Академический Документы
Профессиональный Документы
Культура Документы
Poly Propylene
Загружено:
Akhil AbrahamОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Poly Propylene
Загружено:
Akhil AbrahamАвторское право:
Доступные форматы
POLYMER
MAJOR
PROJECT
POLYPROPYLENE
A
VERSATILE
POLYMER
COURSE
INSTRUCTOR
ASSISTANT
PROFESSOR,
CHEMISTRY
Dr.
SULAFUDIN
VUKUSIC
SUBMITTED
BY
AKHIL
MAMMOOTTIL
ABRAHAM
920018779
TABLE OF CONTENTS
INTRODUCTION
......................................................................................................................................
4
STRUCTURE
...............................................................................................................................................
4
METHOD OF SYNTHESIS
...................................................................................................................
5
Gas phase reactor
....................................................................................................................................................
6
Liquid phase polymerization (Slurry process)
..............................................................................................
7
Bulk Polymerization
..............................................................................................................................................
8
PHYSICAL AND CHEMICAL PROPERTIES
...............................................................................
9
APPLICATIONS
.......................................................................................................................................
11
Rigid Packaging
...................................................................................................................................................
11
Polypropylene in Automotive
.........................................................................................................................
12
Polypropylene in Consumer Products
..........................................................................................................
12
Polypropylene Fibres
.........................................................................................................................................
13
Polypropylene in Industries
.............................................................................................................................
13
Polypropylene in medical applications
........................................................................................................
13
POLYPROPYLENE PROCESSING TECHNIQUE
....................................................................
14
Injection Molding
................................................................................................................................................
14
Processing of polypropylene fibers by melt blown process
..................................................................
15
Thermoforming
....................................................................................................................................................
15
REFERENCES
...........................................................................................................................................
17
LIST OF FIGURES
Figure 1: - Monomer propene ............................................................................................ 4
Figure 2: - Polypropylene polymer structure ..................................................................... 4
Figure 3: - Stereospecific Polypropylene ........................................................................... 5
Figure 4: - Metallocene catalyst ......................................................................................... 6
Figure 5: - Zirconium catalyst responsible for syndiotactic polypropylene ...................... 6
Figure 6: - Gas Phase reactor .............................................................................................. 7
Figure 7: - Slurry phase reactor ......................................................................................... 8
Figure 8: - Polypropylene application globally ............................................................... 11
Figure 9: - Injection molding, Polypropylene .................................................................. 14
Figure 10: - Polypropylene fibre production ................................................................... 15
Figure 11: - Thermoforming polypropylene .................................................................... 16
LIST OF TABLES
Table 1: - Polypropylene Processing Condittions ............................................................... 9
Table 2: - Polypropylene general properties ..................................................................... 10
INTRODUCTION
A linear hydrocarbon polymer, which has similar properties to that polyethylene and
polybutene is conquering the world of polymers with its wide range of applications.
CnH2n is the general expression of this hydrocarbon polymer. Because of its versatility in
application as a plastic and a fibre, the plastic market has lot of room for this polymer [5].
STRUCTURE
The monomer or the repeating unit is propylene or propene as shown in the figure 1
Figure 1: - Monomer propene [10]
Addition polymerization of propene or propylene results in long chain polypropylene
polymer as shown in figure 2.
Figure 2: - Polypropylene polymer structure [10]
Commercially, the repeating unit or the monomer polymerizes in the presence of Ziegler
natta catalyst to produce a stereospecific polypropylene as shown in the figure 3. The
position of methyl group determines the stereo regularity of the polymer formed [10]. If
the substituent methyl group is on the same side, we get a isotactic polypropylene, if the
methyl groups are alternating, a syndiotactic polymer. If the substituent is irregularly
arranged we get an atactic polymer.
