Академический Документы
Профессиональный Документы
Культура Документы
Inoue (2012) - ADHD Hemodynamic Response Go:NoGo & FNIRS
Загружено:
ddb5013Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Inoue (2012) - ADHD Hemodynamic Response Go:NoGo & FNIRS
Загружено:
ddb5013Авторское право:
Доступные форматы
Reduced prefrontal hemodynamic response in children with
ADHD during the Go/NoGo task: a NIRS study
Yuki Inoue
a,c
, Kotoe Sakihara
a
, Atsuko Gunji
a
, Hiroshi Ozawa
c
,
Satoshi Kimiya
b
, Haruo Shinoda
d
, Makiko Kaga
a
and Masumi Inagaki
a
The current study examined the hemodynamic response
during the Go/ NoGo t ask in chil dren with/ without att enti on
deficit / hyperactivity disorder (ADHD). Using near-infrared
spectroscopy, oxy-Hb and deoxy-Hb concentrati on
changes in the frontal areas were compared during the
conditi ons with/ without inhi bitory demand. Compared
with typically devel oping chil dren, chil dren with ADHD
showed si gnificantly reduced activati on during the
conditi ons with inhi bitory demand (NoGo-conditi on)
in the front al areas. However, no si gnificant diff erences
in activati on during the conditi ons without inhi bitory
demand (Go-conditi on) were found between the two
groups. The current findings reveal ed that chil dren
with ADHD exhi bit an alt ered hemodynamic response
specifically during response inhi biti on, but not
during response executi on, and suggest ed the clinical
usefulness of near-infrared spectroscopy for the
eval uati on of response inhi biti on deficits in chil dren
with ADHD. NeuroReport 23:5560 c 2012 Wolt ers Kl uwer
Health | Li ppincott Willi ams & Wil kins.
NeuroReport 2012, 23:5560
Keywords: att enti on-deficit / hyperactivity disorder, Go/ NoGo paradi gm,
near-infrared spectroscopy, response inhi biti on
a
Department of Developmental Disorders, National Institute of Mental Health,
National Center of Neurology and Psychiatry (NCNP),
b
Shimada Ryoiku Center,
c
Department of Child Psychiatry, Shimada Ryoiku Center Hachiouji and
d
Faculty
of Psychology, Rissho University, Tokyo, Japan
Correspondence to Dr Yuki Inoue, MD, PhD, Department of Child Psychiatry,
Shimada Ryoiku Center Hachiouji, 4-33-13 Dai-machi, Hachiouji,
Tokyo 193-0931, Japan
Tel: +81 42 634 8511; fax: +81 42 634 8512;
e-mail: yu.inoue@shimada-ryoiku.or.jp
Received 21 September 2011 accept ed 20 October 2011
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a
developmental/behavioral disorder characterized by poor
attention, hyperactivity, and impulsivity that affects 37%
of the pediatric population [1]. A neuro-cognitive model
of ADHD has been based on disrupted behavioral
inhibition. Barkley developed a conceptual model of
ADHD that links behavioral inhibition, which comprises
three inhibitory functions: inhibition of a prepotent
response; stopping of an ongoing response; and inter-
ference control [2]. More recently, Sonuga-Barke [3]
postulated the Dual pathway model, which comprises
two functional pathways: in one, ADHD is a disorder of
executive function dysregulation, and in the other, it is a
motivational deficit with delay aversion. In both models,
the deficit of inhibitory function is regarded as a key
feature of ADHD. Besides these models, working
memory deficit is also considered to be one of the core
deficits of ADHD. Some neuroimaging studies have
examined the neural basis of working memory deficit of
the disorder [4,5].
To evaluate the cognitive features of ADHD, a series of
neurophysiological and neuroimaging studies involving
inhibitory tasks have been reported. The event-related
potential has been repeatedly examined in children with
ADHD, who show attenuated amplitudes of inhibitory
components (NoGo-N200 and P300) [68]. Moreover, a
one-source localization study of inhibitory event-related
potential revealed a group effect in the ADHD and
control groups in the right dorsolateral prefrontal cortex
(PFC) [9]. Also, functional MRI (fMRI) studies revealed
reduced activation in inhibitory trials in the ventrolateral
and dorsolateral prefrontal cortical areas [10,11], the
striatal region [12], and the presupplementary motor
area [13] of children with ADHD. Suskauer et al. [13]
also reported that these activation differences between
the ADHD and the control groups were not observed
for Go-stimuli, showing that this activation difference for
NoGo-stimuli is associated with response inhibition, not
the motor response.
