Академический Документы
Профессиональный Документы
Культура Документы
ddg12418 PDF
Загружено:
Vidia AsriyantiИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
ddg12418 PDF
Загружено:
Vidia AsriyantiАвторское право:
Доступные форматы
Minireview
Submitted: 5.5.2014
Accepted: 12.6.2014
Conflict of interest
None.
Stina Schiller 1*, Christina
Seebode1*, Hans Christian
Hennies2, Kathrin Giehl3,
Steffen Emmert1
(1) Department of Dermatology,
Venereology, and Allergology,
University Medical Center Gttingen,
Germany
(2) Center for Dermatogenetics,
Division of Human Genetics and
Department of Dermatology, Innsbruck Medical University, and the
Cologne Center for Genomics,
University of Cologne, Germany
(3) Department of Dermatology and
Allergology at the Medical Center of
the University of Munich, Germany
DOI: 10.1111/ddg.12418
Palmoplantar keratoderma (PPK):
acquired and genetic causes of a not
so rare disease
Summary
Palmoplantar keratodermas (PPK) comprise a heterogeneous group of keratinization
disorders with hyperkeratotic thickening of palms and soles. Sporadic or acquired
forms of PPKs and genetic or hereditary forms exist. Differentiation between acquired and hereditary forms is essential for adequate treatment and patient counseling.
Acquired forms of PPK have many causes. A plethora of mutations in many genes can
cause hereditary PPK. In recent years several new causative genes have been identified. Individual PPK may be quite heterogeneous with respect to presentation and
associated symptoms. Since the various hereditary PPK like many other monogenic
diseases exhibit a very low prevalence, making of the correct diagnosis is challenging and often requires a molecular genetic analysis. Knowledge about the large but
quite heterogeneous group of hereditary PPK is also important to dissect the molecular mechanisms of epidermal differentiation on palms and soles, ultimately leading to
targeted corrective therapies in the future.
*Both authors contributed equally to
this work.
Clinical problem
Palmoplantar keratodermas (PPK) comprise a very heterogenous group of diseases. This applies to their symptoms,
as well as in genetic palmoplantar keratoderma the type
of mutation and affected genes. Due to the heterogenicity of
PPK and the rarity of individual variants, especially certain
genetic ones, the course of disease, from the onset of symptoms to a precise diagnosis (and thus promising therapy) can
be long and burdensome for the patient. In spite of growing
use of molecular testing methods, the clinical appearance of
PPK is at the forefront when diagnosing patients in everyday
practice [1]. An overview of diagnosis and therapy of PPK is
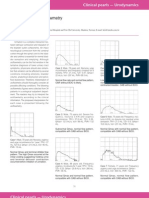
shown in Figure1.
Clinical appearance of palmoplantar
keratoderma
PPK may be divided into acquired [2] and genetic types [3];
other classifications are also possible. Based on the clinical
morphology, a distinction is made between diffuse/plane,
focal (patchy, striate, filiform, or discoid) or punctate with
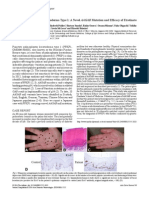
small, round hyperkeratotic lesions (Figure2). In addition,
the severity of disease, involvement of areas other than the
palms or soles (transgredient PPK), and the onset of other
symptoms, e.g., as part of a syndrome, are used to classify
or diagnosis the disorder. In genetic PPK, molecular genetic
methods are used to identify the mutated gene, allowing for
2014 Deutsche Dermatologische Gesellschaft (DDG). Published by John Wiley & Sons Ltd. | JDDG | 1610-0379/2014/1209
781
Minireview Palmoplantar keratoderma
Figure 1 History, diagnosis and therapy
of palmoplantar keratodermas.
Figure 2 Different manifestations of palmoplantar keratodermas (PPK). Focal/filiform PPK: spiny keratosis (unclear origin, an
underlying malignant disease is possible) (a). Focal/discoid PPK: hyperkeratotic papules, coalescing in mechanically stressed
areas (punctate PPK Type 1/Buschke-Fischer-Brauer type) (b). Focal PPK, distinct in mechanically stressed areas, dystrophic nails
(pachyonychia congenita) (c). Diffuse PPK: plane white to yellowish keratosis (palmoplantar keratoderma Vrner-Unna-Thost)
(d). Diffuse PPK (Papillon-Lefvre syndrome) (e).
