Академический Документы
Профессиональный Документы
Культура Документы
Chapter 23: Hyperuricemia and Gout: Background
Загружено:
sharu4291Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Chapter 23: Hyperuricemia and Gout: Background
Загружено:
sharu4291Авторское право:
Доступные форматы
Chapter 23
Chapter 23: Hyperuricemia and Gout
23.1: We suggest treating hyperuricemia in KTRs when
there are complications, such as gout, tophi, or
uric acid stones. (2D)
23.1.1: We suggest colchicine for treating acute
gout, with appropriate dose reduction for
reduced kidney function and concomitant CNI use. (2D)
23.1.2: We recommend avoiding allopurinol in
patients receiving azathioprine. (1B)
23.1.3: We suggest avoiding NSAIDs and COX-2
inhibitors whenever possible. (2D)
CNI, calcineurin inhibitor; COX-2, cyclo-oxygenase2; KTRs, kidney transplant recipients; NSAID, nonsteroidal anti-inflammatory drug.
Background
Definitions of hyperuricemia differ widely. Local laboratories often report the upper normal range as a population
mean plus two standard deviations (gender-specific), and
this performs well in clinical practice (804). An international task force recommends that a level of >0.36 mmol/L
(6.0 mg/dL) be defined as hyperuricemia in the general population (804). For each 0.06 mmol/L (1.0 mg/dL) increase
above 0.06 mmol/L, the adjusted RR of gout increases by
2.33 (95% CI 2.002.71). The threshold of 0.36 mmol/L
is associated with 67% sensitivity and 78% specificity for
diagnosing gout. A threshold of 0.42 mmol/L (7.0 mg/dL)
is associated with a 57% sensitivity and 92% specificity
(805). However, because of gender differences, men are
less likely to experience gout at level between 0.36 and
0.42 mmol/L (6.0 and 7.0 mg/dL) and a higher level (>0.42
mmol/L [7.0 mg/dL]) is generally used for men (804). Detailed information is not available in KTRs, but the Work
Group chose to define hyperuricemia as >0.36 mmol/L
(6.0 mg/dL) in women and >0.42 mmol/L (7.0 mg/dL) in
men.
Rationale
Hyperuricemia is very common in KTRs.
Hyperuricemia increases the incidence of gout and
other complications in KTRs, and it may be associated
with loss of kidney function and CVD.
Important drug interactions and precautions will alter
treatment strategies in KTRs with gout.
S102
The incidence of hyperuricemia approaches 80% in KTRs
(806,807). A recent analysis of 29 597 US Medicare recipients found that the cumulative incidence of gout was
7.6% at 3 years after transplantation (808). This relatively
high incidence is consistent with a number of smaller reports (809812).
The mechanisms responsible for hyperuricemia and gout
are complex. Several studies have shown rates to be
higher with CNIs, and especially CsA, when compared
to azathioprine (806,808,809,811). The incidence of hyperuricemia appears to be similar with CsA and tacrolimus
regimens, both being higher compared to regimens without CNIs. For example, in a recent large RCT, uric acid
levels were similar between patients treated with lowdose CsA and tacrolimus at the end of 1 year, and significantly higher in comparison to patients on sirolimus and
MMF (813). Consistent with these results is a study in
which 35 patients were converted from CsA to tacrolimus
had no change in uric acid levels (814). However, in another report of patients converted from CNIs to sirolimus,
there was a significant reduction in uric acid levels (815).
Similarly, in a small (n = 28) RCT of liver transplant recipients, conversion from CNIs to MMF was associated
with a 1520% reduction in uric acid levels (816). Other
risk factors associated with hyperuricemia and gout are
prior history, higher BMI, diuretics, older age, more recent
year of transplantation and hypertension (806809,812,
817).