Also, previous near-infrared spectroscopy (NIRS) studies
have investigated the frontal lobe function in chil-
dren [1416] and adults with ADHD [5]. Negoro et al.
and Jourdan Moser et al. reported that the prefrontal
hemodynamic response was altered in children with
ADHD [14,16] during the Stroop task, which involves
interference control [2]. However, to our knowledge, no
NIRS studies of children with ADHD have ever involved
the Go/NoGo task, which involves inhibition of the
prepotent response [2]. However, Herrmann et al. [17]
succeeded in detecting frontal lobe activation in normal
adults engaging with the Go/NoGo task by NIRS
measurement. They found significantly higher increase
in [oxy-Hb] and decrease in [deoxy-Hb] in the bilateral
inferior frontal cortex during the inhibition phase of the
Go/NoGo task. In the current study, we examined the
hemodynamic response during the Go/NoGo task in
children with and without ADHD. If we could detect the
Cognitive neuroscience and neuropsychology 55
0959-4965 c 2012 Wolters Kluwer Health | Lippincott Williams & Wilkins DOI: 10.1097/WNR.0b013e32834e664c
Copyright Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited.
altered NIRS finding in the prefrontal area of ADHD
children, which would be consistent with previous fMRI
studies, the current study would increase the possibility
of the clinical usefulness of NIRS measurement for the
evaluation of inhibitory function in children with ADHD.
Methods
Participants
Forty children, inclusive of 20 with ADHD (six females
and 14 males; eight with predominantly inattentive type
and 12 with combined type; mean age, 9 years 7 months;
range, 614 years) and 20 with typical development (six
females and 14 males; mean age, 9 years 9 months; range,
614 years), participated in this study. Children with
ADHD were recruited from among patients who were
referred to the Department of Child Neurology, National
Center Hospital, National Center of Neurology and
Psychiatry, Kodaira, Tokyo, Japan, and the Shimada
Ryoiku Center, Tama, Tokyo, Japan. The diagnosis was
confirmed by two child neurologists and one child
psychiatrist according to the Diagnostic and Statistical
Manual of Mental Disorders, fourth edition criteria [1].
Paid volunteers were recruited from neighboring ordinary
elementary and junior high schools as typically developing
children (TDC). The intelligence quotients of the
ADHD children were determined by means of the
Wechsler Intelligence Scale for Children, third edition [18].
Raven Colored Progressive Matrices [19] were used in
both groups. No participants exhibited significant sensory
or neurological limitation, epilepsy, mental retardation
(FIQ<70, Wechsler Intelligence Scale for Children, third
edition), or a pervasive developmental disorder. No
ADHD children had undergone pharmacotherapy before
the NIRS experiment. The research protocols were
approved by the ethical committees of the National
Center of Neurology and Psychiatry and the Shimada
Ryoiku Center. Written informed consent was obtained
from the children and their parents following a full
explanation of the experiment.
Task procedure
We used the Go/NoGo task software MOGRAZ
(NoruPro Light Systems Inc., Tokyo, Japan) [20]. Every
stimulus picture (a mole with/without sunglasses), with a
visual angle of 101, appeared in the center of a 21-inch
CRT monitor. The stimulus duration was 500 ms and the
interstimulus interval was randomly varied between 750
and 1250 ms. Each participant was instructed to respond
with the index finger of his/her dominant hand as soon as
possible whenever he/she saw a mole with sunglasses
(Go-stimulus) and not to respond to a mole without
sunglasses (NoGo-stimulus).
The current task involved two conditions (Fig. 1). In the
Go-condition, all stimuli were Go-stimuli throughout the
2-min task. In the NoGo-condition, Go-stimuli appeared
with a probability of 50% throughout the 2-min task. The
participants were asked to focus on the CRT monitor with
their minds blank while a 15-s baseline recording was
performed. Then, after completion of a certain condition
(Go-condition or NoGo-condition), they were told to focus
on the CRT again for another 30s. The order of the two
conditions of the task was counter-balanced. Four behavioral
variables [reaction time (RT, ms), reaction time variability
(RTV, ms), omission error rate (OE, %), and commission
error rate (CE, %)] were automatically recorded.
NIRS measurements
We used a 16-channel NIRS device cognoscope (NIM
Inc., Philadelphia, Pennsylvania, USA) to measure the
relative changes in [oxy-Hb] and [deoxy-Hb]. The NIRS
probe consisted of four light sources and ten detectors. The
detectors covered an area of 14 3.5cm on the forehead.
The probe was positioned as it was in the previous
study [21], which involved the same type of NIRS
equipment as that in the current study. The line of four
light sources was set on the FP1FP2 line (International
1020 System). Also, the very center of the probe was
positioned on Fpz (Fig. 2). The setup was designed to
image the PFC including the frontal pole, orbitofrontal
cortex, and the ventromedial-prefrontal and ventrolateral-
prefrontal cortices [22].
Each light source contained two LEDs (wavelengths: 730
and 850 nm; sampling rate: 3 Hz). The measurement
principles were based on the modified BeerLambert law,
for which the [oxy-Hb] and [deoxy-Hb] changes are
calculated from the change in light attenuation at a given
measured point. The sourcedetector distance was
2.5 cm. To correct a drift change of [Hb] over time,
baseline correction by linear fitting was performed for
each channel based on the two baseline data: the mean of
10-s periods before/after the task section. For statistical
analysis, we averaged all the time points of [oxy-Hb] and
[deoxy-Hb] changes during the task section. Then, as
performed in a previous NIRS study on children [23],
[oxy-Hb] and [deoxy-Hb] data from 16 channels were
averaged into four regions. The four regions were named
as left lateral (channel 14), left medial (channel 58),
right medial (channel 912), and right lateral (channel
1316) (Fig. 2).