782
2014 Deutsche Dermatologische Gesellschaft (DDG). Published by John Wiley & Sons Ltd. | JDDG | 1610-0379/2014/1209
Minireview Sclerotherapy of varicose veins
precise genetic classification. Autosomal dominant, recessive,
and X-chromosomal patterns of heredity are all possible [3].
Especially in patients with hyperhidrosis, there is maceration of the keratoses, accompanied by a typical fetor. Mild
Bacterial and mycotic superinfections are also often associated
with the disease. These require adequate therapy, including
oral treatment as needed.
Diagnosis of acquired palmoplantar
keratoses
Acquired PPK lesions have a wide range of clinical appearances: diffuse, focal, and punctate. The most notable
histological feature of acquired palmoplantar keratosis is
hyperkeratosis, which may occur with acanthosis and/or
varying degrees of parakeratosis, hyperplasia of the stratum spinosum and granulosum, and perivascular infiltration of inflammatory cells. The clinical appearance is usually unspecific; hyperkeratosis of the stratum corneum is the
most certain clinical feature. The onset of acquired PPK is
typically later in life, and it may affect patients who do not
have a family history of disease, despite having a corresponding etiology. Questions concerning the patients occupation
can aid diagnosis, as can asking whether the symptoms improve during vacation. Other clues are the presence of allergies or infections. Another possible sign of acquired PPK
is symptoms that are limited to the palms and soles, and/
or are restricted to specific areas on the palms or soles. The
causes of acquired PPK vary, and include exposure to certain
chemicals (e.g., arsenic, chlorinated hydrocarbon fluids) [4,
5]; side effects of certain drugs (e.g., beta-glucan, lithium,
chemotherapy agents) and metabolic disorders (gravidity,
menopause, hypothyroidism, myxedema) are other possible
causes [2]. Common types are acquired PPK due to contact
allergy or toxic irritants as well as PPK in the framework of
atopic dermatitis or psoriasis [6].
Diagnosis of paraneoplastic palmoplantar
keratoses (acquired and/or genetic)
In patients with PPK where the etiology is not clear, the physician should consider the possibility of underlying malignant
disease. Since PPK may be the first visible symptom of a malignancy, the physicians awareness of this manifestation may
be crucial. Examples of paraneoplastic palmoplantar keratoderma include Szary syndrome, Bazex syndrome, and hereditary Howel-Evans syndrome [79]. In patients with Bazex
or Szary syndrome, treatment of the underlying malignancy
leads to improvement of cutaneous symptoms. Esophageal
cancer is common in patients with Howel-Evans syndrome,
and if the doctor is aware of the association, the tumor can
be diagnosed more quickly.
Diagnosis of genetic palmoplantar
keratoderma
In regard to successful corrective therapy (treatment/
elimination of the cause of acquired PPK), as well as for
genetic counseling for pregnant women and patients wishing
to conceive, it is important to distinguish hereditary PPK
from acquired disease. Various features should raise suspicion of hereditary PPK: initial manifestation of disease in
childhood, a positive family history, persistent clinical appearance with little variation in the type and severity of symptoms, and relative treatment resistance. A negative family
history, or initial manifestation during adulthood, does not
exclude the possibility of hereditary PPK. Other signs of hereditary PPK include symptoms in the framework of other
syndromes (such as deafness or premature tooth loss). In patients with hereditary PPK, there is no obvious cause; tests
for allergies and infections yield negative results. If there is
suspicion of hereditary PPK, the patient should be referred to
a specialized unit for treatment and also for genetic testing.
Clinical diagnosis of hereditary palmoplantar
keratoderma
In addition to the above-mentioned features, in hereditary
PPK, the morphology of the lesions may aid clinical diagnosis:
If PPK occurs in isolation, as the cardinal or accompanying symptom (in a syndrome): are other organs or organ
systems (CNS, ears, eyes, immune system, nails, hair,
teeth) affected?
Are the keratoses diffuse/plane or focal/patchy (punctate,
striate, filiform)?
Are the keratoses only located on pressure points?
Are other areas involved, aside from the palms and soles
(transgredient)?
