Академический Документы
Профессиональный Документы
Культура Документы
4335 03 4437 08 MSC 20101210
Загружено:
gkawsar22Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
4335 03 4437 08 MSC 20101210
Загружено:
gkawsar22Авторское право:
Доступные форматы
Mark Scheme (Results)
November 2010
IGCSE
IGCSE Chemistry (4335) Paper 1F
IGCSE Science (Double Award) (4437) Paper 08
Edexcel Limited. Registered in England and Wales No. 4496750
Registered Office: One90 High Holborn, London WC1V 7BH
Edexcel is one of the leading examining and awarding bodies in the UK and throughout the
world. We provide a wide range of qualifications including academic, vocational, occupational
and specific programmes for employers.
Through a network of UK and overseas offices, Edexcels centres receive the support they need
to help them deliver their education and training programmes to learners.
For further information, please call our GCE line on 0844 576 0025, our GCSE team on 0844 576
0027, or visit our website at www.edexcel.com.
If you have any subject specific questions about the content of this Mark
Scheme that require the help of a subject specialist, you may find our Ask
The Expert email service helpful.
Ask The Expert can be accessed online at the following link:
http://www.edexcel.com/Aboutus/contact-us/
Alternately, you can speak directly to a subject specialist at Edexcel on our
dedicated Science telephone line: 0844 576 0037
(If you are calling from outside the UK please dial + 44 1204 770 696 and
state that you would like to speak to the Science subject specialist).
November 2010
All the material in this publication is copyright
Edexcel Ltd 2010
IGCSE CHEMISTRY 4335/03 4437/08 NOVEMBER 2010
Question Mark
Acceptable answers
Notes Total
1 a M1 C 1
M2 D 1
M3 B 1
b M1 A 1
M2 C 1
c i M1 A / E / F 1
Accept correct names for all of above
ii M1 to neutralise /use up the acid Accept complete the reaction 1
iii M1 zinc carbonate / ZnCO
3
1
Question Mark
Acceptable answers
Notes Total
2 a M1 20.60 Final zero needed 1
M2 1.75 1
Award 1 for two correct readings in
wrong order
M3 18.85 CQ on M1 and M2 1
b i M1 ticks under 2nd and 4th columns 1
ii M1 26.1(0) + 26.2(0)
2
No penalty for missing zeroes
CQ on ticked values
1
M2 26.15 Ignore unit
M2 DEP on ticked values or on M1
Correct final answer scores M1 and M2
1
If other than 2nd and 4th values ticked,
see separate table showing acceptable
answers for possible combinations of
ticked values
All answers must be to 2 dp except for
26.6 from averaging all four titres
Question Mark
Acceptable answers
Notes Total
3 a M1 sulphur / S / S
8
1
b i M1 10 (cm
3
) Accept value written in table 1
ii M1 temperature / conical flask / swirling / cross /
concentration of acid
Accept concentration of original
thiosuphate solution
1
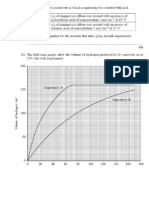
c M1 scale of 1 cm rep 5 s Scale must start at zero or 5 but starting
zero not required
Accept 1 cm rep 4 s
Must be at least two numbers
1
M2
M3
five points correctly plotted Must be to the nearest gridlines
Deduct 1 mark for each error
2
M4 curve of best fit including 80% or 100% but not both Line must not go to the origin 1
d i M1 1 9 1
M2 0.11 (s
1
) No ECF 1
