Академический Документы
Профессиональный Документы
Культура Документы
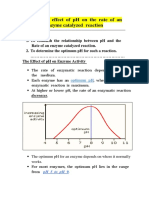
Effect of PH On Solvent Flux During Stirred Ultrafiltration of Proteins
Загружено:
Roxana ElenaИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Effect of PH On Solvent Flux During Stirred Ultrafiltration of Proteins
Загружено:
Roxana ElenaАвторское право:
Доступные форматы
COMMUNICATIONS TO THE EDITOR
Effect of pH on Solvent Flux during Stirred Ultrafiltration of Proteins
INTRODUCTION
Membrane ultrafiltration (UF) is a simple and effective process of concentration, fractionation, and separation of macromolecular solutions. Although separation is primarily
affected by the macrosolute size, v i s - h i s the membrane pore size distribution at a given
condition of stirring, macrosolute solution behavior as well as the solute-membrane interactions are of considerable importance in UF system performance. It is known that pH of
the solution significantly influences the solution conformations of protein molecules which
are dipolar. For example, the solubility of proteins is, in general, minimum at the isoelectric
point (IEP) and the agglomerating tendency is maximum. In addition, it has been reported
that protein adsorption on synthetic surfaces. and on surfaces of both cation exchange
and neutral polymer membranes3 increases as the IEP is approached. One would therefore
expect the solvent flux in protein ultrafiltration to be a strong function of solution pH.
Vilker, Smith, and Colton4 have observed that the UF flux of albumin solutions through a
total retention membrane in an unstirred batch cell increases at pH levels above the IEP.
We have therefore investigated the UF flux behavior of a number of different protein
solutions in a stirred batch cell with membranes of widely different permeabilities under
varying pH conditions. Fluxes were measured in two ways: 1) time-dependent initial transient flux achieving rapidly a steady state over a period of 0-15 mid-; and 2) steady-state
measurements over a period of 1 h., We report the results in this communication.
MATERIALS AND METHODS
Suspending Media
All the protein solutions of this study were prepared in appropriate buffer solutions made
from stock solutions of 0.1M citric acid and 0.2M disodium phosphate in double-distilled
water according to Table I.9
Solutes
The proteins investigated were bovine serum albumin (BSA, Cohn fraction V from V.P.
Chest Research Institute, New Delhi), ovalbumin (Wilson), hemoglobin (EM), and egg
albumin (Wilson). The last three solutes were supplied by Chempure Ltd., Bombay. Properties of these proteins relevant for this study are given in Table 11.
UF membranes
The membranes used were anisotropic synthetic membranes Diaflo PM30, XMIOOA, and
XM300 of Amicon Corp. (USA) with molecular weight cutoffs of 3 x lo4, 10 x lo4, and
30 x lo4, respectively. While PM30 is based on an aromatic polymer, the other two are
based on a substituted olefin polymer.
Biotechnology and Bioengineering, Vol. XXIII, Pp. 1873-1880 (1981)
0 1981 John Wiley & Sons, Inc.
CCC 0006-3592181/081873-08$01.OO
1874
BIOTECHNOLOGY AND BIOENGINEERING VOL. XXIII (1981)
TABLE I
McIlvain's Citric Acid-Phosphate Buffer"
PH
X
3.0
79.45
4.6
53.25
4.8
50.70
6.0
36.85
6.8
22.75
8.0
2.75
mL of O.IM citric acid (21 g C6H*07.H20/L)+ (100 0.2M disodium phosphate (35.6 g Na2HPO4.2H2O/L).
X)
mL of
Experiments were conducted in a batch system employing a magnetically stirred and
baffled 250-mL acrylic plastic U F cell over a pH range 3-8. A buffer solution reservoir was
always connected to the U F cell and maintained at the same pressure to replenish any
solvent lost from the cell during ultrafiltration conducted at a constant stirring speed of 800
rpm. Identical vortex-free stirring conditions thus were maintained in each experiment.