Is there no scale, scale without redness (no epidermolysis) or additional redness, inflammatory components or
blistering (epidermolysis)?
Histological diagnosis
Along with the usually unspecific main histological characteristic of palmoplantar keratosis hyperkeratosis other
histological features, such as epidermolysis, may be helpful
in distinguishing epidermolytic from non-epidermolytic PPK.
They may also be helpful in distinguishing between individual entities.
2014 Deutsche Dermatologische Gesellschaft (DDG). Published by John Wiley & Sons Ltd. | JDDG | 1610-0379/2014/1209
783
Minireview Palmoplantar keratoderma
Genetic diagnosis
Newer molecular genetic testing methods allow for the classification of hereditary PPK based on the causal gene defects.
These types of PPK are heterogenous in terms of genetics,
but generally the affected genes either code for epidermal
structure proteins or for proteins which regulate or influence
various processes during epidermal differentiation. In recent
years, a number of various genetic mutations have been identified which may trigger PPK. For instance, in 2013 autosomal dominant mutations in the AQP5 gene (chromosome
12q13), which codes for aquaporin 5, was found to trigger
aquagenic, non-epidermolytic PPK (Bothnia type) [10]. The
identification of loss-of-function mutations in SERPINB7,
whose genetic product belongs to the family of serine-protease inhibitors, has made it possible to distinguish PPK
(Nagashima type), based on the underlying mutations, from
Mal de Meleda [11]. An association between PPK and hearing loss in the inner ear has also been associated with the
identification of triggering mutations in GJB2, which codes
for connexin 26. These examples show that the identification of underlying mutations is enormously important for the
correct diagnosis. Table1 provides a summary of hereditary
PPK, its clinical appearance, related genetic defects, and altered genetic products.
Treatment of acquired and hereditary
palmoplantar keratoses
There is as yet no cure for hereditary palmoplantar keratoses.
In patients with acquired PPK, the cause should be treated
(toxins, infection, other factors.) or eliminated, if possible. In
both instances, optimized treatment can lead to a significant
improvement in symptoms. A wide range of treatments are
available which aim to fortify the skin barrier and remove
hornification. Treatment may be local and/or systemic [6].
Regular baths cleanse and hydrate areas of keratinization. Routine use of hand and foot baths lead to keratolysis and facilitates the mechanical removal of hyperkeratotic
areas (e.g., during medical foot care procedures). Mechanical
keratolysis should be performed as needed. After balneotherapy, a moisturizer should be applied in order to ensure optimal hydration of the skin. Topical therapy with urea-based
ointments improves the skins absorption of moisture and has
keratolytic effects; urea can be readily combined with other
agents such as lactic acid, sodium chloride, and vitamin A
acid. Topical vitamin D therapy is another option. The choice
of treatment is made on an individual basis and should be accompanied by topical antibacterial and antifungal prophylaxis. Primary therapy may continue to be supported by a basic
therapy for rehydration and skin care. In patients with severe
784
hereditary disease, medical foot care is indicated (AWMF
guideline no. 013/043, http://www.ichthyose.de).
Systemic therapy with retinoids (generally acitretin),
taking into account the side effects, may lead to marked
improvement of palmoplantar hyperkeratosis. In patients
with blistering or epidermolytic PPK, however, an expectant
approach should be taken, given that retinoid therapy can
cause detachment of large areas of the skin and erosions.
Retinoids can also cause birth defects, and the drug is stored
in adipose tissue for up to 24 months after discontinuing its
use. Regardless of whether systemic therapy is given, aggressive topical therapy is advisable [12].
Promising curative therapies are available for keratinopathies due to dominant mutations. This involves using RNA
interference to switch off dominant negative alleles. Mutation-specific siRNA are introduced into the cell using various
methods; the expression of the wild-type allele is not affected
and is sufficient for the formation of intact keratin intermediate filaments [13, 14]. Advanced forms of such corrective
measures are available for pachyonychia congenita, as well as
other keratin diseases. Yet, questions on long-term transport
of interfering RNA molecules in the epidermal keratinocytes and duration of molecule activity have not yet been fully
answered.