ii M1 times are short(er) / reaction is fast 1
M2 big(ger) (percentage) error in measuring time/using
clock
Accept difficult to measure time
(accurately)
1
e i M1 rate (directly) proportional to concentration Accept "concentration proportional to
rate"
Accept quantitative answer such as
"rate doubles when concentration
doubles"
Do not accept "they are proportional"
Award 1 mark for qualitative expression
such as "rate increases as
concentration increases"
2
ii M1 collisions OWTTE 1
M2 more frequent Must be some reference to time, eg
more per second
1
If atoms or molecules used in place of
particles/ions, award max 1
Do not award M2 if any reference to
increasing speed /energy/activation
energy
Ignore references to effectiveness of
collisions
Question Mark
Acceptable answers
Notes Total
4 a M1 18.8 1
M2 24.2 1
Award 1 for two correct readings in
wrong order
M3 5.4 CQ on M1 and M2 1
b M1 repeat 1
c i M1 4 1
M2 not stated whether temperature increase or decrease DEP on M1 1
ii M1 (headings) salt/student number AND start temp AND
end temp
Ignore mass of salt and student column
headings
1
M2 (units) C for both temperature columns Accept C in one column if start and end
temps included
1
M3
M4
(results) all three salts/student numbers and six temps
included
Deduct 1 mark for each error and
omission
Two correct temperatures for one
experiment in wrong order loses 1 mark
2
If results for student identified in (c)(i)
included, then only M1 and M2 can be
awarded
d i M1 insulate / use a polystyrene cup (instead of a beaker) 1
ii M1 salt name cannot be plotted / no connection between
name and temperature change
Accept only one continuous variable /
one variable is categoric / only one set
of numbers
1
iii M1 100 Ignore units 1
Question Mark
Acceptable answers
Notes Total
5 a M1 Q 1
b M1 R 1
c M1 R and S 1
d M1 ()1.7 (V) 1
e M1 V V
2+
+ 2e 1
M2 W
2+
+ 2e W 1
Accept in either order
f i M1 no reaction 1
ii M1 T + W
2+
T
2+
+ W 1
PAPER TOTAL: 50 MARKS
Further copies of this publication are available from
International Regional Offices at www.edexcel.com/international
For more information on Edexcel qualifications, please visit www.edexcel.com
Alternatively, you can contact Customer Services at www.edexcel.com/asktheexpert or on + 44 1204 770 696
Edexcel Limited. Registered in England and Wales no.4496750
Registered Office: One90 High Holborn, London, WC1V 7BH
Вам также может понравиться
- Mark Scheme (Results) January 2014Документ14 страницMark Scheme (Results) January 2014Lalith77Оценок пока нет
- Mark Scheme (Results) June 2011: International GCSE Chemistry (4CH0) Paper 1C Science Double Award (4SC0) Paper 1CДокумент22 страницыMark Scheme (Results) June 2011: International GCSE Chemistry (4CH0) Paper 1C Science Double Award (4SC0) Paper 1CCisum ErupОценок пока нет
- 4CH0 1C MSC 20120124Документ28 страниц4CH0 1C MSC 20120124avishkabandaraОценок пока нет
- Mark Scheme (Results) January 2008: GCE Chemistry (6242) Paper 1Документ11 страницMark Scheme (Results) January 2008: GCE Chemistry (6242) Paper 1Chong Cheng NamОценок пока нет
- Mark Scheme (Results) January 2014Документ24 страницыMark Scheme (Results) January 2014Maoga2013Оценок пока нет