The initial time experiments were carried out with 0.5% protein solution for 15 min starting
with flux data at 0.5 min. The steady-state experiments were conducted with 0.25% protein
solutions for 1 h with flux data being presented at 6-min intervals except for the data at 3
min from start. The slope of the plot of the ultrafiltrate volume versus time of filtration was
utilized to calculate U F flux at any instant of time. An effective applied pressure of 1.38
X lo5 Pa (20 psig) was used in all experiments. The details of the experimental system and
procedure are available in a thesis by Swaminathan.' The initial time and steady-state
ultrafiltration procedures are described elsewhere.'.'
RESULTS
The results of initial time ultrafiltration studies of BSA and ovalbumin solutions through
PM30 membranes are shown in Figure I . In this, as well as in Figures 2 , 3, and 4, the
variation of filtrate volume with time has been shown and the steady-state ultrafiltration
rate achieved has been indicated by its value in each case. The steady-state ultrafiltration
of a 0.25% BSA solution through a PM30 membrane is given in Figure 2. Whereas these
results represent the behavior of an almost total rejection membrane for the two solutes
BSA and ovalbumin, we show in Figures 3 and 4 the steady-state ultrafiltration of several
solutes through membranes XMIOOA and XM300 which are quite open. Figure 3 indicates
the filtration rate of BSA and egg albumin solutions through XMIOOA membranes. The
ultrafiltration rates of a hemoglobin solution through an XM300 membrane are shown in
Figure 4. The pH of the solution was varied in each case from 3.0-8.0 with an intermediate
data point corresponding to the IEP of the protein.
It can be observed from these figures that the rate of ultrafiltration decreases with time
and reaches a steady value due to the dynamically forming polarized layer of proteins. It
is further evident that although concentration polarization takes place at all pH values
studied resulting in a substantially reduced steady-state flux. the extent of flux reduction
due to concentration polarization varies significantly with solution pH. One further notes
in each figure that the flux value at the IEP pH is almost always lower than those at other
TABLE I1
Properties of Solutes
Solute
BSA
Molecular weight
lsoelectric pH
69,000
4.8
Hemoglobin Ovalbumin
67,000
6.8
45,000
4.6
Egg albumin
44,000
4.6
1875
COMMUNICATIONS TO THE EDITOR
50
45
40
35
I,
30
:
3
-l
t
2
25
;
3
>
>
w
I-
a 4
a
20 w
IQ
LL
3
-LL
LL
15
10
I 0
2.0
3-0
4 . 0
T I M E , MINUTEE
5 0
6.0
7 0
Fig. 1. Initial time ultrafiltration of BSA and ovalbumin through PM 30 membrane as
a function of pH. Ce = 0.05%, pressure = 20 psig. pH 3.0: (-)BSA, (A) ovalbumin; pH 8.0:
(o)BSA, ( 7 ) ovalbumin; pH 6.0: (-I)BSA,)(. ovalbumin; pH 4.8:(o)BSA, ( 0 ) ovalbumin.
pH values. This behavior becomes clearer when the steady-state UF flux for both steadystate experiments and initial time experiments are plotted against pH for each case. As
Figures 5(a) and 5(b) show, the solvent flux reaches a minimum at the isoelectric pH in
each case and increases at pH values on both sides of the isoelectric point.