Statistical analysis
Behavioral variables (RT, RTV, OE, and CE), age, and the
Raven score were compared between the TDC and the
ADHD groups by means of an unpaired t-test. For
statistical analysis of the NIRS data, the mean [oxy-Hb]
and [deoxy-Hb] changes detected in the four regions (left
lateral, left medial, right medial, and right lateral) during
the two task conditions (Go-conditions and NoGo-condi-
tions) were included. These NIRS measures were analyzed
using a mixed analysis of variance (ANOVA) design
(Diagnosis Region Task Condition), with repeated
measures over the latter two factors. Post-hoc analysis
56 NeuroReport 2012, Vol 23 No 2
Copyright Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited.
was conducted using an unpaired t-test (between-group
comparison: Diagnosis) and a paired t-test (within-group
comparison: Region and Task Condition), when main
effects or significant interactions were found. Bonferroni
correction was used for multiple post-hoc tests.
Finally, Pearsons correlation coefficients were calculated
for the relationship between the mean [oxy-Hb] (or
[deoxy-Hb]) changes and all demographic and behavioral
parameters in the ADHD and TDC groups.
Results
Behavioral and demographic data
There were no significant differences in age (t = 0.3,
P=0.80) or the total Raven Colored Progressive Matrices
score (ADHD vs. TDC; 28.6 vs. 29.6, t =0.56, P=0.58)
between the ADHD and the TDC groups. Regarding the
performance data, the ADHD group showed a signifi-
cantly larger RTV (98.4 vs. 72.7 ms, t =2.5, P<0.05) and
a higher CE (16.1% vs. 2.8%, t =3.9, P<0.001) during
the NoGo-condition and a larger RTV (115.5 vs. 64.4 ms,
t =2.4, P<0.05) during the Go-condition compared with
the TDC group.
NIRS data
On mixed-ANOVA analysis of the [oxy-Hb] changes, a
significant interaction (Diagnosis Task condition) was
observed [F(1,38) =7.0, P<0.05]. Within-group compari-
son revealed that [oxy-Hb] changes were significantly
Fig. 2
RL RM LM LL
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
Fpz
Near-infrared spectroscopy probe placement onto a participants
forehead. The very middle of the probe was placed at the Fpz position.
Small squares represent emitters, whereas white circles represent
detectors. Each number represents a channel. The 16 channels were
averaged into four regions; left lateral (LL) in orange, left medial (LM) in
yellow, right medial (RM) in green, and right lateral (RL) in blue.
Fig. 1
Go-probability=100%
NoGo-condition
(a)
(b)
Go-probability=50%
Go-condition
At rest
(15s)
At rest
(30s)
Task block
(120s)
Two task conditions in the current study. Moles with sunglasses (Go-stimuli) appeared with a 50% probability in (a) the NoGo-condition and a 100%
probability in (b) the Go-condition.
Reduced hemodynamic response in ADHD chil dren Inoue et al. 57
Copyright Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited.
larger during the NoGo-condition versus the Go-condition
in the TDC group (0.83 vs. 0.37 mmol; t =2.2, P<0.05),
whereas such an effect was not observed in the ADHD
group (0.20 vs. 0.32 mmol; t = 1.5, P=0.15). Between-
group comparison revealed that [oxy-Hb] changes during
the NoGo-condition were significantly lower in the
ADHD group than in the TDC group (0.20 vs. 0.83 mmol;
t = 3.1, P<0.01), whereas such an effect was not ob-
served during the Go-condition (ADHD vs. control; 0.32
vs. 0.37 mmol; t =0.27, P=0.78) (Figs 3 and 4).
On mixed-ANOVA analysis of the [deoxy-Hb], main ef-
fects and significant interactions were not observed, except
for the main effect of the Task condition [F(1,38) =14.5,
P<0.001], which revealed that the [deoxy-Hb] change
was significantly smaller during the NoGo-condition versus
in the Go-condition across the diagnostic groups ( 0.21 vs.
0.14mmol).
As a main effect of Region was not found on NIRS data
analysis, the mean [oxy-Hb] and [deoxy-Hb] changes during
the NoGo-condition/Go-condition were averaged across the
regions and then subjected to correlational analysis. The
mean [oxy-Hb] and [deoxy-Hb] changes during the NoGo-
condition/Go-condition were not significantly correlated
with age (NoGo-[oxy-Hb] r =0.132, P=0.411; NoGo-
[deoxy-Hb] r =0.01, P=0.95; Go-[oxy-Hb] r =0.019,
P=0.906; Go-[deoxy-Hb] r = 0.171, P=0.286), Raven
score (NoGo-[oxy-Hb] r =0.117, P=0.568; NoGo-[deoxy-
Hb] r =0.078, P=0.706; Go-[oxy-Hb] r = 0.035,
P=0.864; Go-[deoxy-Hb] r = 162, P=0.4), RT (NoGo-
[oxy-Hb] r = 0.16, P=0.308; NoGo-[deoxy-Hb] r =
0.135, P=0.401; Go-[oxy-Hb] r = 0.225, P=0.157; Go-
[deoxy-Hb] r = 0.087, P=0.588), RTV (NoGo-[oxy-Hb]
r = 0.291, P=0.065; NoGo-[deoxy-Hb] r =0.131, P=
0.416; Go-[oxy-Hb] r = 0.044, P=0.783; Go-[deoxy-Hb]
r =0.191, P=0.232), OE (NoGo-[oxy-Hb] r = 0.45,
P=0.782; NoGo-[deoxy-Hb] r =0.099, P=0.539), or CE
(NoGo-[oxy-Hb] r = 0,299, P=0.057 ; NoGo-[deoxy-Hb]
r =0.164, P=0.307).