Figure 3: - Stereospecific Polypropylene [10]
The stereospecific isotactic and syndiotactic structures are crystalline because the
structure has a regularity in its packing. Hence of its regularity in structure polymer is
found to be hard, a rigid material and in its pure form the melting point is found to be
440K [10].
However the non-stereospecific atactic polypropylene donot crystallize and high
molecular weight atactic polypropylene is rubbery [10].
Commercially available polypropylene is isotactic about 1-5% of atactic content [10].
METHOD OF SYNTHESIS
Slurry, solution or gas phase polymerization could be employed for the manufacture of
polypropylene. Repeating unit or the monomer is propylene, which is pressurized and
heated in the presence of catalyst. Polymerization happens at relatively low pressure and
temperature, because of the presence of Ziegler-natta catalyst. Upon changing the
conditions used and catalyst employed, the property of the polymer could be varied [10].
Polypropylene production takes place either in a gas phase (fluidized bed or stirred
reactor) or a liquid phase reactor (slurry or solution).
Gas phase reactor
Gas phase reactor is often employed in the production of polypropylene with the help of
following catalyst.
1. Ziegler Natta
2. Metallocene
Stereospecific Ziegler-Natta catalyst was a revolution indeed in the manufacture of
polypropylene, as polymerization could be carried out at a lower pressure and
temperature. The polymer manufactured in the presence of Ziegler-natta catalyst would
be isotactic polypropylene. Before we dispatch the final product, which is in the pellet
form, the catalyst has to be destroyed. This could be done by addition of water or alcohol.
Hence gas phase process could be completed with out much residues in the final product
[10].
Metallocene is being used these days for the manufacture of polypropylene.
Metallocenes are transition metals forming ligands with cyclopentadienyl [10], one such
example is metallocene based on zirconium as shown in the figure 4.
Figure 4: - Metallocene catalyst [10]
The specific orientation of zirconium, facilitate the production of isotactic polypropylene.
However once we employ a different zirconium compound as shown in figure 5
syndiotactic polypropylene could be produced, which is the only way of producing
syndiotactic polypropylene [10].
Figure 5: - Zirconium catalyst responsible for syndiotactic polypropylene [10]
Figure 6 and 7 demonstrates both the process (gas phase and slurry phase respectively).
Figure 6: - Gas Phase reactor [7]
Fig above is a flow sheet for gas phase reactor. We have Ziegler Natta as the catalyst
along with the monomer in the gas-phase reactor, where the reactor is operated at 70800C and at a pressure of 8-35atm [10] [7].
Propylene, nitrogen and hydrogen contribute the inlet stream. They act as heat transfer
medium and reactants for the growing polypropylene chain in the fluidized bed reactor.
Co-catalyst is continuously supplied into the reactor to activate the reactants and produce
polymer particles. Another function of co-catalyst is to ensure that moisture stays below
2ppm and keep the catalyst activated, which is required for the production of industrial
grade polypropylene. Once the bed is fluidized, disengaging section of the plant
separates the left over gases, which is recycled and combined with the initial feed
consisting of propylene, nitrogen and hydrogen. Heat exchanger is employed to remove
the excess heat in the recycle stream. The propylene conversion to polypropylene per
pass is 2-3%, while the overall conversion can sum up to 98%. The whole plant is
designed to run at 30bar [7].
Liquid phase polymerization (Slurry process)
Liquid phase polymerization happens in a slurry reactor, which consist of a pipe usually
in the form a loop. Propene and catalyst are fed into the reactor, which is filled with
diluent such as isobutene. Polypropylene produced in small pellet circulates around the
loop as slurry where the loop reactor is operated at 75-1100C and 8-35 atm [10]. Slurry is
continuously removed and the polypropylene pellets removed are dried and transported to
the extrusion area.
Figure 7: - Slurry phase reactor [4]
The only difference from the gas-phase reactor is the introduction of a loop reactor.
In other words a semi-plug flow reactor. Catalyst is added to the reactor with the diluent.
