3-18me0h0angОценок пока нет
- The Social Revolution: Connecting with Today's CustomerДокумент55 страницThe Social Revolution: Connecting with Today's Customerrahuldutt1Оценок пока нет
- BeReal Product Tear DownДокумент8 страницBeReal Product Tear DownAnkith naiduОценок пока нет
- FinalPublicWriting01 MirandaDuckworthДокумент1 страницаFinalPublicWriting01 MirandaDuckworthMiranda DuckworthОценок пока нет
- Apple Innovation A Case Study: Ahmad Al-RubaieДокумент13 страницApple Innovation A Case Study: Ahmad Al-RubaieMegat Azwan100% (1)
- Human Cloning: A Social Ethics PerspectiveДокумент4 страницыHuman Cloning: A Social Ethics PerspectiveJecel Jean Gaudicos GabatoОценок пока нет
- Final Synthesis EssayДокумент5 страницFinal Synthesis Essayapi-617074664Оценок пока нет
- Reflection PaperДокумент3 страницыReflection PaperCurt sah jie SolermoОценок пока нет
- Bill Gates SummaryДокумент10 страницBill Gates SummaryArslanОценок пока нет
- Zuckerberg Responses To Commerce Committee QFRs1Документ229 страницZuckerberg Responses To Commerce Committee QFRs1TechCrunch100% (2)
- Heavens Gate Presentation Final CorrectedДокумент20 страницHeavens Gate Presentation Final Correctedapi-249435848Оценок пока нет
- Dl2011 Bioethics LegislationДокумент36 страницDl2011 Bioethics Legislationjeff_quinton5197Оценок пока нет
- Putting Families First: Good Jobs For AllДокумент52 страницыPutting Families First: Good Jobs For AllsarahcommchangeОценок пока нет
- Taco Bell CorporationДокумент4 страницыTaco Bell CorporationSiddharth KaushikОценок пока нет
- BAE Systems PresentationДокумент19 страницBAE Systems PresentationSuyash Thorat-GadgilОценок пока нет
- How Do You Suppose Milken Maintains His Self-Esteem When So Many People Despise Him As A Very Wealthy White Color Criminal?Документ3 страницыHow Do You Suppose Milken Maintains His Self-Esteem When So Many People Despise Him As A Very Wealthy White Color Criminal?Karma GurungОценок пока нет
- 2018.05.04 - Passers ListДокумент280 страниц2018.05.04 - Passers ListTheSummitExpress100% (1)
- 1-2. Red. Nature of TradlДокумент6 страниц1-2. Red. Nature of TradldewpraОценок пока нет
- COSCA Partner Organizations and ProjectsДокумент5 страницCOSCA Partner Organizations and ProjectsKaye BaguilodОценок пока нет
- Exploratory EssayДокумент5 страницExploratory EssayMahadeo SinghОценок пока нет
- Cardiac PacemakersДокумент3 страницыCardiac PacemakersDhruv SridharОценок пока нет
- ENGL 200 Technical Description Paper (Cardiac Pacemaker) Final DraftДокумент7 страницENGL 200 Technical Description Paper (Cardiac Pacemaker) Final DraftSara FarheenОценок пока нет
- 4 2 3 A Ekg Student Response Sheet (Revised 2 23 15)Документ8 страниц4 2 3 A Ekg Student Response Sheet (Revised 2 23 15)api-281824634100% (1)
- Recent Trends in Medical Imaging by Using VLSIДокумент4 страницыRecent Trends in Medical Imaging by Using VLSIinventyОценок пока нет
- Pacemakers and Implantable Cardioverter DefibrillatorsДокумент10 страницPacemakers and Implantable Cardioverter Defibrillatorsnoorhadi.n10Оценок пока нет
- Pacemaker InformatiiДокумент4 страницыPacemaker InformatiiAndreea MitranОценок пока нет
- Pulse Oximetry Design for Developing WorldДокумент54 страницыPulse Oximetry Design for Developing WorldrijowanОценок пока нет
- SBM1403 unit1Документ64 страницыSBM1403 unit1Balasaraswathi SОценок пока нет
- Unit 1 - Cardiac Care UnitsДокумент29 страницUnit 1 - Cardiac Care UnitssitalekshmiОценок пока нет
- What is a defibrillator and how does it workДокумент8 страницWhat is a defibrillator and how does it workRajib RajuОценок пока нет
- PacemakersДокумент12 страницPacemakersNixter TurnОценок пока нет
- Ekg Ecg Interpretation by DR Jason Lowe PDF Bb3 DR NotesДокумент79 страницEkg Ecg Interpretation by DR Jason Lowe PDF Bb3 DR NotesGuru RewriОценок пока нет
- Ecg SimulatorДокумент47 страницEcg SimulatorSafwan ShaikhОценок пока нет
- Implantable CardioverterДокумент5 страницImplantable CardioverterpratonyОценок пока нет
- Ei 65 Unit 5Документ31 страницаEi 65 Unit 5karthiha12Оценок пока нет