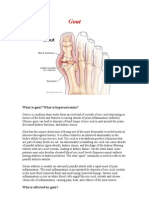
Of the clinical manifestations of hyperuricemia, gout is
the most common. It can be disabling and is associated with lost time from work. Impressive tophaceous
deposits in the hands can occur (806,812). Evidence that
hyperuricemia causes or contributes to progressive kidney disease or CVD is weak, even in the general population (804,818,819). Acute kidney injury from very high uric
acid levels has been reported (820). A large registry cohort study recently demonstrated an association of gout
with elevated mortality (adjusted hazard ratio 1.26, 95%
CI 1.081.47) and graft loss (adjusted hazard ratio 1.22,
95% CI 1.011.49) (808). This association with mortality,
though, has not been observed in other studies. There are
no RCTs to show that lowering uric acid levels is associated with better graft survival, kidney function or patient
survival. There is one small (n = 54) recent RCT in nontransplant patients with kidney impairment, however, in which
improved function with uric acid reduction failed to reach
statistical significance (821). Case series have not shown a
consistent benefit of uric acid reduction on kidney function
in CKD (822).
American Journal of Transplantation 2009; 9 (Suppl 3): S102S103
Chapter 23
Monitoring patients for hyperuricemia at the time of other
routine blood monitoring might help prevent further increases in uric acid levels and greater risks for gout. There
is evidence that dietary interventions (losing weight and
reduced meat and alcohol consumption) and avoiding diuretics in the general population can lower uric acid levels
(804). There are no studies in KTRs. Several medications
used in KTRs can lower uric acid levels. For example, in
a randomized crossover trial of 26 KTRs, losartan was associated with an 8% fall in uric acid levels (823). The uric
acid lowering effect would not be the sole reason for using these medications, but could be substituted if these
medications were needed for other indications. Monitoring might also give clinicians an increased level of suspicion for dealing with atypical symptoms of gout. Measuring
uric acid levels is indicated in patients with suspected gout;
however, during an acute gouty attack, levels may be normal (804). Treatment of asymptomatic hyperuricemia has
not been generally recommended in the general population
or KTRs, but it is advocated in those with recurrent symptomatic episodes of gout, tophi or radiographic changes of
gout (627,804,824).
term colchicine therapy (829,830). Therefore, prolonged
use of colchicine should be avoided in patients with eGFR
<60 mL/min/1.73 m2 . However, colchicine can be used
at reduced doses for <1 week in patients with eGFR
>10 mL/min/1.73 m2 not requiring dialysis. In patients
with eGFR <60 mL/min/1.73 m2 , avoid doses higher than
0.6 mg/day. Intraarticular or short-term systemic steroids
have also been used if the above therapies are contraindicated or not tolerated (824).
Treatment of gout is beyond the scope of these guidelines. There are evidence-based reviews on the treatment
of hyperurcemia and gout (824). Briefly, oral colchicines
and/or nonsteroidal anti-inflammatory agents are recommended as first-line agents for gout (824). Nonsteroidal
anti-inflammatory agents and cyclo-oxygenase-2 inhibitors
can be associated with significant reductions in kidney
function and acute kidney injury (825827). Patients with
normal kidney function may use these agents in moderate doses for short periods of time, but nonsteroidal antiinflammatory agents should be avoided in KTRs whenever
possible (806).
The American Society of Transplantation guidelines recommended measuring uric acid levels once 23 months after
transplantation, with additional screening in patients with
reduced function and on diuretics (627). The Caring for Australasians with Renal Impairment guidelines for patients
with CKD state that treating hyperuricemia does not retard progression and cannot be recommended; patients on
protein-restricted diets treated with allopurinol may require
dose reductions (822). The European Best Practice guideline on kidney transplantation recommends that the combination of allopurinol and azathioprine be avoided (708).
Cochicine levels may be increased in patients with reduced
kidney function and in patients treated with CsA (and
presumably tacrolimus). Life-threatening colchicine toxicity has been described in patients with reduced kidney
function receiving colchicine 1 mg/day for only 58 days
(828). A disabling myoneuropathy has also been described
in patients with reduced kidney function receiving long-
Research Recommendations
Allopurinol is a common uric acid lowering agent (804).
However, allopurinol and azathioprine used together can
result in profound, life-threatening pancytopenia (627,753),
and thus this combination should be used with extreme
caution, or not at all. If used together, azathioprine should
be reduced by at least 50% and frequent complete blood
counts should be used to monitor the interaction (806).