The purpose of loop reactor is to avoid deposits, provide high surface to volume ratio
enhancing heat removal and provides shorter residence time. Gas phase reactor follows
the loop reactor, polymerization happens in the following reactor at certain temperature
and pressure and the product is dispatched. Copolymerization of polypropylene is carried
out in the gas phase reactors.
Bulk Polymerization
Monomer and solvent is liquid propene itself. Polymerization happens at a temperature of
340-360K and at a pressure of 30-40atm. The high pressure is because; propene has to be
in liquid state. After polymerization solid particles are collected, and liquid separated is
recycled [10].
Polypropylene processing conditions are as shown in table 1
Parameters
Homopolymer
Polypropylene
Copolymers
polypropylene
Melt temperature (0C)
210-290
210-290
Mould temperature (0C)
20-60
20-60
Pre-drying
Not required
Not required
Typical Mould Shrinkage 1.5
(%)
Reactor
Operating 70-80
0
temperature ( C)
70-80
Operating pressure (atm)
8-35
8-35
of
Table 1: - Polypropylene Processing Condittions [5] [10]
PHYSICAL AND CHEMICAL PROPERTIES
Commercial polypropylene is a semi-crystalline polymer, as the polymer structure is
isotactic. The crystallinity of the polypropylene lies between low-density polyethylene
(LDPE) and high-density polyethylene (HDPE). When copolymerized with ethylene, the
polymer has extra toughness and flexibility, hence polypropylene find lot of applications
in engineering plastic, and has the potential to replace material like acrylonitrile
butadiene styrene (ABS) [8]. Polypropylene is economical and is not as transparent as
polymers like polystyrene, acrylic or other plastics. It shows good fatigue resistance,
Perfect isotactic polypropylene has melting point around 1700C, depending on the
tacticity commercially available polypropylene has melting point ranging from 160 to
1660C. Molecular weight is a function of Melt Flow Index (MFI), enabling us to
determine the flow of polymer melts while processing. Polypropylene having high MFI ,
would have less impact strength [8].
Polypropylene shows moderate barrier resistance to moisture, gases and odours. Though
its flexibility is less than of polyethylene, it finds similar application of that of lowdensity polyethylene (LDPE) [5] [8] [10].
10
Polypropylene has no stress-cracking issues and shows good chemical and electrical
resistance. Though the properties of polypropylene are similar to polyethylene, there
some differences as well, one such is softening point; polypropylene has a higher
softening point than polyethylene making it rigid and hard. Additives are often used for
better end-use performance [8].
Polypropylene properties are summarized in table 2.
Properties
homopolymer
Co-polymer
Density (kg/m3)
905
905
Tensile strength (Mpa)
33
25
Tensile Modulus (Gpa)
1.4
Elongation at break (%)
150
300
Heat distortion temperature 65
@ 1.8 Mpa/0C
Volume Resistivity (logUm) 19
60
Oxygen Index (%)
17
17
19
Table 2: - Polypropylene general properties [5]
11
APPLICATIONS
Crates, toys, medical equipments, bottle caps and many day-to-day products are made of
polypropylene. Propylene film in package industry, fibres in clothing and carpet are all
made of polypropylene [10] [3].
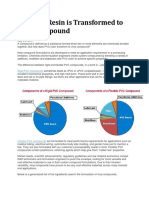
Figure 8 shows the applications of polypropylene in the global market
Figure 8: - Polypropylene application globally [10]
As observed from figure 8, major portion of the polypropylene is used in the manufacture
of crates, CD and DVD covers etc , which comes under the roof of rigid packaging
(28%), Followed by textile (21%) and technical industries (20%). 15% of polypropylene
produced is used as consumer products and 16% in food packaging respectively
Superior puncture resistance, low sealing threshold and competitive price has benefited
and helped Polypropylene gain ground over cellophane, metals and paper in the
manufacture of material to be used in film extrusion. Available polypropylene films are
Cast or bi-axially oriented. Film market has three divisions [5].