- 5 Identifying ECG IrregularitiesДокумент37 страниц5 Identifying ECG IrregularitiesRahmat MuliaОценок пока нет
- PacemakerДокумент26 страницPacemakerAswathy RCОценок пока нет
- A Review On Pacemakers: Device Types, Operating Modes and Pacing Pulses. Problems Related To The Pacing Pulses DetectionДокумент12 страницA Review On Pacemakers: Device Types, Operating Modes and Pacing Pulses. Problems Related To The Pacing Pulses DetectionsdekapureОценок пока нет
- Pacemaker Basics ExplainedДокумент5 страницPacemaker Basics ExplainedPoonam ThakurОценок пока нет
- Major Project Phase 1 Report CloudДокумент35 страницMajor Project Phase 1 Report CloudPraj CОценок пока нет
- The Heart'S Conduction System and "Natural Pacemaker "Документ8 страницThe Heart'S Conduction System and "Natural Pacemaker "merin sunilОценок пока нет
- Q: Characterize The Different Types of Pacemaker. Explain The Various Steps of PacingДокумент4 страницыQ: Characterize The Different Types of Pacemaker. Explain The Various Steps of PacingKRUPALITHAKKAR100% (1)
- Ecg en LabviewДокумент61 страницаEcg en LabviewHarold David Gil MuñozОценок пока нет
- Design a Cellphone-based ECG MonitorДокумент22 страницыDesign a Cellphone-based ECG MonitorMona NaiduОценок пока нет
- Interpreting AV (Heart) Blocks: Breaking Down The Mystery: 2 Contact HoursДокумент29 страницInterpreting AV (Heart) Blocks: Breaking Down The Mystery: 2 Contact HoursAsri RachmawatiОценок пока нет
- Real Time Ecg & Body Temperature Monitoring Device: Mini Project ReportДокумент73 страницыReal Time Ecg & Body Temperature Monitoring Device: Mini Project ReportLadvine D'AlmeidaОценок пока нет
- Biology Investegatory Project: Artificial PacemakerДокумент18 страницBiology Investegatory Project: Artificial PacemakerNirupama DesaiОценок пока нет
- ++ 437-1692-1-PBДокумент9 страниц++ 437-1692-1-PBAli TarkashvandОценок пока нет
- Ch-9 Equity Problem CaseДокумент6 страницCh-9 Equity Problem CaseShifa Fauzia AfrianneОценок пока нет
- Bab #12Документ13 страницBab #12Shifa Fauzia AfrianneОценок пока нет
- 18E0CF000004A6.Filename.15 Succession Planning FMI GMA 1-12-2011 To Send To ConferenceДокумент45 страниц18E0CF000004A6.Filename.15 Succession Planning FMI GMA 1-12-2011 To Send To ConferenceShifa Fauzia AfrianneОценок пока нет
- Bab #7Документ36 страницBab #7Shifa Fauzia AfrianneОценок пока нет
- Bab #7Документ36 страницBab #7Shifa Fauzia AfrianneОценок пока нет
- Problem 6-5Документ1 страницаProblem 6-5Shifa Fauzia AfrianneОценок пока нет
- Ch-9 Equity Problem CaseДокумент6 страницCh-9 Equity Problem CaseShifa Fauzia AfrianneОценок пока нет
- Stern Corp Balance Sheet Case StudyДокумент6 страницStern Corp Balance Sheet Case StudyShifa Fauzia AfrianneОценок пока нет
- Bab #8Документ35 страницBab #8Shifa Fauzia AfrianneОценок пока нет
- Kuliah Sesi-1 04 Sept 2013Документ44 страницыKuliah Sesi-1 04 Sept 2013Shifa Fauzia AfrianneОценок пока нет
- Bab #11 Analysis and DesignДокумент19 страницBab #11 Analysis and DesignShifa Fauzia AfrianneОценок пока нет
- Silabus SCM (Sep-Des 2013) 1Документ6 страницSilabus SCM (Sep-Des 2013) 1Shifa Fauzia AfrianneОценок пока нет
- Bab #9 Customer Relationship ManagementДокумент33 страницыBab #9 Customer Relationship ManagementShifa Fauzia AfrianneОценок пока нет
- Bab #10Документ20 страницBab #10Shifa Fauzia AfrianneОценок пока нет
- Materi PDB Pertemuan 1Документ6 страницMateri PDB Pertemuan 1Shifa Fauzia AfrianneОценок пока нет
- Bab #11 Analysis and DesignДокумент19 страницBab #11 Analysis and DesignShifa Fauzia AfrianneОценок пока нет
- 08 Managerial Decision MakingДокумент40 страниц08 Managerial Decision MakingHanniedya Violina BalinОценок пока нет
- 06 The Strategic & Operational Planning ProcessДокумент49 страниц06 The Strategic & Operational Planning ProcessShifa Fauzia AfrianneОценок пока нет
- Silabus ERP (Sep-Des2013) 1Документ4 страницыSilabus ERP (Sep-Des2013) 1Shifa Fauzia AfrianneОценок пока нет
- Bab #12Документ13 страницBab #12Shifa Fauzia AfrianneОценок пока нет
- Catatan Ira. Accounting AftermidДокумент26 страницCatatan Ira. Accounting AftermidShifa Fauzia AfrianneОценок пока нет