Fig. 3
[oxy-Hb]_Control
2.0
(a)
(b)
NoGo-condition
[deoxy-Hb]_Control
[oxy-Hb]_ADHD
[deoxy-Hb]_ADHD
0.0
LL LM
RM RL
[
H
b
]
c
h
a
n
g
e
(
M
)
2.0
2.0
Go-condition
0.0
[
H
b
]
c
h
a
n
g
e
(
M
)
2.0
LL LM
RM RL
The averaged near-infrared spectroscopy waveforms of [oxy-Hb] and [deoxy-Hb] elicited during (a) NoGo-condition and (b) Go-condition. Two
vertical lines represent the task block (2-min duration). ADHD, attention deficit/hyperactivity disorder; LL, left lateral; LM, left medial; RL, right lateral;
RM, right medial.
Fig. 4
1
0.8
AD/HD
TDC
0.6
0.4
0.2
0
NoGo-condition
M
e
a
n
[
o
x
y
-
H
b
]
c
h
a
n
g
e
(
M
)
Go-condition
The average [oxy-Hb] changes in children with attention-deficit/
hyperactivity disorder (ADHD) and the group of typically developing
children (TDC) across the four regions.
58 NeuroReport 2012, Vol 23 No 2
Copyright Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited.
Discussion
In the current study, the hemodynamic response of
prefrontal regions during a Go/NoGo task was compared
between TDC and children with ADHD. The mean
change in [oxy-Hb] was clearly reduced in the ADHD
group compared with that in the TDC group. This char-
acteristic change was confined to the NoGo-condition,
for which prepotent inhibition is involved. Our NIRS data
for TDC indicated that activation of the prefrontal area
during an inhibitory task could be detected on NIRS
measurement even in children 614 years of age. As the
current and previous study [17] compared two task
conditions (Go-conditions and NoGo-conditions), in
both, the condition-specific brain activity in healthy
participants (children and adults) caused by inhibitory
demand but not by simple response execution could be
detected. Therefore, the current results overcome the
methodological limitations in the early NIRS study
involving a Go/NoGo task [24], in which the hemody-
namic response without an adequate control condition
equivalent to the Go-condition used in the current study
was measured.
Therefore, the mean [oxy-Hb] and [deoxy-Hb] changes
were not influenced by the regions of NIRS probes,
suggesting that bilateral PFC regions were equally
involved in the response inhibition. Tamm et al. [25]
conducted an fMRI study on children/adolescents,
showing that significant activation during a Go/NoGo
task was observed in the right superior/inferior frontal gyri
and the left middle frontal gyrus, which indicates
activation in more confined areas compared with the
currently described one. This may be due to the higher
mean age of the participants in the fMRI study (14 years
5 months) than in the current study (9 years 9 months),
which implies that younger participants show more
extensive brain activity than older participants because
of inefficient recruitment of brain regions. Moreover, the
[oxy-Hb] changes observed in the current study may
reflect activation in broader brain areas compared with
that in the former fMRI study, which was based on the
BOLD signal [25].
Thus, the reduced [oxy-Hb] response across PFC regions
in ADHD children observed in the current study is
consistent with the former fMRI studies involving an
inhibitory task [10,11], which showed a significant
reduction in brain activity in the bilateral PFC of children
with ADHD. These NIRS/fMRI findings during the
Go/NoGo task presumably reflect the deficit of inhibi-
tion of a prepotent response in children with ADHD [2].
Considering the characteristic findings in the previous
NIRS studies on ADHD children involving the task of
interference control [14,16], the clinical usefulness of
NIRS measurement for investigation of the inhibitory
function in ADHD patients is indicated.
Conclusion
The current study showed that NIRS measurement is a
powerful and handy tool for evaluation of the inhibitory
function in children with ADHD. Future NIRS studies on
ADHD children should include investigation of the
effects of pharmacotherapy on the inhibitory function
and clinical outcome.
Acknowledgements
This study was supported in part by a grant from the
Hayao Nakayama Foundation for Science & Technology
and Culture and a Grant for Research on Psychiatric and
Neurological Diseases and Mental Health (H19-Kokoro-
Ippan-006), and a Research Grant (19A-8) for Nervous
and Mental Disorders from the Ministry of Health,
Labour, and Welfare.
Conflicts of interest
There are no conflicts of interest.
References
1 American Psychiatric Association. . Diagnostic and statistical manual of
mental disorders Text revision. 4th ed. Washington, DC: American
Psychiatric Press; 2000.
2 Barkley RA. Behavioral inhibition, sustained attention, and executive
functions: Constructing a unifying theory of AD/HD. Psychol Bull 1997;
121:6594.