1. Food and confectionaries
2. Tobacco
3. Clothing
Tobacco products contribute major share for polypropylene after food and
confectionaries. Food and confectionaries holds the film market with applications ranging
from confectionaries to crisps and biscuits. Another market polypropylene holds share is
rigid packaging, which is subdivided into applications varying from caps to crates [5].
Various applications in detail are discussed below.
Rigid Packaging
Polypropylene packaging is efficient because of the following properties it possesses [3].
12
Polypropylene in natural state doesnt violate FDA regulations for food contact
applications
Good mechanical and barrier properties
As the product are thin walled, the raw material can be saved, hence reducing the
cost.
Good impact resistance at lower temperatures.
Simple and safe storage of products without affecting the features of the product is
definitely the primary concern. Reusable and stackable crates made of Polypropylene
provide excellent application in transport and storage products without disturbing the
content inside [5] [1]. Hence very good solution for storage and transport of products as
polypropylene posses good mechanical and barrier properties [1], which has attracted
supermarkets, groceries and automotive supply chain as well
Blow moulded polypropylene bottles are employed for the packing of condiments,
detergents and toiletries. Yoghurt pots available in the market are made of thin-walled
polypropylene containers, because of its inherent properties, which helps in preserving
the yoghurt [5].
Polypropylene in Automotive
In the field of automotives, polypropylene materials are used in interior designing,
especially as a monomaterial solution. For example mono material dashboards are very
popular in the house of automotives, polypropylene film cushioning, film skins and
powder slush moulding integrated with polypropylene textile covers are gainig ground in
automotive industries [5].
Now moving to the outer body of the automotives, parts such as bumpers, exterior trim
and cladding are all made of polypropylene. Because of the properties listed below, it is
gaining ground in such applications [5].
Low coefficient of linear thermal expansion and specific gravity.
High chemical resistance and good weatherability.
Processability and impact balance.
Pre-colored polypropylene is readily available, avoiding painting of products in some
applications [5].
Polypropylene in Consumer Products
Properties that make polypropylene a good consumer product are [3].
High stiffness and durability.
Good heat resistance and stack strength
Outstanding performance and lightweight
A very good cycle time.
13
Housewares, luggage, toys, battery cases, furniture, appliances and other durable
products for garden and leisure use come under this title. The moulding technique that
prevails in this sector is Injection moulding [5].
Polypropylene Fibres
Products such as tapes, bulk continuous filaments, staple fibres, spun bound and
continuous filament find very much useful for lots of applications that we come across.
Polypropylene fibres are used in the manufacture of above-mentioned commodities [5].
Following properties make polypropylene a good non-woven fabric [3]
Lightweight.
Good resistance to chemicals, weather, mildew, insects etc.
Good abrasion resistance.
Sunlight resistance.
Good wash ability, quick drying and unique wicking.
Low moisture absorption.
Polypropylene in Industries
Polypropylene is a) chemically stable b) When heated only carbon dioxide and water
vapor is produced c) Calorific value same as of oil d) Abrasion resistance and light
weight [3], hence used in industries for applications mentioned below.
Different varieties of sheets, pipes, Returnable Transport Packages (RTP), which
constitutes the major portion of the industries are manufactured from polypropylene.
Extrusion moulding is, the technique employed in the manufacture these products, except
RTP, where Injection Moulding is used. Construction sectors, especially domestic
drainage pipes are made of polypropylene [5].
Polypropylene in medical applications
As a cost effective and environmentally friendly alternative, polypropylene is conquering
the market of medical equipment. Its resistance to a) cleaning agents, b) disinfectants and
c) many solvents makes it apt for the manufacture of surgical trays, syringes and lab
equipment such as pipette tips, centrifuge and blood collection tubes [5].