Conclusions for practice
When diagnosing and treating PPK, it is important to distinguish between acquired and hereditary types. In acquired
and paraneoplastic PPK, treatment of the underlying disease
or its trigger leads to improvement of symptoms. Hereditary
forms of PPK, which are not all that rare, may be identified
and diagnosed genetically, but treatment is only symptomatic. An exact diagnosis is essential for providing genetic
counseling to the patient and their family, in order to predict
the course of disease and the risk of passing it on (Kster
2006). The identification of new mutations that trigger PPK
illustrates the complexity of disease and incomplete picture
of palmoplantar epidermal differentiation.
About the authors
Christina Seebode is a doctoral student, and Stina Schiller a
post-doctoral student, in Steffen Emmerts working group.
They are currently investigating the role of SNAP29 in epidermal differentiation and have established suitable mouse
models. An SNAP29 deficiency triggers the rare CEDNIK
(cerebral dysgenesis, neuropathy, ichthyosis, and keratoderma) syndrome. Steffen Emmert is a professor in the Department of Dermatology at University Medical Center Gttingen with a focus on dermato-oncology. Kathrin Giehl is a
lecturer at the Department of Dermatology and Allergology
2014 Deutsche Dermatologische Gesellschaft (DDG). Published by John Wiley & Sons Ltd. | JDDG | 1610-0379/2014/1209
Minireview Sclerotherapy of varicose veins
Table 1 List of hereditary palmoplantar keratodermas, affected genes, relevant gene products and inheritances [3, 15].
Disease
Pattern of
heredity
Gene (protein)
Signs
Associated symptoms
Vrner-Unna-Thost
disease
AD
KRT9
(Keratin 9)
(KRT1)
(Keratin 1)
Isolated diffuse, non-
transgredient PPK, histologically acanthokeratolytic
Greither disease
AD
KRT1 (Keratin 1)
Isolated diffuse, partly
transgredient PPK
Manifestation of hyperkeratosis
during infancy, hyperhidrosis,
possible regression during 4th or 5th
decade of life
Mal de Meleda
AR
SLURP1 (SLURP1
(Ly6/uPAR family)
Diffuse, massively transgredient PPK, mutilating, rarely
constrictions
Nail changes, hyperhidrosis, maceration of keratosis/unpleasant fetor,
tendency toward bacterial superinfections
Huriez syndrome
AD
Unknown
Diffuse, transgredient PPK
Scleroatrophy of the distal extremities, nail changes, growth delays
affecting the hand, increased risk of
squamous cell carcinoma
KID syndrome
AD
GJB2
(GJB6)
(Connexin 26
(Connexin 30)
Diffuse PPK
Ichthyosiform erythroderma in
infants,
Progressive verruciform hyperkeratoses (including scalp, face, backs of
hands and dorsal aspect of the feet,
buttocks)
Nail dystrophies, alopecia
ichthyosis, corneal clouding,
hearing affecting inner ear, tendency toward bacterial superinfections,
risk of squamous cell carcinoma
Bart-Pumphrey
syndrome
AD
GJB2
(Connexin 26)
diffuse PPK
Loss of hearing affecting inner ear,
knuckle pads, leukonychia
PPK (Bothnia type)
AD
AQP5
(Aquaporin)
Diffuse PPK
Swelling of areas after contact with
water
PPK (Nagashima
type)
AR
SERPINB7
(serine protease
inhibitor)
Mild diffuse PPK (circumscribed hyperkeratosis with redness), not progressive
Diffuse PPK
Diffuse mutilating PPK
Vohwinkel
syndrome
AD
GJB2
(Connexin 26)
Diffuse, severe, yellowish
PPK, mutilating
Loss of hearing affecting inner
ear, hyperhidrosis, alopecia, nail
dystrophy, (myopathy, possible
spastic paraplegia)
Vohwinkel syndrome, ichthyosiform
variant (Camisa
syndrome)
AD
LOR,
(Loricrin)
PPK resembling classic
Vohwinkel syndrome
Mild ichthyosis
2014 Deutsche Dermatologische Gesellschaft (DDG). Published by John Wiley & Sons Ltd. | JDDG | 1610-0379/2014/1209
785
Minireview Palmoplantar keratoderma
Table 1 Continued.
Disease
Pattern of
heredity
Gene (protein)
Signs
Associated symptoms
Olmsted syndrome AD
X-ch.