3 Sonuga-Barke EJ. Psychological heterogeneity in AD/HD: a dual
pathway model of behavior and cognition. Behav Brain Res 2002; 130:
2936.
4 Bayerl M, Dielenthesis TF, Vucurevic G, Gesierich T, Vogel F, Fehr C, et al.
Disturbed brain activation during a working memory task in drug-na ve adult
patients with ADHD. Neuroreport 2010; 21:442446.
5 Ehlis AC, Bahne CG, Jacob CP, Herrman MJ, Fallgatter AJ. Reduced lateral
prefrontal activation in adult patients with attention-deficit/hyperactivity
disorder (ADHD) during a working memory task: a functional near-infrared
spectroscopy (fNIRS) study. J Psychiatr Res 2008; 42:10601067.
6 Pliszka SR, Liotti M, Woldorff MG. Inhibitory control in children with
attention-deficit/hyperactivity disorder: event-related potentials identify the
processing component and timing of an impaired right-frontal response-
inhibition mechanism. Biol Psychiatry 2000; 48:238246.
7 Dimoska A, Johnstone SJ, Barry RJ, Clarke AR. Inhibitory motor control in
children with attention-deficit/hyperactivity disorder: event-related potentials
in the Stop-Signal paradigm. Biol Psychiatry 2003; 54:13451354.
8 Fallgatter AJ, Ehlis AC, Seifert J, Stirk WK, Scheuerpflug P, Zillessen KE,
et al. Altered response control and anterior cingulate function in
attention-deficit/hyperactivity disorder boys. Clin Neurophysiol 2004;
115:973981.
9 Liotti M, Pliszka SR, Perez R III, Luus B, Glahn D, Semrud-Clikeman M.
Electrophysiological correlates of response inhibition in children and
adolescents with AD/HD: influence of gender, age, and previous treatment
history. Psychophysiology 2007; 44:936948.
10 Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response
inhibition in pediatric bipolar disorder and attention deficit hyperactivity
disorder. Psychiatry Res 2010; 181:3643.
11 Rubia K, Cubillo A, Smith AB, Woolley J, Heyman I, Brammer MJ. Disorder-
specific dysfunction in right inferior prefrontal cortex during two inhibition
tasks in boys with attention-deficit hyperactivity disorder compared
to boys with obsessive-compulsive disorder. Hum Brain Mapp 2010;
31:287299.
12 Epstein JN, Casey BJ, Tonev ST, Davidson MC, Reiss AL, Garrett A, et al.
ADHD- and medication-related brain activation effects in concordantly
affected parent-child dyads with ADHD. J Child Psychol Psychiatry 2007;
48:899913.
13 Suskauer SJ, Simmonds DJ, Fotedar S, Blankner JG, Pekar JJ, Denckla MB,
et al. Functional magnetic resonance imaging evidence for abnormalities in
response selection in attention deficit hyperactivity disorder: differences in
Reduced hemodynamic response in ADHD chil dren Inoue et al. 59
Copyright Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited.
activation associated with response inhibition but not habitual motor
response. J Cogn Neurosci 2008; 20:478493.
14 Negoro H, Sawada M, Iida J, Ota T, Tanaka S, Kishimoto T. Prefrontal
dysfunction in attention-deficit/hyperactivity disorder as measured
by near-infrared spectroscopy. Child Psychiatry Hum Dev 2010;
41:193203.
15 Weber P, Lutschg J, Fahnenstich U. Cerebral hemodynamic changes in
response to an executive function task in children with attention-deficit
hyperactivity disorder measured by near-infrared spectroscopy. J Dev Behav
Pediatr 2005; 26:105111.
16 Jourdan Moser S, Cutini S, Weber P, Schroeter ML. Right prefrontal brain
activation due to Stroop interference is altered in attention-deficit
hyperactivity disorder: a functional near-infrared spectroscopy study.
Psychiatry Res 2009; 173:190195.
17 Herrmann MJ, Plichta MM, Ehlis AC, Fallgatter AJ. Optical topography
during a Go-NoGo task assessed with multi-channel near-infrared
spectroscopy. Behav Brain Res 2005; 160:135140.
18 Wechsler D. Wechsler Intelligence Scale for Children-III. San Antonio, TX:
The Psychological Corporation; 1991.
19 Raven JC, Court JH, Raven J. Manual for Ravens progressive matrices and
vocabulary scales. London: H.K. Lewis; 1985.
20 Inoue Y, Inagaki M, Gunji A, Furushima W, Kaga M. Response switching
process in children with attention-deficit/hyperactivity disorder on the novel
continuous performance test. Dev Med Child Neurol 2008; 50:462466.
21 Leon-Carrion J, Damas-Lopez J, Martin-Rodriguez JF, Dominguez-Roldan JM,
Murillo-Cabezas F, Barroso Y, et al. The hemodynamics of cognitive control:
the level of concentration of oxygenated hemoglobin in the superior
prefrontal cortex varies as a function of performance in a modified
stroop task. Behav Brain Res 2008; 193:248256.
22 Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, et al. Three-
dimensional probabilistic anatomical cranio-cerebral correlation via the
International 10-20 System oriented for transcranial functional brain
mapping. NeuroImage 2004; 21:99111.