14
POLYPROPYLENE PROCESSING TECHNIQUE
Injection Molding
Standard screw molding equipment is employed without much alteration. Pre-drying is
not required but filled resins may require. Feed is fed into the hopper thereafter feed
enters the heating phase in the cylindrical section where cylinder temperature is
maintained at 400-5700F. The reciprocating motion of the screw pushes the melt forward
and then to the mold through nozzle at a pressure of 1000-1500 psi, the frictional motion
of the screw also heats polymer. The hopper or feed section is maintained at 30-500F.
Good results are obtained at 75% of press capacity. Pressure has to be maintained if not
can result in flashing, burning and can stick inside the mold. Mold temperature is kept at
60-1500F. Other parameters that affect the processing are injection time, cure time, back
pressure, and screw speed [6].
Figure 9: - Injection molding, Polypropylene [11]
15
Processing of polypropylene fibers by melt blown process
Initially the polymer is melted in an extruder and it is done through a spinneret, which
contains 200-1000 holes at a spinning rate of 800-4000rpm, followed by quenching
filaments for spin-finished applications. The drawn filaments are then heated, stretched,
crimped and cut for various applications [9].
Figure 10: - Polypropylene fibre production [9]
Extruder which operates at 430-4900F (1) pushes the melt polymer into the spinning pack
(2) where polymer melt is homogenized and fed to the roller at constant spinning rate.
The melt is cooled inside the quench (3). New filaments are stretched and fed into box (5)
for abrasion resistance and to improve thermo-physical properties. Finally step (7) and
(9) for stabilizing and crimping respectively. Final product is cut using cutter (10) [6] [9].
Thermoforming
The steps involved in thermoforming are [2]
Heating
Vacuum forming
Cooling
Trimming
16
Figure 11 depicts a flow diagram of thermoforming of polypropylene.
Figure 11: - Thermoforming polypropylene [2]
Polypropylene sheets are heated on the clamp, once the polypropylene reaches the
thermoforming temperature (170-1850C). The polypropylene gets molded onto the mold
by applying vacuum pressure. Once the polymer is molded cooling and trimming would
be the concern [2].
The mold temperature determines the appearance, the length of forming cycle and
stability of the polymer. Usually the polypropylene molds are maintained at 30-650C.
Trimming the polymer from mold depends on the sheet temperature and the equipment
employed. With adequate cooling time of 1-2 minutes sheets of thickness less than 1mm
could be generated. Cooling for long time generates sheets of thickness greater than 3mm
[2].
17
REFERENCES
[1]Allahvaisi , S. (2012). Polypropylene in the Industry of Food Packaging. In D. Dogan ,
Polypropylene (pp. 3-21). Croatia: INTECH.
[2]CPC Congdon Plastics Consulting LLC. (n.d.). A Guide to Thermoform Processing of
Polypropylene
.
Retrieved
April
15,
2015,
from
http://congdonplasticsconsulting.com/:http://congdonplasticsconsulting.com/ts/sheet/PP%
20Thermoforming.pdf
[3]ExxonMobil. (n.d.). polypropylene. Retrieved April 17, 2015, from
http://www.exxonmobilchemical.com/:http://www.exxonmobilchemical.com/ChemEnglish/productsservices/polypropylene.aspx
[4]Guichon Valves. (1921). PP Polypropylene Manufacturing process of PP .
Retrieved April 9, 2015, from http://guichon-valves.com/: http://guichonvalves.com/faqs/pp-polypropylene-manufacturing-process-of-pp-polypropylene/
[5]Hindle, C. (1933). British Plastic federation. Retrieved April 7, 2015, from bpf.co.uk:
http://www.bpf.co.uk/plastipedia/polymers/PP.aspx#properties
[6]Ineos olefins and polymers USA. (2007, March). Polypropylene processing guide.