Further dose reductions may be needed. Mycophenolate
does not interact with allopurinol and can be used in place
of azathioprine if an antiproliferative agent is necessary for
immunosuppression (831). Patients allergic to allopurinol
may be given benziodarone (832,833).
A RCT with adequate statistical power is needed
to study the effect of treating asymptomatic hyperuricemia on preventing loss of kidney function, gout
and CVD.
American Journal of Transplantation 2009; 9 (Suppl 3): S102S103
S103
Вам также может понравиться
- Naplex ReviewДокумент76 страницNaplex Reviewapartment4rent_4100% (2)
- Foods To Aid The Kidneys To Eliminate Uric AcidДокумент25 страницFoods To Aid The Kidneys To Eliminate Uric AcidSabbra CadabraОценок пока нет
- GoutДокумент6 страницGoutNader Smadi100% (1)
- Dialysisprescription2 161102121138Документ61 страницаDialysisprescription2 161102121138ZH. omg sar100% (1)
- UW Path NoteДокумент218 страницUW Path NoteSophia Yin100% (4)
- Rheumatology MedicineДокумент19 страницRheumatology MedicineSami Ur RehmanОценок пока нет
- Renal Urology HistoryДокумент131 страницаRenal Urology Historysharu4291Оценок пока нет
- GoutДокумент65 страницGoutChristina BarrogaОценок пока нет
- Rheumatology Cases Fall Clinics 2019Документ85 страницRheumatology Cases Fall Clinics 2019NaziBrola TsivadzeОценок пока нет
- Pediatric Acute Kidney Injury (AKI) Indications, Timing, and Choice of ModalityДокумент32 страницыPediatric Acute Kidney Injury (AKI) Indications, Timing, and Choice of ModalityCarolineОценок пока нет
- Complementary and Alternative Medical Lab Testing Part 8: UrologyОт EverandComplementary and Alternative Medical Lab Testing Part 8: UrologyРейтинг: 3 из 5 звезд3/5 (1)
- Done By: Dana Othman: AscitesДокумент28 страницDone By: Dana Othman: Ascitesraed faisalОценок пока нет
- Drug Interactions in Infectious Diseases Antimicrobial Drug Interactions PDFДокумент576 страницDrug Interactions in Infectious Diseases Antimicrobial Drug Interactions PDFMedy RahmanОценок пока нет
- Wellington ICU Drug Manual 2013Документ444 страницыWellington ICU Drug Manual 2013khangsiean89Оценок пока нет
- D Received Patient Sitting On Bed With Bottle #Документ17 страницD Received Patient Sitting On Bed With Bottle #jaypee100% (2)
- 194 Renal Replacement Therapy in Critical Care PDFДокумент15 страниц194 Renal Replacement Therapy in Critical Care PDFAndreeaIrinaHedes100% (1)
- Urinary Stones: Medical and Surgical ManagementОт EverandUrinary Stones: Medical and Surgical ManagementMichael GrassoОценок пока нет
- Delaying or Halting Progression of Chronic Kidney DiseaseДокумент9 страницDelaying or Halting Progression of Chronic Kidney Diseasehannya manОценок пока нет
- SX Hepatorrenal 2020Документ14 страницSX Hepatorrenal 2020AlexisBallénОценок пока нет
- Case Challenge: Diagnosing and Managing CKD Comorbidities: Literature ReviewДокумент8 страницCase Challenge: Diagnosing and Managing CKD Comorbidities: Literature ReviewPratik TripathiОценок пока нет
- Citrato FígadoДокумент9 страницCitrato FígadopmunizОценок пока нет
- Augmented Renal Clearance and How To AugmentДокумент12 страницAugmented Renal Clearance and How To Augmentlocal5.fsgОценок пока нет
- Subclinical Celiac Disease and Crystal-Induced Kidney Disease Following Kidney TransplantДокумент6 страницSubclinical Celiac Disease and Crystal-Induced Kidney Disease Following Kidney TransplantsigmundmaharajanОценок пока нет