23 Matsuda G, Hiraki K. Sustained decrease in oxygenated hemoglobin during
video games in the dorsal prefrontal cortex: a NIRS study of children.
NeuroImage 2006; 29:706711.
24 Fallgatter AJ, Stirk WK. Right frontal activation during the continuous
performance test assessed with near-infrared spectroscopy in healthy
subjects. Neurosci Lett 1997; 223:8992.
25 Tamm L, Memon V, Reiss AL. Maturation of brain function associated
with response inhibition. J Am Acad Child Adolsec Psychiatry 2002;
41:12311238.
60 NeuroReport 2012, Vol 23 No 2
Copyright Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited. Copyright Lippincott Williams & Wilkins. Unauthorized reproduction of this article is prohibited.
Вам также может понравиться
- Bong Et Al - 2020 - A Strong No-Go Theorem On The Wigner's Friend ParadoxДокумент9 страницBong Et Al - 2020 - A Strong No-Go Theorem On The Wigner's Friend Paradoxchucku100% (1)
- Experimental Psychology PSY 433: Ch. 8, PG 207-209 Reaction Time As A Dependent VariableДокумент15 страницExperimental Psychology PSY 433: Ch. 8, PG 207-209 Reaction Time As A Dependent Variablezahar89Оценок пока нет
- ConflictmanagementДокумент18 страницConflictmanagementmtkhan52-1Оценок пока нет
- AcceptanceДокумент4 страницыAcceptanceJustin VaculaОценок пока нет
- Jensen Chapter 3Документ10 страницJensen Chapter 3api-258575318Оценок пока нет
- A Review of The Motivation Theories in LearningДокумент8 страницA Review of The Motivation Theories in LearningannОценок пока нет
- Unit 9 Communication and ConflictДокумент45 страницUnit 9 Communication and ConflictIshworNeupaneОценок пока нет
- Chapter4 Anova Experimental Design AnalysisДокумент31 страницаChapter4 Anova Experimental Design AnalysisAnup ŠářkarОценок пока нет
- Cognitive and Emotional Processes in Web-Based EducationДокумент568 страницCognitive and Emotional Processes in Web-Based EducationAgus WinarjiОценок пока нет
- The Characteristics of Working MemoryДокумент7 страницThe Characteristics of Working MemoryJan Ivar Kinn EkrollОценок пока нет
- Ksk5024 - Computer System Setup and RepairДокумент3 страницыKsk5024 - Computer System Setup and RepairazzkvbesutОценок пока нет
- Understanding Intelligence: IQ, EI, and MIДокумент33 страницыUnderstanding Intelligence: IQ, EI, and MIMiles WackyОценок пока нет
- Big Five PersonalityДокумент8 страницBig Five PersonalityNicky FernandesОценок пока нет
- AP Psych Final Study Guide - Semester 1Документ8 страницAP Psych Final Study Guide - Semester 1Shane TangОценок пока нет
- Interpersonal SkillsДокумент8 страницInterpersonal Skillshunny861Оценок пока нет
- Assignment Psychology History BaruДокумент31 страницаAssignment Psychology History BaruNurul Syahirah Mohamad AliОценок пока нет
- Variations in Psychological Attributes .: Class Xii Chapter OneДокумент30 страницVariations in Psychological Attributes .: Class Xii Chapter OneJuliet AbrahamОценок пока нет
- Validity and ReliabilityДокумент5 страницValidity and ReliabilityzainalijanjuyaОценок пока нет
- HSG136 Workplace Transport SafetyДокумент143 страницыHSG136 Workplace Transport SafetyMike Mitakidis MichailОценок пока нет
- Social Exchange TheoryДокумент35 страницSocial Exchange TheorynakulpadalkarОценок пока нет
- Fielding-Mus149-Assignment 5Документ16 страницFielding-Mus149-Assignment 5api-510692327Оценок пока нет
- 7 Emotions and CommunicationДокумент16 страниц7 Emotions and CommunicationvickyОценок пока нет
- The Right Person For The JobДокумент5 страницThe Right Person For The JobViet Quang NguyenОценок пока нет
- Emotions ArticlesДокумент116 страницEmotions ArticlesNag VardhanОценок пока нет
- Documentary On Emotional IntelligenceДокумент28 страницDocumentary On Emotional IntelligencelyvabreraОценок пока нет
- PSY3101 Study Guide Exam 2Документ12 страницPSY3101 Study Guide Exam 2Wen RenОценок пока нет
- HCF and LCM No Calculator: Exam-Type Questions A1 A2 A3 A4Документ1 страницаHCF and LCM No Calculator: Exam-Type Questions A1 A2 A3 A4LabeenaОценок пока нет
- Self-Concept in CommunicationДокумент5 страницSelf-Concept in CommunicationJuniorD21Оценок пока нет
- Big 5 Personality ReportsДокумент3 страницыBig 5 Personality ReportsSaumya ShrivastavaОценок пока нет
- Eco 162Документ17 страницEco 162msukri_81Оценок пока нет
- CorsiBlock-TappingTask DetailsДокумент23 страницыCorsiBlock-TappingTask Detailsincredible soulОценок пока нет