Retrieved
April
8,
2015,
from
http://www.ineos.com/:
http://www.ineos.com/global/olefins%20and%20polymers%20usa/products/technical%2
0information/ineos_polypropylene_processing_guide.pdf
[7]Khan , M. H., Hussain , M. A., & Mujtaba , I. M. (2014, March 27). Polypropylene
Production Optimization in Fluidized Bed Catalytic Reactor (FBCR): Statistical
Modeling and Pilot Scale Experimental Validation . materials , 2441-2458.
[8]Maier, C., & Calafut, T. (1998). Morphology and Commercial forms. Bromley, United
Kingdom: Elsevier.
[9]Rong, H., & Kannadaguli, M. (2004, April). Olefin Fiber. Retrieved April 2016, 2015,
from
http://www.engr.utk.edu/:
http://www.engr.utk.edu/mse/pages/Textiles/Olefin%20fibers.htm
[10]The university of york. (2014, january 2). The essential chemical industry online.
Retrieved
April
4,
2015,
from
http://www.essentialchemicalindustry.org:
http://www.essentialchemicalindustry.org/polymers/polypropene.html
[11]AV Plastics. (1977). What is injection molding? Retrieved April 4, 2015, from
http://www.avplastics.co.uk/about-av-plastics:
http://www.avplastics.co.uk/what-isinjection-moulding
18
Вам также может понравиться
- Atomic Layer Deposition (ALD) : Myo Min TheinДокумент28 страницAtomic Layer Deposition (ALD) : Myo Min Theinajays2539Оценок пока нет
- Yang Et Al 2010 ElectroanalysisДокумент6 страницYang Et Al 2010 ElectroanalysisAkhil AbrahamОценок пока нет
- Nanotechnology and NanocompositesДокумент43 страницыNanotechnology and NanocompositesAkhil AbrahamОценок пока нет
- Project Report FinalДокумент20 страницProject Report FinalAkhil AbrahamОценок пока нет
- Project Report FinalДокумент20 страницProject Report FinalAkhil AbrahamОценок пока нет
- Intro to Lab Safety TrainingДокумент20 страницIntro to Lab Safety TrainingAkhil AbrahamОценок пока нет
- HSE-LS-15 Laser Safety Training PDFДокумент23 страницыHSE-LS-15 Laser Safety Training PDFAkhil AbrahamОценок пока нет
- HSE-LS-14 Radiation Safety Training PDFДокумент23 страницыHSE-LS-14 Radiation Safety Training PDFAkhil AbrahamОценок пока нет
- HSE-LS-05 Fume Hood Training Handout PDFДокумент15 страницHSE-LS-05 Fume Hood Training Handout PDFAkhil AbrahamОценок пока нет
- HSE-LS-12 Compressed Gas Safety PDFДокумент21 страницаHSE-LS-12 Compressed Gas Safety PDFAkhil AbrahamОценок пока нет
- HSE-LS-13 Electrical Safety PDFДокумент30 страницHSE-LS-13 Electrical Safety PDFAkhil AbrahamОценок пока нет
- Lab Safety Risk AssessmentДокумент19 страницLab Safety Risk AssessmentAkhil AbrahamОценок пока нет
- HSE-LS-04 PPE Handout PDFДокумент10 страницHSE-LS-04 PPE Handout PDFAkhil AbrahamОценок пока нет
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- Venezuela Asfalto Modificado PDFДокумент12 страницVenezuela Asfalto Modificado PDFkarenОценок пока нет
- PolymersДокумент9 страницPolymersChhavi SharmaОценок пока нет
- Biopolymer - WikipediaДокумент5 страницBiopolymer - WikipediaLizbethОценок пока нет
- Carothers Theory - Step PolymerizationДокумент8 страницCarothers Theory - Step Polymerization891208Оценок пока нет