DISCUSSION AND CONCLUSIONS
Although such results have not been previously reported in literature, related behavior
has been observed in several investigations. In a study on UF of starch factory effluents
BIOTECHNOLOGY AND BIOENGINEERING VOL. XXlIl(1981)
1876
180
160
140
120
z
_I
100
0
>
w
I-
-'i8C
LL
6C
4C
2c
__
12
-18
T I M E , MINUTES
I
30
I
36
I
42
Fig. 2. Steady-state ultrafiltration results for BSA through PM 30 membrane as afunction
of pH. CB = 0.25%. pressure = 20 psig. pH: (T3.0: (43.0; (0)4.8.
through noncellulosic membranes, Fane and Fell" had observed a minimum in flux at pH
4-5 with a maximum at pH 3. They had also pointed out that the minimum in flux that
occurred at the IEP pH was probably due to agglomeration and lowered diffusivities. Friedli
et aI.l3 have reported a sharp reduction in the rate of filtration of dilute albumin solution
as the IEP was reached. ForbesI4 had also observed similar events with proteinaceous
solutions. Chemical treatment of cheese whey to reduce fouling of U F membranes was
studied by Lee and Mersonls and Hayes, Dunkerley, and Muller," wherein acidification
of whey was found to increase the ultrafiltration rates. This was presumably due to pH-
I877
COMMUNICATIONS TO THE EDITOR
360
180
3 20
160
280
140
24c
w2 200
100
3
J
0
>
0
>
180
80 w
4
IX
IJ
I20
60
80
40
40
20
0
0
I2
I8
LL
24
TIME, MINUTES
30
36
42
48
Fig. 3. Steady-state ultrafiltration results for BSA and egg albumin through XMIOOA
membrane as a function of pH. CB = 0.25%. pressure = 20 psig. pH BSA: ( ~ ) 8 . 0(a)3.0:
;
;
(*)4.6 (isoelectric pH).
(0)4.8 (isoelectric pH). pH egg albumin: ( ~ ) 3 . 0(~)8.0;
induced changes in the state of P-lactoglobulin which constitutes about 50% of the whey
protein. The marked effect of pH on the association-dissociation behavior of P-lactoglobulin
is well k n o ~ n . Lee
~ ~and
~ Merson had also shown, from electron micrograph studies of
the fouling deposits on the membrane, that the form of the deposits is strongly dependent
upon pH. Addition of NaOH destabilized the protein, causing thick deposits of a granular
matrix to form a nonporous fouling layer. On the other hand, acidification stabilized the
proteins and only light deposits were found. Thus it would appear that ultrafiltration rates
will be high if the proteins are maintained in a dispersed state by controlling the pH. This
will not only reduce the amount of deposit on the membrane surface due to polarization but
it will also reduce the protein adsorption on membrane s ~ r f a c e sIn
. ~ addition, the deposits
will be much more porous.
The concentration profile measurements of Vilker, Smith, and Colton4 with an unstirred
batch UF cell provide direct evidence in this connection. For BSA ultrafiltration in 0.15%
saline through a total retention membrane, the membrane surface concentration reached the
highest value of 40.2 g % at a pH of 4.5 compared to 31.5 g % at pH 5.4 and 24.9% at pH
1878
BIOTECHNOLOGY AND BIOENGINEERING VOL. XXIII (1981)
---
- _
240
I
12
16
30
24
T I M E , MINUTES
36
42
Fig. 4. Steady-state ultrafiltration results for hemoglobin through XM300 membrane as
a function of pH. CB = 0.25%, pressure = 20 psig. pH: (0)3.0; ( ~ ) 8 . 0(0)6.7.
:
7.4. Although the osmotic pressure at a given concentratlon is lowered as pH approaches
the IEP, a higher wall concentration will ensure a greater reduction in flux. Further, the
diffusion coefficients are lowered significantly when the IEP is approached leading to a
lower flux. In addition, an increase in diffusion potential as the solution goes away from
IEP will increase U F flux significantly as shown recently by Dileo, Smith, and Colton'' for
an unstirred batch ultrafiltration of a BSA solution. Thus we conclude that the results of
these two investigation^^.'^ on unstirred batch U F lend support to our observations in stirred
COMMUNICATIONS TO THE EDITOR
1879
(a)
20
XMIOOA]
16
ISOELECTRIC
0
Y
EGG ALBUMIN
X M IOOA
0
2
PH
( b)
OVALBUMIN
I
8
PH
Fig. 5 .
pressure
Effect of pH on ultrafiltration flux. (a) Steady-state experiments: Cg = 0.2576,
20 psig. (b) Initial time experiments: Cs = 0.0576, pressure = 20 psig.
batch UF of protein solutions. Other conditions remaining constant, the stirred ultrafiltration
flux of a protein solution will be a minimum at the isoelectric pH and will increase as the
pH changes on both sides of the IEP.