TRPV3
(MBTPS2)
(TRPV3
(MBTPS2)
Diffuse, mutilating PPK
Perioral hyperkeratotic plaques,
diffuse alopecia, onychodystrophy,
oral leukokeratosis, corneal lesions,
pseudoainhum
Klick syndrome
AR
POMP
(POMP)
Diffuse, mutilating PPK
Hyperkeratotic plaques, ichthyosis
and papules distributed in a linear fashion on the arm flexures and wrists
Buschke-FischerBrauer disease
AD
AAGAB
(alpha-and
gamma-adaptin
binding protein)
Punctate PPK
Howel-Evans-
syndrome
AD
RHBDF2
(RHBDF2
(rhomboid
protease family)
Diffuse weal-like PPK
Keratosis palmoplantaris striata
(Brnauer-FuhsSiemens syndrome)
AD
DSG1
(Desmoglein)
Striate PPK
Keratosis palmoplantaris striata
AD
DSP
(Desmoplakin)
Striate PPK
Keratosis palmoplantaris striata
AD
KRT1
(Keratin 1)
Striate PPK on the palms, diffuse on
the soles
Pachyonychia
congenita
AD
KRT6A / 6B / 16 / 17 Diffuse PPK
(Keratin 6a / 6b /
16 / 17)
Pachyonychia/nail changes,
hyperhidrosis, oral leukokeratosis,
steatocystoma
Naegeli-Franceschetti-Jadassohn
syndrome
AD
KRT14
(Keratin 14)
Diffuse PPK
Absence of papillary relief, nail
dystrophies, anhidrosis, dental
defects, hyperpigmentation and
loss of pigmentation
Papillon-Lefvre
syndrome
AR
CTSC
(Cathepsin C)
Diffuse PPK
Periodontitis, tooth lost
Haim-Munk
syndrome
AR
CTSC
(Cathepsin C)
Diffuse PPK
Corresponding to Papillon-Lefvre
disease, additional arachnodactyly,
acroosteolysis, pes planus, finger
deformities
Schpf-Schulz-
AR
Passarge syndrome
WNT10A
Diffuse PPK
Symptoms correspond to OODD +
cysts affecting the eyelids, increased
risk of skin tumors
Odonto- onychodermal dysplasia
WNT10A
(Wnt-10a)
Diffuse PPK
Hyperhidrosis, hypodontia, hypotrichosis, dystrophic nails
Focal / punctate PPK
Oral leukoplakia, keratosis pilaris,
esophageal carcinoma
Striated PPK
Ectodermal dysplasia
786
AR
2014 Deutsche Dermatologische Gesellschaft (DDG). Published by John Wiley & Sons Ltd. | JDDG | 1610-0379/2014/1209
Minireview Sclerotherapy of varicose veins
Table 1 Continued.
Disease
Pattern of
heredity
Gene (protein)
Ectodermal
dysplasia with skin
fragility
AR
PKP1
(Plakophilin 1)
Signs
Associated symptoms
Woolly hair
Other types of syndromal PPK
Carvajal syndrome
AR
DSP
(Desmoplakin)
Striate PPK
Cardiomyopathy, woolly hair
Naxos syndrome
AR
JUP
(Plakoglobin)
Diffuse PPK
Cardiomyopathy, woolly hair
PPK as an accompanying symptom
Cowden syndrome
AD
PTEN
(Phosphatase
PTEN)
Hamartoma development,
malignant transformation
Darier disease
AD
ATP2A2
(sarco/endoplasmic
calcium-ATPase
isoform SERCA2)
Palmoplantar disrupted ridge
pattern in the papillary relief, crusty
papules in seborrheic skin areas,
nail changes
AD
Unknown
Other
Filiform
hyperkeratosis
Spiky hyperkeratosis
at the Medical Center of the University of Munich, where her
focus is on rare and hereditary skin diseases. Hans Christian Hennies heads the Center for Dermatogenetics, which is
part of the Division Human Genetics and the Department
of Dermatology at Innsbruck Medical University and at the
Cologne Center for Genomics at the University of Cologne.
Correspondence to
Univ.-Prof. Dr. med. Steffen Emmert
Klinik fr Dermatologie, Venerologie und Allergologie
Universittsmedizin Gttingen
Robert-Koch-Strae 40
37075 Gttingen
E-mail: semmert@gwdg.de
References
1
Emmert S, Kuster W, Hennies HC et al. 47 patients in 14 families
with the rare genodermatosis keratosis punctata palmoplantaris Buschke-Fischer-Brauer. Eur J Dermatol 2003; 13: 1620.