- Mark Scheme (Results) Summer 2010: IGCSE Chemistry (4335) Paper 1FДокумент16 страницMark Scheme (Results) Summer 2010: IGCSE Chemistry (4335) Paper 1FCoolman PoonОценок пока нет
- Mark Scheme (Results) January 2010: GCE Chemistry (6CH02/01)Документ16 страницMark Scheme (Results) January 2010: GCE Chemistry (6CH02/01)raaaaaawrОценок пока нет
- Mark Scheme GCE Chemistry 6245 01Документ17 страницMark Scheme GCE Chemistry 6245 01eeshvariОценок пока нет
- 7081 01 Rms 20080306Документ15 страниц7081 01 Rms 20080306MERCY LAWОценок пока нет
- GCE Chemistry Paper 1 Mark Scheme January 2008Документ11 страницGCE Chemistry Paper 1 Mark Scheme January 2008KelumОценок пока нет
- Chemistry Paper 2 SL MarkschemeДокумент11 страницChemistry Paper 2 SL MarkschemeEDLYN JAIRO JHOWELL CALDERON MONAGOОценок пока нет
- Jan 09 Paper 1 M-SchemeДокумент7 страницJan 09 Paper 1 M-SchemeHugo WongОценок пока нет
- Mark Scheme (Results) January 2008: GCE Chemistry (6246) Paper 1AДокумент8 страницMark Scheme (Results) January 2008: GCE Chemistry (6246) Paper 1Anahian_aziz9050Оценок пока нет
- Mark Scheme (Final) January 2010: GCE Chemistry (6CH02/01)Документ13 страницMark Scheme (Final) January 2010: GCE Chemistry (6CH02/01)AhmedAman565623Оценок пока нет
- Mark Scheme (Results) January 2012: International GCSE Chemistry (4CH0) Paper 2CДокумент16 страницMark Scheme (Results) January 2012: International GCSE Chemistry (4CH0) Paper 2CJohn HopkinsОценок пока нет
- Markscheme Unit5 (6CH05) January2011Документ15 страницMarkscheme Unit5 (6CH05) January2011Vraj PatelОценок пока нет
- 2015 Jan Chem 1 MsДокумент26 страниц2015 Jan Chem 1 Mskosala naveen wijekulasuriyaОценок пока нет
- Mark Scheme (Results) January 2010: GCE Chemistry (6CH01/01)Документ20 страницMark Scheme (Results) January 2010: GCE Chemistry (6CH01/01)Anas BhaiОценок пока нет
- 4CH0 1C Rms ChemistryДокумент32 страницы4CH0 1C Rms ChemistryAlex Smith100% (1)
- Mark Scheme (Results) Summer 2008: GCE Chemistry Nuffield (6254/01)Документ10 страницMark Scheme (Results) Summer 2008: GCE Chemistry Nuffield (6254/01)Danny AdonisОценок пока нет
- GCE Chemistry Mark Scheme Provides Concise GuidanceДокумент19 страницGCE Chemistry Mark Scheme Provides Concise GuidanceNuha NishatОценок пока нет
- Chemistry Unit 1 June 2011 AS EDEXCEL MARK SCHEMEДокумент21 страницаChemistry Unit 1 June 2011 AS EDEXCEL MARK SCHEMEGhaleb W. MihyarОценок пока нет
- Mark Scheme (Results) June 2011: GCE Chemistry (6CH08) Paper 01 Chemistry Laboratory Skills (WA)Документ14 страницMark Scheme (Results) June 2011: GCE Chemistry (6CH08) Paper 01 Chemistry Laboratory Skills (WA)areyouthere92Оценок пока нет
- Mark Scheme (Final) January 2009: GCE Chemistry (6244/01)Документ20 страницMark Scheme (Final) January 2009: GCE Chemistry (6244/01)Aaisha AfaОценок пока нет
- Secondary Progression Test - Stage 7 Science MSДокумент17 страницSecondary Progression Test - Stage 7 Science MSpixelhobo44% (16)
- Fiitjee Solutions Jee Main 2016Документ31 страницаFiitjee Solutions Jee Main 2016TanujОценок пока нет
- Chemistry Unit 3b June 2011 AS EDEXCEL MARK SCHEMEДокумент18 страницChemistry Unit 3b June 2011 AS EDEXCEL MARK SCHEMEGhaleb W. MihyarОценок пока нет