1880
BIOTECHNOLOGY AND BIOENGINEERING VOL. XXlIl(198l)
References
I . A. D. McLaren, J. Phys. Chern., 58, 129 (1954).
2. E . I. F. Pearce and B. G. Bibby, Arch. Oral B i d . , 11, 329 (1966).
3. W. J . Dillman and J. F. Miller, J. Colloid Interface Sci., 44,221 (1973).
4. V. L. Vilker, K. A. Smith, and C. K. Colton, paper No. 102e presented at the 68th
Annual AIChE Meeting at Los Angeles, November 16-20. 1975.
5. T . Swaminathan, Ph.D. thesis. 1.1.T.. Kanpur, India, 1978.
6. T. Swaminathan, M. Chaudhuri, and K. K. Sirkar, paper No. 8b presented at the
86th National Meeting of AlCHE, Houston, Texas, April 2, 1979.
7. T. Swaminathan, M. Chaudhuri, and K. K. Sirkar, J. App!. Polym. Sci., 24, 1581
(1979).
8. T. Swaminathan, M. Chaudhuri, and K. K. Sirkar, J. Colloid Interface Sci., 76(2),
573 (1980).
9. H . A. Sober, Handbook of Biochemistry (CRC, Cleveland, OH, 1968).
10. E. J. Cohn, L. E. Strong, W. L. Hughes, Jr., D. J. Mulford, J. N. Ashworth, M.
Melin, and H. L. Taylor, J . Am. Chem. Soc., 68, 459 (1946).
11. J . S. Fruton and S. Simmonds, General Biochemistry (Asia Publishing House, New
Delhi, 1960), p. 102.
12. A. G. Fane and C. J. D. Fell, AlChE Symp. Ser. 73(163), 198 (1977).
13. H . Friedli, E. Fournier, T. Volk, and P. Kistler, Vox Sang., 31, 283 (1976).
14. F. Forbes, Chem. Eng. (London),257, 29 (1972).
15. D. N. Lee and R. L. Merson, J. Food Sci.,41, 778 (1976).
16. J. F. Hayes. J. A. Dunkerley. and L. L. Muller, Aust. J. Dairy Technol., 29, 132
( 1974).
17. D. N. Lee and R. L . Merson, J. Dairy Sci., 58, 1423 (1975).
18. H. A. McKenzie, Milk Protein I1 (Academic, New York, 1971).
19. A. J. Dileo, K. A. Smith, and C. K. Colton, paper No. 126a presented at the 71st
Annual AIChE Meeting at Miami, November 16, 1978.
T. SWAMINATHAN*
M. CHAUDHURI
K. K. SIRKAR
Indian Institute of Technology
Kanpur-208016, U.P., India
Accepted for Publication March 12, 1981
* Present address: National Environmental Engineering Research Institute, Nagpur440020, India.
r Correspondence should be sent to: Department of Chemistry and Chemical Engineering,
Stevens Institute of Technology, Hoboken, NJ 07030.