Patel S, Zirwas M, English JC 3rd. Acquired palmoplantar
keratoderma. Am J Clin Dermatol 2007; 8: 111.
Braun-Falco M. Hereditary palmoplantar keratodermas.
J Dtsch Dermatol Ges 2009; 7: 97184; quiz 845.
4 Hsu LI, Chen GS, Lee CH et al. Use of arsenic-induced
palmoplantar hyperkeratosis and skin cancers to predict risk
of subsequent internal malignancy. Am J Epidemiol 2013; 177:
20212.
5 Passarini B, Infusino SD, Kasapi E. Chloracne: still cause for
concern. Dermatology 2010; 221: 6370.
6 Emmert B, Hallier E, Schon MP et al. Disease management
guidelines in dermatology: implementation, potentials and
limitations exemplified by the guidelines for the management
of hand eczema. Hautarzt 2011; 62: 30814.
7 Blaydon DC, Etheridge SL, Risk JM et al. RHBDF2 mutations
are associated with tylosis, a familial esophageal cancer
s yndrome. Am J Hum Genet 2012; 90: 3406.
8 Delavari D, Zywica M, Hartmann M. Sudden onset of acral
erythema with hyperkeratosis and pityriasiform scales on
soles, fingertips, nose and ear helices. J Dtsch Dermatol Ges
2013; 11: 3602.
9 Jawed SI, Myskowski PL, Horwitz S et al. Primary cutaneous
T-cell lymphoma (mycosis fungoides and Sezary syndrome):
part I. Diagnosis: clinical and histopathologic features and
new molecular and biologic markers. J Am Acad Dermatol
2014; 70: 205 e116; quiz 212.
10 Blaydon DC, Lind LK, Plagnol V et al. Mutations in AQP5,
encoding a water-channel protein, cause autosomal-dominant
2014 Deutsche Dermatologische Gesellschaft (DDG). Published by John Wiley & Sons Ltd. | JDDG | 1610-0379/2014/1209
787
Minireview Palmoplantar keratoderma
11
12
788
diffuse nonepidermolytic palmoplantar keratoderma. Am J
Hum Genet 2013; 93: 3305.
Kubo A, Shiohama A, Sasaki T et al. Mutations in SERPINB7,
encoding a member of the serine protease inhibitor superfamily,
cause Nagashima-type palmoplantar keratosis. Am J Hum Genet
2013; 93: 94556.
Vahlquist A, Ganemo A, Virtanen M. Congenital ichthyosis:
an overview of current and emerging therapies. Acta Derm
Venereol 2008; 88: 414.
13
14
15
Hickerson RP, Flores MA, Leake D et al. Use of self-delivery
siRNAs to inhibit gene expression in an organotypic pachyonychia congenita model. J Invest Dermatol 2011; 131: 103744.
Leachman SA, Hickerson RP, Schwartz ME et al. First-in-human
mutation-targeted siRNA phase Ib trial of an inherited skin
disorder. Mol Ther 2010; 18: 4426.
Seebode C, Schiller S, Emmert S, Giehl K. Hautvernderungen
an Hnden und Fen: Wann muss man an die Gene denken?
Hautarzt 2014; in press.