- 10 1016@j Ijcard 2015 05 023Документ3 страницы10 1016@j Ijcard 2015 05 023Choi Eva Young ShineeОценок пока нет
- Hepatorenal SyndromeДокумент14 страницHepatorenal SyndromeVerónica Duménez JofréОценок пока нет
- S64 FullДокумент3 страницыS64 FullkarenafiafiОценок пока нет
- 11 Chapter 87 Renal Insufficiency and Ischemic NephropathyДокумент19 страниц11 Chapter 87 Renal Insufficiency and Ischemic NephropathyfransiskaОценок пока нет
- Acute Kidney InjuryДокумент17 страницAcute Kidney InjuryCha-cha UzumakiОценок пока нет
- Aki and CKD Therapy 2021Документ46 страницAki and CKD Therapy 2021Alfathri YunediОценок пока нет
- Novel Aspects of Pharmacological Therapies For Acute Renal FailureДокумент16 страницNovel Aspects of Pharmacological Therapies For Acute Renal FailureproluvieslacusОценок пока нет
- Renal Disease: Acute Kidney Injury (AKI)Документ5 страницRenal Disease: Acute Kidney Injury (AKI)api-142637023Оценок пока нет
- Acute Renal Failure in The ICU PulmCritДокумент27 страницAcute Renal Failure in The ICU PulmCritchadchimaОценок пока нет
- Perioperative Acute Kidney Injury: DR Mukul Kapoor Director Anesthesia, Max Smart Super Specialty Hospital, Saket, DelhiДокумент46 страницPerioperative Acute Kidney Injury: DR Mukul Kapoor Director Anesthesia, Max Smart Super Specialty Hospital, Saket, DelhiChiragОценок пока нет
- #4 (Sofà A)Документ6 страниц#4 (Sofà A)Sofia OrduñoОценок пока нет
- Renal Replacement Therapy in Acute Kidney Injury: Review: Blood Research & Transfusion JournalДокумент4 страницыRenal Replacement Therapy in Acute Kidney Injury: Review: Blood Research & Transfusion JournalNova SuryatiОценок пока нет
- Causes: Ascites (/Ə S Ti Z/) (FromДокумент10 страницCauses: Ascites (/Ə S Ti Z/) (FromlupiОценок пока нет
- 10 Glycemic Control in Perioperative PeriodДокумент5 страниц10 Glycemic Control in Perioperative PeriodAna-Maria CroitoruОценок пока нет
- Akizawa 2009Документ9 страницAkizawa 2009PROF. ERWIN M. GLOBIO, MSITОценок пока нет
- Chronic Kidney Disease (CKD) : Provider's Guide To Diagnose and Code CKDДокумент2 страницыChronic Kidney Disease (CKD) : Provider's Guide To Diagnose and Code CKDAry Rio PambudiОценок пока нет
- 22Документ7 страниц22Evaa Michizane NurtanioОценок пока нет
- Clinical Case Reports - 2020 - Schnaubelt - Hyperkalemia A Persisting Risk A Case Report and Update On Current ManagementДокумент6 страницClinical Case Reports - 2020 - Schnaubelt - Hyperkalemia A Persisting Risk A Case Report and Update On Current ManagementDesi MeliaОценок пока нет
- Piis1548559512001784 PDFДокумент11 страницPiis1548559512001784 PDFDevi NugrahaОценок пока нет
- Indications For Initiation of Dialysis in Chronic Kidney DiseaseДокумент11 страницIndications For Initiation of Dialysis in Chronic Kidney DiseaseLiliana WoodОценок пока нет
- Version of Record:: ManuscriptДокумент23 страницыVersion of Record:: Manuscriptmiko balisiОценок пока нет
- Acute Kidney Injury (AKI) : BackgroundДокумент22 страницыAcute Kidney Injury (AKI) : BackgroundDeif TunggalОценок пока нет
- Joacp 28 386Документ11 страницJoacp 28 386KrisztinaОценок пока нет
- Ann Burns and Fire Disasters 26 16Документ10 страницAnn Burns and Fire Disasters 26 16plastic guardiansОценок пока нет