Вам также может понравиться
- Polyphenol Oxidase Activity of Bananas 2Документ5 страницPolyphenol Oxidase Activity of Bananas 2Victor Nyarugwe100% (3)
- MSDS Strata Bond W S Comp A 2020-09-03 enДокумент7 страницMSDS Strata Bond W S Comp A 2020-09-03 encarlos_salas80Оценок пока нет
- A Geoscientist's Guide To PetrophysicsДокумент363 страницыA Geoscientist's Guide To PetrophysicsGunturОценок пока нет
- Salt Spray TestДокумент13 страницSalt Spray TestSreedhar Patnaik.M100% (1)
- Precipitation ChapterДокумент12 страницPrecipitation ChapterMaricica Gorceag50% (2)
- Liposome EvaluationДокумент32 страницыLiposome EvaluationSajesh Joseph100% (1)
- Ozone Layer DepletionДокумент32 страницыOzone Layer DepletionroshanjsachanОценок пока нет
- 4.10 Shaft SealingДокумент11 страниц4.10 Shaft SealingSandi AslanОценок пока нет
- DGCA Meteorology Question PaperДокумент4 страницыDGCA Meteorology Question PaperKrithika R68% (28)
- HPA Aerobic Colony CountДокумент14 страницHPA Aerobic Colony CountHMVMCОценок пока нет
- PID NotesДокумент40 страницPID Notesmegha ingulkarОценок пока нет
- Life Cycle Assessment PneuДокумент44 страницыLife Cycle Assessment PneuatpontesОценок пока нет
- UOP-Mercury-Removal-From-Natural-Gas-and-Liquid-Streams-Tech-Paper 2 PDFДокумент9 страницUOP-Mercury-Removal-From-Natural-Gas-and-Liquid-Streams-Tech-Paper 2 PDFPedraza Velandia JhonОценок пока нет
- Determination of Protein Content SpectrophotometricallyДокумент10 страницDetermination of Protein Content SpectrophotometricallyTsabit AlbananiОценок пока нет
- Foam Separation of ProteinsДокумент9 страницFoam Separation of ProteinsHaile GetachewОценок пока нет
- Layer-By-Layer Films of Poly (O-Ethoxyaniline), Chitosan and Chitosan-Poly (Methacrylic Acid) Nanoparticles and Their Application in An Electronic TongueДокумент9 страницLayer-By-Layer Films of Poly (O-Ethoxyaniline), Chitosan and Chitosan-Poly (Methacrylic Acid) Nanoparticles and Their Application in An Electronic TongueCihan AykasОценок пока нет
- Paper 1 Bio 1 LabДокумент4 страницыPaper 1 Bio 1 Labheidia92Оценок пока нет
- Determining The Concentration of Protein On Egg Albumin Through Lowry MethodДокумент6 страницDetermining The Concentration of Protein On Egg Albumin Through Lowry MethodMuslimah Anggun100% (5)
- Effect of PH On Enzyme ActivityДокумент12 страницEffect of PH On Enzyme ActivityAb AbОценок пока нет
- Ref (Ellman - 1961)Документ9 страницRef (Ellman - 1961)Tasso SalesОценок пока нет
- Penentuan Kadar Protein Dalam Albumin Telur Melalui Metode LowryДокумент7 страницPenentuan Kadar Protein Dalam Albumin Telur Melalui Metode LowryKadek Anggra SupraptaОценок пока нет
- BioTek Instruments, Inc., P.O. Box 998, Highland Park, Winooski, Vermont 05404-0998 USAДокумент6 страницBioTek Instruments, Inc., P.O. Box 998, Highland Park, Winooski, Vermont 05404-0998 USAarnaud.fillaudeau44Оценок пока нет
- tmp1B1 TMPДокумент6 страницtmp1B1 TMPFrontiersОценок пока нет
- An Experiment in Enzyme Characterization: Banana Polyphenoloxidase Michael C. Archer and James K. PalmerДокумент3 страницыAn Experiment in Enzyme Characterization: Banana Polyphenoloxidase Michael C. Archer and James K. PalmerThu HàОценок пока нет
- Exercise 5.1 & 5.2 Techniques For Protein Analysis Protein Isolation & Determination of Protein Concentration by SpectrophotometryДокумент7 страницExercise 5.1 & 5.2 Techniques For Protein Analysis Protein Isolation & Determination of Protein Concentration by SpectrophotometryJulie Ann Estaras FelicesОценок пока нет