- Buttermilk Fried Chicken (Anna Olson)Документ2 страницыButtermilk Fried Chicken (Anna Olson)Shifa Fauzia AfrianneОценок пока нет
- French MacaronsДокумент2 страницыFrench MacaronsShifa Fauzia AfrianneОценок пока нет
- Buttermilk Fried Chicken (Michael Smith)Документ2 страницыButtermilk Fried Chicken (Michael Smith)Shifa Fauzia AfrianneОценок пока нет
- Macaron TowerДокумент3 страницыMacaron TowerShifa Fauzia AfrianneОценок пока нет
- Molten Chocolate CakeДокумент3 страницыMolten Chocolate CakeShifa Fauzia AfrianneОценок пока нет
- Molten Centre Chocolate CakesДокумент2 страницыMolten Centre Chocolate CakesShifa Fauzia AfrianneОценок пока нет
- Oaxacan Hot ChocolateДокумент2 страницыOaxacan Hot ChocolateShifa Fauzia AfrianneОценок пока нет
- Research Methodology ExampleДокумент8 страницResearch Methodology Exampletrueguyme0825Оценок пока нет
- Kritik jurnal kuantitatif surveyДокумент18 страницKritik jurnal kuantitatif surveyLuthi PratiwiОценок пока нет
- Detection of Brain HemorrhageДокумент8 страницDetection of Brain HemorrhageIJRASETPublicationsОценок пока нет
- Ergonomics and Health Issues Caused by Working With ComputerДокумент7 страницErgonomics and Health Issues Caused by Working With ComputerprviОценок пока нет
- The Role of Social Worker in Community Development: ArticleДокумент4 страницыThe Role of Social Worker in Community Development: ArticleTushar GautamОценок пока нет
- 3710Документ9 страниц3710Asad KhanОценок пока нет
- John Deere Performance Handbook 2012Документ484 страницыJohn Deere Performance Handbook 2012Martin100% (3)
- Unit 10-Maintain Knowledge of Improvements To Influence Health and Safety Practice RGДокумент10 страницUnit 10-Maintain Knowledge of Improvements To Influence Health and Safety Practice RGAshraf EL WardajiОценок пока нет
- White Mineral Oil SDSДокумент4 страницыWhite Mineral Oil SDSAreIf Cron BmxStreetОценок пока нет
- Frcpath Part 1 Previous Questions: Document1Документ11 страницFrcpath Part 1 Previous Questions: Document1Ali LaftaОценок пока нет
- Doshic Balance WorksheetДокумент2 страницыDoshic Balance WorksheetMidi GuruОценок пока нет
- JCI Booklet Final EditedДокумент40 страницJCI Booklet Final EditedRei IrincoОценок пока нет
- A General Introduction To PsychoanalysisДокумент18 страницA General Introduction To PsychoanalysisTrần Nhật Anh0% (1)
- Work Related Learning Form for Medical InternshipДокумент4 страницыWork Related Learning Form for Medical InternshipSanjog ChoudharyОценок пока нет
- The Concept of Intersubjectivity - BohleberДокумент19 страницThe Concept of Intersubjectivity - BohleberJanina BarbuОценок пока нет
- Most Common HSE DefinitionsДокумент15 страницMost Common HSE DefinitionsdillooseОценок пока нет
- Dukungan Leadership-LEAN - PPNIДокумент34 страницыDukungan Leadership-LEAN - PPNIBUKU PASIENОценок пока нет
- Conducting Qualitative ResearchДокумент7 страницConducting Qualitative ResearchIca RamosОценок пока нет
- WATO EX-65/55: Order InformationДокумент2 страницыWATO EX-65/55: Order InformationVladimir OsunaОценок пока нет
- Jeffrey Ansloos - Indigenous Peoples and Professional Training in Psychology in CanadaДокумент17 страницJeffrey Ansloos - Indigenous Peoples and Professional Training in Psychology in CanadaleoОценок пока нет
- Letter of Invitation For The ReactorДокумент2 страницыLetter of Invitation For The ReactorImmah PinedaОценок пока нет
- Soac 2021 First AnnouncementДокумент13 страницSoac 2021 First AnnouncementHendi PrihatnaОценок пока нет
- Google CL Ebony ThomasДокумент2 страницыGoogle CL Ebony Thomasebony thomasОценок пока нет
- Paths Pre-Health Intro Presentation 2021-2022Документ15 страницPaths Pre-Health Intro Presentation 2021-2022api-521535528Оценок пока нет
- FST556 - Dietary Fibre ObeДокумент37 страницFST556 - Dietary Fibre Obeinshirahizham100% (1)
- Armitage Shanks Blue Book MENA Edition 1Документ277 страницArmitage Shanks Blue Book MENA Edition 1josОценок пока нет
- 952-Article Text-3024-1-10-20221113Документ13 страниц952-Article Text-3024-1-10-20221113Audi GaluhОценок пока нет