- The Association Between Urine Output, Creatinine Elevation, and DeathДокумент9 страницThe Association Between Urine Output, Creatinine Elevation, and DeathTrahmono SrОценок пока нет
- AKI TreatДокумент2 страницыAKI TreatNama ManaОценок пока нет
- Management of Ascites in Cirrhosis: AdvancesinclinicalpracticeДокумент10 страницManagement of Ascites in Cirrhosis: AdvancesinclinicalpracticeSergiu ManОценок пока нет
- Hepatorenal Syndrome 2022Документ19 страницHepatorenal Syndrome 2022palomisgarcia1995Оценок пока нет
- Síndrome Hepatorrenal Cirrosis Eur Gast J 2021Документ9 страницSíndrome Hepatorrenal Cirrosis Eur Gast J 2021Anabel GonzalezОценок пока нет
- Electrolyte and Acid-Base Disorders in Chronic Kidney Disease and End-Stage Kidney FailureДокумент7 страницElectrolyte and Acid-Base Disorders in Chronic Kidney Disease and End-Stage Kidney FailureFarra JansyeОценок пока нет
- Acute Hemodialysis PrescriptionДокумент18 страницAcute Hemodialysis PrescriptionOlga BabiiОценок пока нет
- Recent Trends in ESRD: Presented By: DR Sayyed Ahmad Moderator: DR Poonam DalalДокумент58 страницRecent Trends in ESRD: Presented By: DR Sayyed Ahmad Moderator: DR Poonam DalalSayyed Ahmad KhursheedОценок пока нет
- Acute Kidney InjuryДокумент15 страницAcute Kidney Injurykuchaibaru90Оценок пока нет
- Cefotaxime Renal ImpairmentДокумент6 страницCefotaxime Renal ImpairmentChristine LilyanaОценок пока нет
- NGAL Como Biomarcador en La Enfermedad Renal CrónicaДокумент13 страницNGAL Como Biomarcador en La Enfermedad Renal CrónicaventasОценок пока нет
- Section 26Документ19 страницSection 26syahrun22Оценок пока нет
- HCQ CardiotoxicityДокумент28 страницHCQ CardiotoxicitypaidisuriОценок пока нет
- Aki Inn EmergencyДокумент52 страницыAki Inn EmergencyHamza DossaОценок пока нет
- Acute Kidney Injury 2Документ15 страницAcute Kidney Injury 2Manish VijayОценок пока нет
- Surgery in The Patient With Renal DysfunctionДокумент11 страницSurgery in The Patient With Renal DysfunctionAps CnjОценок пока нет
- Jurnal AkiДокумент7 страницJurnal AkiNining Komala SariОценок пока нет
- Dialysis Free Protocol For Some End Stage Renal Disease PatientsДокумент7 страницDialysis Free Protocol For Some End Stage Renal Disease PatientsDannieCiambelliОценок пока нет
- The MERCURY I OpenДокумент12 страницThe MERCURY I OpenSharmil IyapillaiОценок пока нет
- New Insights Into The Pa Tho Physiology of Idiopathic Nephrotic SyndromeДокумент9 страницNew Insights Into The Pa Tho Physiology of Idiopathic Nephrotic SyndromeMartina Alarcon AcevedoОценок пока нет
- Use of Diuretics in CKDДокумент19 страницUse of Diuretics in CKDyulibudhyОценок пока нет
- Urologi Jurnal InternasionalДокумент9 страницUrologi Jurnal InternasionalMyisha UfairaОценок пока нет
- UntitledДокумент8 страницUntitledIgnacio Enrique Squella TroncosoОценок пока нет
- Renal Pharmacotherapy: Dosage Adjustment of Medications Eliminated by the KidneysОт EverandRenal Pharmacotherapy: Dosage Adjustment of Medications Eliminated by the KidneysОценок пока нет
- Hyperglycemic Hyperosmolar Nonketotic Syndrome: R. Venkatraman and Sunit C. SinghiДокумент6 страницHyperglycemic Hyperosmolar Nonketotic Syndrome: R. Venkatraman and Sunit C. Singhisharu4291Оценок пока нет
- Comparison CarsДокумент5 страницComparison Carssharu4291Оценок пока нет
- ChoiДокумент19 страницChoiLuciana RafaelОценок пока нет