- Mark Scheme (Results) June 2008: GCE Chemistry (6243/01A)Документ7 страницMark Scheme (Results) June 2008: GCE Chemistry (6243/01A)UncleBulgariaОценок пока нет
- GCE Chemistry (6245) Paper 1 Mark SchemeДокумент11 страницGCE Chemistry (6245) Paper 1 Mark SchemeeeshvariОценок пока нет
- Marking Scheme ChemistryДокумент14 страницMarking Scheme ChemistryIsuru Udana AbeysekaraОценок пока нет
- Aqa Chem5 W MS Jan10Документ12 страницAqa Chem5 W MS Jan10Christopher WilliamsОценок пока нет
- 2009 Jan MS OLD SPECДокумент70 страниц2009 Jan MS OLD SPECJames SmithОценок пока нет
- Qs Ans NEET 2023 Code E5 FinalДокумент44 страницыQs Ans NEET 2023 Code E5 FinalY AОценок пока нет
- 6CH04 MS 2011Документ25 страниц6CH04 MS 2011areyouthere92Оценок пока нет
- June 2011 MS - Paper 1C Edexcel Chemistry IGCSEДокумент22 страницыJune 2011 MS - Paper 1C Edexcel Chemistry IGCSEVideesha AmunugamaОценок пока нет
- M09CДокумент12 страницM09CMiriam LópezОценок пока нет
- 9701 s10 Ms 31 PDFДокумент6 страниц9701 s10 Ms 31 PDFtess_15Оценок пока нет
- Chemistry_paper_3_SL_markschemeДокумент17 страницChemistry_paper_3_SL_markschemeEDLYN JAIRO JHOWELL CALDERON MONAGOОценок пока нет
- AQA CHEM2 W MS Jan11Документ32 страницыAQA CHEM2 W MS Jan11roses17Оценок пока нет
- 6CH02 MS 2011Документ20 страниц6CH02 MS 2011areyouthere92Оценок пока нет
- Mark Scheme (Results) Summer 2013Документ15 страницMark Scheme (Results) Summer 2013lolomg90Оценок пока нет
- Mock IIT Advanced Test - 3/2014/paper-1: Read The Following Instructions Very Carefully Before You ProceedДокумент28 страницMock IIT Advanced Test - 3/2014/paper-1: Read The Following Instructions Very Carefully Before You ProceedShaliniОценок пока нет
- Mark Scheme: Chemistry 1421Документ9 страницMark Scheme: Chemistry 1421Fedrich De MarcusОценок пока нет
- 9701 s10 Ms 35 PDFДокумент8 страниц9701 s10 Ms 35 PDFtess_15Оценок пока нет
- Markscheme: M12/4/PHYSI/HP2/ENG/TZ1/XX/MДокумент21 страницаMarkscheme: M12/4/PHYSI/HP2/ENG/TZ1/XX/MAhmad OmarОценок пока нет
- Model Answers in Ordinary National Certificate Mathematics for EngineersОт EverandModel Answers in Ordinary National Certificate Mathematics for EngineersОценок пока нет
- The Classical Stefan Problem: Basic Concepts, Modelling and AnalysisОт EverandThe Classical Stefan Problem: Basic Concepts, Modelling and AnalysisОценок пока нет
- The Thermodynamics of Phase and Reaction EquilibriaОт EverandThe Thermodynamics of Phase and Reaction EquilibriaРейтинг: 3.5 из 5 звезд3.5/5 (5)
- Code of Safe Working Practices for Merchant Seafarers Consolidated 2015 edition, including amendments 1-7От EverandCode of Safe Working Practices for Merchant Seafarers Consolidated 2015 edition, including amendments 1-7Оценок пока нет
- Code of Safe Working Practices for Merchant Seafarers: Consolidated edition (incorporating amendments 1-6)От EverandCode of Safe Working Practices for Merchant Seafarers: Consolidated edition (incorporating amendments 1-6)Оценок пока нет
- Phase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringОт EverandPhase Equilibrium in Mixtures: International Series of Monographs in Chemical EngineeringОценок пока нет
- Practice Makes Perfect in Chemistry: Oxidation-ReductionОт EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionРейтинг: 5 из 5 звезд5/5 (1)