- Analyzing The Innovative Challenges and Possible Solutions of Polymer and Related Material Based On AI Chatbot (Chat GPT) ResponsesДокумент7 страницAnalyzing The Innovative Challenges and Possible Solutions of Polymer and Related Material Based On AI Chatbot (Chat GPT) ResponsesInternational Journal of Innovative Science and Research TechnologyОценок пока нет
- Lesson #4: The Polymer Materials and Products Learning ObjectivesДокумент14 страницLesson #4: The Polymer Materials and Products Learning ObjectivesMartin John RamirezОценок пока нет
- PVC CompoundingДокумент3 страницыPVC CompoundingAndrek Queck100% (1)
- Loxiol® A 2 PDFДокумент24 страницыLoxiol® A 2 PDFXuân Giang NguyễnОценок пока нет
- Universal Polyamide Overmold Thermoplastic Elastomer PDFДокумент11 страницUniversal Polyamide Overmold Thermoplastic Elastomer PDFjasonОценок пока нет
- Polymerization HP enДокумент13 страницPolymerization HP enDr BNBОценок пока нет
- Emulsion Polymerization PDFДокумент13 страницEmulsion Polymerization PDFprabhat singhОценок пока нет
- Order Form: Shipping Sub-Total Sub-TotalДокумент1 страницаOrder Form: Shipping Sub-Total Sub-TotalTheodore LiwonganОценок пока нет
- Pneumatic Seal Catalogue 2022Документ141 страницаPneumatic Seal Catalogue 2022Septyan kurnia ardiansyahОценок пока нет
- Rubber, 3. Synthetic 1Документ146 страницRubber, 3. Synthetic 1John Patrick DagleОценок пока нет
- Sizing Chemicals - Sizing of Warp Yarns, Sizing Chemicals For Textile WeavingДокумент2 страницыSizing Chemicals - Sizing of Warp Yarns, Sizing Chemicals For Textile WeavingTholkappiyan GanesanОценок пока нет
- Textile FibreДокумент34 страницыTextile Fibrekavi RAJ SenОценок пока нет
- 95 731 01 SilanesSelectorGuideДокумент4 страницы95 731 01 SilanesSelectorGuideAelya SanОценок пока нет
- PDFДокумент2 страницыPDFAndhdОценок пока нет
- Fox Equation For Polymer Blend TGДокумент6 страницFox Equation For Polymer Blend TGchiuchan888Оценок пока нет
- Hall Wise List of Exhibitiors Hiplex HyderabadДокумент7 страницHall Wise List of Exhibitiors Hiplex HyderabadLakshay UniplarОценок пока нет
- Public Expose 2019 (ADMG) PT POLYCHEM INDONESIA TBKДокумент32 страницыPublic Expose 2019 (ADMG) PT POLYCHEM INDONESIA TBKFauzan ShodiqОценок пока нет
- CE336 12 Polymer CompositesДокумент34 страницыCE336 12 Polymer CompositesAmit karОценок пока нет
- Understanding ESCRДокумент9 страницUnderstanding ESCRimru2Оценок пока нет
- Baoli: Dry-Cut Strand Pelletizer For Compounding and RecyclingДокумент2 страницыBaoli: Dry-Cut Strand Pelletizer For Compounding and Recyclingrong duОценок пока нет
- Glossary of Basic Terms in Polymer ScienceДокумент25 страницGlossary of Basic Terms in Polymer ScienceDownload_from_ScribdОценок пока нет
- Iso 1874 1 2010 en FR PDFДокумент8 страницIso 1874 1 2010 en FR PDFShilpa shriОценок пока нет
- PolymerДокумент15 страницPolymerspiraldaoОценок пока нет
- Project CHEMISTRYДокумент9 страницProject CHEMISTRYNishant NischayaОценок пока нет
- Arlanxeo TSR Product BrochureДокумент16 страницArlanxeo TSR Product BrochureErwin ErwinОценок пока нет
- 1 s2.0 S2772397621000319 MainДокумент13 страниц1 s2.0 S2772397621000319 MainJOSHUA MEDRANOОценок пока нет