- Molecules 18 08976Документ18 страницMolecules 18 08976sharu4291Оценок пока нет
- Prospectus MDMSJULY2015Документ39 страницProspectus MDMSJULY2015sharu4291Оценок пока нет
- Approach To A Case of Hyperuricemia: Singh V, Gomez VV, Swamy SGДокумент7 страницApproach To A Case of Hyperuricemia: Singh V, Gomez VV, Swamy SGsharu4291Оценок пока нет
- ABC Emergency Differential DiagnosisffДокумент3 страницыABC Emergency Differential Diagnosisffsharu4291Оценок пока нет
- ABC Emergency Differential DiagnosisdrererДокумент3 страницыABC Emergency Differential Diagnosisdrerersharu4291Оценок пока нет
- ABC Emergency Differential DiagnosisewwwwweДокумент3 страницыABC Emergency Differential Diagnosisewwwwwesharu4291Оценок пока нет
- Intern ACOG Bulletin Episiotomy and Repair3Документ3 страницыIntern ACOG Bulletin Episiotomy and Repair3sharu4291Оценок пока нет
- ABC Emergency Differential DiagnДокумент3 страницыABC Emergency Differential Diagnsharu4291Оценок пока нет
- ABC Emergency Differential Diagnosis1Документ4 страницыABC Emergency Differential Diagnosis1sharu4291Оценок пока нет
- BIOCHEMISTRY 2nd SessionДокумент116 страницBIOCHEMISTRY 2nd SessionShane CoquillaОценок пока нет
- Bp503t Pcol Unit-IIIДокумент38 страницBp503t Pcol Unit-IIIAakkkОценок пока нет
- Anti GoutДокумент4 страницыAnti GoutStephanie ReyesОценок пока нет
- CPT Post TestДокумент38 страницCPT Post TestUlrich TiamОценок пока нет
- Drug Study LeukemiaДокумент12 страницDrug Study LeukemiaLiana CervantesОценок пока нет
- 4.+panji+36052 FINALДокумент6 страниц4.+panji+36052 FINALRecky PatalaОценок пока нет
- Non-Protein Nitrogenous Constituents of Blood - Urea, Uric Acid EtcДокумент50 страницNon-Protein Nitrogenous Constituents of Blood - Urea, Uric Acid EtcBobskinnyОценок пока нет
- Acute LeukemiaДокумент4 страницыAcute LeukemiaRonald Cszar Fabian VillanoОценок пока нет
- Non ProteinДокумент7 страницNon ProteinMelanie ParisОценок пока нет
- F. Case Study Thesis-Drug Study (Revised)Документ5 страницF. Case Study Thesis-Drug Study (Revised)Lopirts NiganiОценок пока нет
- Office of The Infection Control Committee: Allied Care Experts (Ace) Medical Center - PaterosДокумент5 страницOffice of The Infection Control Committee: Allied Care Experts (Ace) Medical Center - PaterosCris GalendezОценок пока нет
- Kualitas Hidup Pasien GoutДокумент10 страницKualitas Hidup Pasien GoutShared LifeОценок пока нет
- New Approaches To An Old Disease: GoutДокумент27 страницNew Approaches To An Old Disease: GoutDini HanifaОценок пока нет
- Febuxostat: Febo UloricДокумент23 страницыFebuxostat: Febo Uloricabd hamzaОценок пока нет
- Tumor Lysis SyndromeДокумент5 страницTumor Lysis SyndromeSusan RamosОценок пока нет
- NEET PG 2023 Solved PaperДокумент139 страницNEET PG 2023 Solved PapersaravananОценок пока нет
- APNC 520 Exam 10 Study Guide Musculoskeletal DisordersДокумент15 страницAPNC 520 Exam 10 Study Guide Musculoskeletal DisordersMariaОценок пока нет
- ArthritisДокумент69 страницArthritisKavya sriОценок пока нет
- MR Abdul Muthalib OAДокумент20 страницMR Abdul Muthalib OAfadli padoОценок пока нет
- Tumor Lysis Syndrome: Practice GapsДокумент9 страницTumor Lysis Syndrome: Practice GapsMichelleHanОценок пока нет
- Krok 1 - 2018 (General Medicine) - EneutronДокумент57 страницKrok 1 - 2018 (General Medicine) - EneutronHarsh NimavatОценок пока нет