- Types of Theories and ModelsДокумент26 страницTypes of Theories and ModelsRichard AlboroОценок пока нет
- Conflict at WorkДокумент25 страницConflict at Workkelemework dagneОценок пока нет
- MB0034 (Research Methodology) ...Документ22 страницыMB0034 (Research Methodology) ...Faiha Afsal100% (1)
- Assignment 3Документ13 страницAssignment 3Thurei IIОценок пока нет
- Interpersonal ConflictДокумент4 страницыInterpersonal Conflictapi-437432128Оценок пока нет
- Chapter 6 AP Psych OutlineДокумент8 страницChapter 6 AP Psych OutlineJenny Gan100% (1)
- Personality Definitions and MeaningsДокумент34 страницыPersonality Definitions and Meaningsscribdpersona100% (1)
- Cross Cultural Communication-1Документ10 страницCross Cultural Communication-1Nikita SangalОценок пока нет
- Personality TheoriesДокумент23 страницыPersonality Theoriesyav007Оценок пока нет
- Barriers To Effective CommunicationДокумент13 страницBarriers To Effective CommunicationBenazir QureshiОценок пока нет
- A Structural Model of Self-Worth Protection and AchievementДокумент10 страницA Structural Model of Self-Worth Protection and Achievementdanyela_12sep5506Оценок пока нет
- Assignment Two (2) The Body Shop International PLC MST 3871 March 2012Документ8 страницAssignment Two (2) The Body Shop International PLC MST 3871 March 2012Christofdash MbangoОценок пока нет
- Stages of Interpersonal RelationshipДокумент33 страницыStages of Interpersonal RelationshipSanjida DorothiОценок пока нет
- Managing Multicultural Teams-Assignment - Frank XiaДокумент2 страницыManaging Multicultural Teams-Assignment - Frank XiaJian Xia100% (1)
- Com100 r3 Introduction To Communication WorksheetДокумент8 страницCom100 r3 Introduction To Communication WorksheetTami Martin100% (1)
- Introduction DatabaseДокумент181 страницаIntroduction DatabaseAmerulzai SyazaОценок пока нет
- Correct sentences before a good essay ! 掌握 CCC-Check (查) -动词; Cut (切) ; Count 和 TSVC table-Time; Subject; Verb; ComplementДокумент6 страницCorrect sentences before a good essay ! 掌握 CCC-Check (查) -动词; Cut (切) ; Count 和 TSVC table-Time; Subject; Verb; ComplementHenry LowОценок пока нет
- Limitations and Conclusion IntroДокумент3 страницыLimitations and Conclusion Intropasign inОценок пока нет
- The NEO Personality Inventory (NEO-PI-R)Документ2 страницыThe NEO Personality Inventory (NEO-PI-R)Mohammed Abdul QayumОценок пока нет
- Communication ChannelsДокумент11 страницCommunication ChannelsSheila692008Оценок пока нет
- Oumm3203 P EthicsДокумент10 страницOumm3203 P Ethicszakuan79Оценок пока нет
- Chapter 6.emotionalДокумент20 страницChapter 6.emotionalMurni AbdullahОценок пока нет
- Alemi 2011 PDFДокумент17 страницAlemi 2011 PDFTri Lestari Nela VRОценок пока нет
- Communication Interpersonal SkillsДокумент24 страницыCommunication Interpersonal SkillsInderjot SinghОценок пока нет
- Neuro FeedbackДокумент14 страницNeuro FeedbackMar LindaОценок пока нет
- Transcranial Direct Current Stimulation Improves CДокумент13 страницTranscranial Direct Current Stimulation Improves CCarolina Robledo CastroОценок пока нет
- Biomedical Journal: SciencedirectДокумент8 страницBiomedical Journal: SciencedirectCarlosToledoОценок пока нет
- Múnger Etal (2021) Behavioral and Neurophygiological Markers of ADHDДокумент10 страницMúnger Etal (2021) Behavioral and Neurophygiological Markers of ADHDVander PereiraОценок пока нет
- Brain Development in ADHDДокумент6 страницBrain Development in ADHDalexmeduernОценок пока нет
- A Case Study of Balance Rehabilitation in Parkinson's DiseaseДокумент8 страницA Case Study of Balance Rehabilitation in Parkinson's Diseaseddb5013Оценок пока нет
- Developmental Milestones Birth To 5 YearsДокумент2 страницыDevelopmental Milestones Birth To 5 Yearsddb5013Оценок пока нет
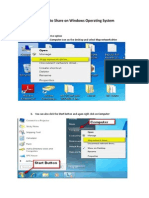
- Connecting To Share On Windows OSДокумент4 страницыConnecting To Share On Windows OSddb5013Оценок пока нет
- Neuropsych Eval Report Sample MTBI 2Документ4 страницыNeuropsych Eval Report Sample MTBI 2ddb5013Оценок пока нет
- Reaction Paper - in ProgressДокумент6 страницReaction Paper - in Progressddb5013Оценок пока нет
- Power Up Your PowerPointДокумент2 страницыPower Up Your PowerPointddb5013Оценок пока нет
- Anatomy of A ProposalДокумент13 страницAnatomy of A Proposalddb5013Оценок пока нет