2014 Deutsche Dermatologische Gesellschaft (DDG). Published by John Wiley & Sons Ltd. | JDDG | 1610-0379/2014/1209
Вам также может понравиться
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- Gene Hunting: J. PongraczДокумент15 страницGene Hunting: J. PongracztahirnaeemkhanОценок пока нет
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- NBME Quests ActualДокумент66 страницNBME Quests ActualDeborah Peters100% (3)
- Inheritance - How Our Genes Change Our Lives - and Our Lives Change Our Genes (PDFDrive)Документ216 страницInheritance - How Our Genes Change Our Lives - and Our Lives Change Our Genes (PDFDrive)Alberto SotoОценок пока нет
- Radio SensitivityДокумент29 страницRadio SensitivitykarthikhrajvОценок пока нет
- Cranial Nerves: DR Dyan Roshinta Laksmi Dewi, SP.S SMF Saraf Rsud DR Soedarso PontianakДокумент19 страницCranial Nerves: DR Dyan Roshinta Laksmi Dewi, SP.S SMF Saraf Rsud DR Soedarso PontianakVidia AsriyantiОценок пока нет
- Biological Anthropology The Natural History of Humankind 4th Edition Stanford Test BankДокумент28 страницBiological Anthropology The Natural History of Humankind 4th Edition Stanford Test Bankashleebernardgikfndpcxw100% (17)
- Reviewer in Science 10Документ2 страницыReviewer in Science 10fan girlОценок пока нет
- Genetic CounselingДокумент33 страницыGenetic CounselingSAYMABANU100% (29)
- PNDT Act RulesДокумент32 страницыPNDT Act RulesVeerendra BaliОценок пока нет
- Multiple Disability Original HandoutДокумент42 страницыMultiple Disability Original HandoutHabtamu DebasuОценок пока нет
- URODYNAMICSДокумент5 страницURODYNAMICSdoddah83Оценок пока нет
- Dubowitz ScoreДокумент3 страницыDubowitz ScorentadudulОценок пока нет
- Meliani, 2011 PDFДокумент4 страницыMeliani, 2011 PDFVidia AsriyantiОценок пока нет
- Kasus DM PDFДокумент22 страницыKasus DM PDFVidia AsriyantiОценок пока нет
- Tsuda, 2004 PDFДокумент9 страницTsuda, 2004 PDFVidia AsriyantiОценок пока нет
- Kumari, 2012 PDFДокумент4 страницыKumari, 2012 PDFVidia AsriyantiОценок пока нет
- Meliani, 2011 PDFДокумент4 страницыMeliani, 2011 PDFVidia AsriyantiОценок пока нет
- Veterinaria Medika Vol 7, No. 1, Pebruari 2014Документ10 страницVeterinaria Medika Vol 7, No. 1, Pebruari 2014Vidia AsriyantiОценок пока нет
- Journal Pone 0002069 PDFДокумент11 страницJournal Pone 0002069 PDFVidia AsriyantiОценок пока нет
- Alloxan 2Документ22 страницыAlloxan 2Jerónimo TortiОценок пока нет
- Activities of antioxidants, α-Glucosidase inhibitors and aldose reductase inhibitors of the aqueous extracts of four Flemingia species in TaiwanДокумент10 страницActivities of antioxidants, α-Glucosidase inhibitors and aldose reductase inhibitors of the aqueous extracts of four Flemingia species in TaiwanVyshali PingleОценок пока нет
- Alexandru, 2011 PDFДокумент5 страницAlexandru, 2011 PDFVidia AsriyantiОценок пока нет
- Aguirre L. Et Al.Документ10 страницAguirre L. Et Al.vesna_tasevaОценок пока нет
- Choi, 2008 PDFДокумент16 страницChoi, 2008 PDFVidia AsriyantiОценок пока нет
- Animal Models in Type 2 Diabetes Research: An OverviewДокумент22 страницыAnimal Models in Type 2 Diabetes Research: An OverviewVidia AsriyantiОценок пока нет
- Ta 33 05082002 Keloids Print - Proc PDFДокумент6 страницTa 33 05082002 Keloids Print - Proc PDFVidia AsriyantiОценок пока нет
- Tsuda, 2004 PDFДокумент9 страницTsuda, 2004 PDFVidia AsriyantiОценок пока нет
- Palmoplantar Keratodermas (PPK) : Foundation For Ichthyosis & Related Skin Types, Inc.™Документ4 страницыPalmoplantar Keratodermas (PPK) : Foundation For Ichthyosis & Related Skin Types, Inc.™Vidia AsriyantiОценок пока нет
- PDFДокумент6 страницPDFVidia AsriyantiОценок пока нет
- Ahmed, 2009 PDFДокумент5 страницAhmed, 2009 PDFVidia AsriyantiОценок пока нет