- Calculations in Furnace Technology: Division of Materials Science and TechnologyОт EverandCalculations in Furnace Technology: Division of Materials Science and TechnologyРейтинг: 3 из 5 звезд3/5 (2)
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersОт EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersОценок пока нет
- Types of ExtractionДокумент5 страницTypes of ExtractionMohammad NafaiОценок пока нет
- Report of Preparatory Mockigcse SCДокумент55 страницReport of Preparatory Mockigcse SCgkawsar22Оценок пока нет
- Qualifying Test Result (2014-15) Subject: Chemistry: Bangladesh School MuscatДокумент2 страницыQualifying Test Result (2014-15) Subject: Chemistry: Bangladesh School Muscatgkawsar22Оценок пока нет
- Crude OilДокумент4 страницыCrude Oilgkawsar22Оценок пока нет
- Relative Atomic MassДокумент8 страницRelative Atomic Massgkawsar22Оценок пока нет
- Periodic TableДокумент3 страницыPeriodic Tablegkawsar22Оценок пока нет
- Acid & BaseДокумент3 страницыAcid & Basegkawsar22Оценок пока нет
- Energy From ChemicalsДокумент4 страницыEnergy From Chemicalsgkawsar22Оценок пока нет
- RadiationДокумент3 страницыRadiationgkawsar22Оценок пока нет
- Acid & BaseДокумент3 страницыAcid & Basegkawsar22Оценок пока нет
- BondingДокумент5 страницBondinggkawsar22Оценок пока нет
- Test Nichrome Wire Wire Wire ColourДокумент2 страницыTest Nichrome Wire Wire Wire Colourgkawsar22Оценок пока нет
- AtomsДокумент4 страницыAtomsgkawsar22Оценок пока нет
- ElectrolysisДокумент7 страницElectrolysisgkawsar22Оценок пока нет
- HeinemannIGCSE Chemistry Chapter1Документ3 страницыHeinemannIGCSE Chemistry Chapter1gkawsar22Оценок пока нет
- Classifying Materials Activity 5.1 - Physical Properties TestДокумент1 страницаClassifying Materials Activity 5.1 - Physical Properties Testgkawsar22Оценок пока нет
- Q 1Документ36 страницQ 1gkawsar22Оценок пока нет
- Making SaltsДокумент1 страницаMaking Saltsgkawsar22Оценок пока нет
- Activity 13.3: How Does Concentration Affect Reaction Rate?Документ2 страницыActivity 13.3: How Does Concentration Affect Reaction Rate?gkawsar22Оценок пока нет
- GCSE CHEMISTRY ACIDS, BASES & SALTS HIGH DEMAND QUESTIONSДокумент21 страницаGCSE CHEMISTRY ACIDS, BASES & SALTS HIGH DEMAND QUESTIONSeeenusОценок пока нет
- Introducing Energy Changes in ReactionsДокумент2 страницыIntroducing Energy Changes in Reactionsgkawsar22Оценок пока нет
- A Guide To Practical QuestionsДокумент10 страницA Guide To Practical Questionsgkawsar22Оценок пока нет
- Seperatting and AnalysisДокумент3 страницыSeperatting and Analysisgkawsar22Оценок пока нет
- The Aqueous Chemistry of CationsДокумент11 страницThe Aqueous Chemistry of Cationsgkawsar22Оценок пока нет
- 0906 O Level Grade BoundariesДокумент2 страницы0906 O Level Grade Boundariesryuzaki589Оценок пока нет
- Q 2Документ25 страницQ 2gkawsar22Оценок пока нет
- Kinetic Theory of DiffusionДокумент1 страницаKinetic Theory of Diffusiongkawsar22Оценок пока нет
- Introducing Reversible ReactionsДокумент3 страницыIntroducing Reversible Reactionsgkawsar22Оценок пока нет
- Rate of ReactionДокумент3 страницыRate of Reactiongkawsar22Оценок пока нет
- Oxygen and OxidesДокумент5 страницOxygen and Oxidesgkawsar22Оценок пока нет