- Cartas Contra La HumanidadДокумент31 страницаCartas Contra La Humanidadjohn_200000080% (5)
- Greenberger 2008 - Self-Entitled College Students - Contributions of Personality, Parenting, and Motivational FactorsДокумент12 страницGreenberger 2008 - Self-Entitled College Students - Contributions of Personality, Parenting, and Motivational Factorsddb5013Оценок пока нет
- Concussions in Sports: What Is A Concussion?Документ5 страницConcussions in Sports: What Is A Concussion?api-447282632Оценок пока нет
- NCP GrievingДокумент3 страницыNCP GrievingAecee Jose-Macayan100% (4)
- Client Centered TherapyДокумент9 страницClient Centered TherapymysticaladyОценок пока нет
- 3connectionism TheoryДокумент1 страница3connectionism TheoryShianne RacadioОценок пока нет
- Chapter 3 Developing The Whole PersonДокумент35 страницChapter 3 Developing The Whole PersonMarielle Cusi100% (1)
- Anatomy and Physiology Tetanus FinalДокумент17 страницAnatomy and Physiology Tetanus Finalfelicisimo039690175% (4)
- Subdural Hygroma Different Treatment Modalities AnДокумент13 страницSubdural Hygroma Different Treatment Modalities AnDewan Shamsul AsifОценок пока нет
- C. Raymond Lake Auth. Schizophrenia Is A Misdiagnosis Implications For The DSM-5 and The ICD-11 2012Документ442 страницыC. Raymond Lake Auth. Schizophrenia Is A Misdiagnosis Implications For The DSM-5 and The ICD-11 2012Romulus-Dan Nicoara100% (1)
- E45 Anecdotal Record E FormДокумент2 страницыE45 Anecdotal Record E FormShafi MuhammadОценок пока нет
- Multiple-Choice Questions: I Toward Self-Assessment CME. Category 1 CME Credits Not DesigДокумент9 страницMultiple-Choice Questions: I Toward Self-Assessment CME. Category 1 CME Credits Not DesigManish MauryaОценок пока нет
- Liceo de Cagayan University Senior High School Department The Problem and Its ScopeДокумент19 страницLiceo de Cagayan University Senior High School Department The Problem and Its ScopeJohn Stephen BacolОценок пока нет
- NeurotransmittersДокумент19 страницNeurotransmittersJelly Millabangco Baloncio100% (1)
- Autism PDD Fact SheetДокумент6 страницAutism PDD Fact SheetNational Dissemination Center for Children with DisabilitiesОценок пока нет
- Handbook of Mindfulness and Self-RegulationДокумент299 страницHandbook of Mindfulness and Self-RegulationBea Arce Bass Werner100% (10)
- 33 34 35 Test BankДокумент24 страницы33 34 35 Test Bankanimepenguin_610Оценок пока нет
- OpenAI GlossaryДокумент1 страницаOpenAI GlossaryAsuper122Оценок пока нет
- Department of Elderly Care: What Is Delirium?Документ2 страницыDepartment of Elderly Care: What Is Delirium?mutiaraОценок пока нет
- Rewards and Punishments Inside The Classroom (Academic Paper)Документ6 страницRewards and Punishments Inside The Classroom (Academic Paper)Roma Blaise G FloresОценок пока нет
- Chapter 17Документ54 страницыChapter 17Cameron HannaОценок пока нет
- Graded Exposure WorksheetДокумент2 страницыGraded Exposure WorksheetEmily AndersonОценок пока нет
- 01 Introduction To NeuroanatomyДокумент49 страниц01 Introduction To NeuroanatomySara HajjajОценок пока нет
- Social Thinking PresentationДокумент87 страницSocial Thinking PresentationLaura Bocsa100% (1)
- AI Lect8 NeuralДокумент84 страницыAI Lect8 NeuralMenna SaedОценок пока нет
- Session 5 (Learner Types. Learning Styles)Документ5 страницSession 5 (Learner Types. Learning Styles)Sophia NazarenkoОценок пока нет
- 04.0 HydrocephalusДокумент41 страница04.0 HydrocephalusBaraka SayoreОценок пока нет
- A Parent'S Guide To Applied Behavior Analysis (ABA) : Who We AreДокумент2 страницыA Parent'S Guide To Applied Behavior Analysis (ABA) : Who We AreRubí Corona TápiaОценок пока нет
- B.Pharmacy-4 Semester (New PCI Syllabus) : Pharmacology of Central Nervous SystemДокумент3 страницыB.Pharmacy-4 Semester (New PCI Syllabus) : Pharmacology of Central Nervous SystemGuma KipaОценок пока нет
- Bella - CA 04 Emotion and CognitionДокумент4 страницыBella - CA 04 Emotion and Cognition陳芷萍Оценок пока нет
- Principles of Teaching and Learning Related To Health Education 2019-2020Документ106 страницPrinciples of Teaching and Learning Related To Health Education 2019-2020Lyssa Marie Ege100% (2)
- Development of The Concept of MindДокумент15 страницDevelopment of The Concept of MindRenzo Villanueva GomezОценок пока нет