- Dermeng 1203 PDFДокумент6 страницDermeng 1203 PDFVidia AsriyantiОценок пока нет
- Firearms PDFДокумент5 страницFirearms PDFVidia AsriyantiОценок пока нет
- Keratoderma PDFДокумент5 страницKeratoderma PDFVidia AsriyantiОценок пока нет
- Ta 33 05082002 Keloids Print - Proc PDFДокумент6 страницTa 33 05082002 Keloids Print - Proc PDFVidia AsriyantiОценок пока нет
- Palmoplantar Keratodermas (PPK) : Foundation For Ichthyosis & Related Skin Types, Inc.™Документ4 страницыPalmoplantar Keratodermas (PPK) : Foundation For Ichthyosis & Related Skin Types, Inc.™Vidia AsriyantiОценок пока нет
- 4141 PDFДокумент2 страницы4141 PDFVidia AsriyantiОценок пока нет
- bjd12927 PDFДокумент4 страницыbjd12927 PDFVidia AsriyantiОценок пока нет
- 4141 PDFДокумент2 страницы4141 PDFVidia AsriyantiОценок пока нет
- Microcephaly - Developmental and Behavioral Pediatrics - Golisano Children's Hospital - University of Rochester Medical CenterДокумент7 страницMicrocephaly - Developmental and Behavioral Pediatrics - Golisano Children's Hospital - University of Rochester Medical CenterTony FernandezОценок пока нет
- Genetice Disorders: Genetic Disorder Is A Disease or Disorder Caused by Damage or Change and Mutation On Individual's DNAДокумент46 страницGenetice Disorders: Genetic Disorder Is A Disease or Disorder Caused by Damage or Change and Mutation On Individual's DNAAna Subol AñonuevoОценок пока нет
- Human Genome BasicsДокумент12 страницHuman Genome BasicsPradeep Kumar. NagisettyОценок пока нет
- Genetic DisorderДокумент5 страницGenetic DisorderD GarciaОценок пока нет
- Chapter 6 - Nursing Care For The Family in Need of Reproductive Life PlanningДокумент7 страницChapter 6 - Nursing Care For The Family in Need of Reproductive Life PlanningBern NerquitОценок пока нет
- Bio 410 Entire CourseДокумент2 страницыBio 410 Entire CourseJohn TaylorОценок пока нет
- Genetic Diseases in HumansДокумент3 страницыGenetic Diseases in Humansjosh chavezОценок пока нет
- Genetic Disorders: Mendelian Inheritance PatternsДокумент14 страницGenetic Disorders: Mendelian Inheritance PatternskanagadurgaОценок пока нет
- Genetics Lec ReviewerДокумент4 страницыGenetics Lec ReviewerdiezelОценок пока нет
- Module 3 ReviДокумент35 страницModule 3 ReviJohn Van Dave TaturoОценок пока нет
- Child Development An Active Learning Approach 3Rd Edition Levine Test Bank Full Chapter PDFДокумент36 страницChild Development An Active Learning Approach 3Rd Edition Levine Test Bank Full Chapter PDFmatthew.templeton879100% (10)
- Types of Collagen and Associated Disorders: Osteogenesis ImperfectaДокумент2 страницыTypes of Collagen and Associated Disorders: Osteogenesis ImperfectaAnjana J PrathapОценок пока нет
- Jan Mar 2019Документ92 страницыJan Mar 2019DrNishanthОценок пока нет
- Principles of Inheritance & Variation-Neet Pyqs-6290457667-SampleДокумент95 страницPrinciples of Inheritance & Variation-Neet Pyqs-6290457667-Sampleseetharaman8341Оценок пока нет
- Pathology Biochemical and Molecular Basis of Some Mendelian DisordersДокумент5 страницPathology Biochemical and Molecular Basis of Some Mendelian DisordersAmna Baig0% (1)
- Gene MutationДокумент39 страницGene MutationJanhaji PlotadoОценок пока нет
- Child India NewsletterДокумент65 страницChild India NewsletterAnsu MaliyakalОценок пока нет
- Oral GenodermatosesДокумент4 страницыOral GenodermatosesAkshatОценок пока нет
- Childhood Leukodystrophies A Literature Review ofДокумент18 страницChildhood Leukodystrophies A Literature Review ofPosadasLoezaJosueKarlaОценок пока нет
- Curriculum and Syllabus for Classes XI & XII BiologyДокумент11 страницCurriculum and Syllabus for Classes XI & XII Biologyaleena'100% (1)
- Bio 1Документ3 страницыBio 1James TolentinoОценок пока нет