- Lysozyme On Apatites: A Model of Protein Adsorption Controlled by Electrostatic InteractionsДокумент17 страницLysozyme On Apatites: A Model of Protein Adsorption Controlled by Electrostatic InteractionsOumaima BenОценок пока нет
- Jarzebski 1989 PDFДокумент6 страницJarzebski 1989 PDFblooom_00Оценок пока нет
- Research SampleДокумент11 страницResearch SamplesuryasanОценок пока нет
- Hildegarde Esther Allen: City of Medical AND Division OF OFДокумент7 страницHildegarde Esther Allen: City of Medical AND Division OF OFJulien Patrick CebrianОценок пока нет
- SPM Operational DefinitionДокумент6 страницSPM Operational DefinitionMark CwmОценок пока нет
- BIOL2016 Sea Anemone ReportДокумент8 страницBIOL2016 Sea Anemone ReportZahra PerwinОценок пока нет
- Vol. 7I Human Seminal Acid Phosphatase 233: TransportДокумент10 страницVol. 7I Human Seminal Acid Phosphatase 233: TransportAlishba KaiserОценок пока нет
- The Effect of Protein Protein and Protei PDFДокумент12 страницThe Effect of Protein Protein and Protei PDFRoxana ElenaОценок пока нет
- Test Instructions For Measuring The Microbial Metabolic Activity in Water Samples - ObstДокумент3 страницыTest Instructions For Measuring The Microbial Metabolic Activity in Water Samples - ObstgotcanОценок пока нет
- PH and Salt Effects On Chiral Separations Using Affinity UltrafiltrationДокумент6 страницPH and Salt Effects On Chiral Separations Using Affinity UltrafiltrationJosé CatalánОценок пока нет
- Lecture 4: Basic Chromatography: HPLC (RP & IEC) & Capillary ElectrophoresisДокумент21 страницаLecture 4: Basic Chromatography: HPLC (RP & IEC) & Capillary Electrophoresisaamer_shahbaazОценок пока нет
- An Experiment in Enzyme Characterization-Banana PolyphenoloxidaseДокумент3 страницыAn Experiment in Enzyme Characterization-Banana PolyphenoloxidaseKristiani SuhermanОценок пока нет
- J. Biol. Chem.-2000-Avilan-9447-51Документ5 страницJ. Biol. Chem.-2000-Avilan-9447-51Fatma ZorluОценок пока нет
- Peroxidase Activity of Cytochrome CДокумент4 страницыPeroxidase Activity of Cytochrome CNigel LoewОценок пока нет
- Full Lab Report On: Exercise No. 4 Protein DenaturationДокумент8 страницFull Lab Report On: Exercise No. 4 Protein DenaturationElaine FaloОценок пока нет
- 1 s2.0 0168365989900734 MainДокумент7 страниц1 s2.0 0168365989900734 MainJôsy SouzaОценок пока нет
- Of Three Acid: Isolation Phosphatases From Wheat GermДокумент6 страницOf Three Acid: Isolation Phosphatases From Wheat GermBarry WhiteОценок пока нет
- GI DissolutionДокумент8 страницGI DissolutionZainab Eassa JassimОценок пока нет
- Comparison of Tryptophan Interactions To Free and Grafted BSA ProteinДокумент7 страницComparison of Tryptophan Interactions To Free and Grafted BSA ProteinangeljosechuquiureОценок пока нет
- Practical Biochemistry (STBP2012) : Experiment 3: Purification & Characterization Of α -Lactalbumin, A Milk ProteinДокумент20 страницPractical Biochemistry (STBP2012) : Experiment 3: Purification & Characterization Of α -Lactalbumin, A Milk ProteinfaizzudDENT100% (2)
- Department of Chemical & Biomolecular Engineering The National University of SingaporeДокумент14 страницDepartment of Chemical & Biomolecular Engineering The National University of Singaporerussell_mahmoodОценок пока нет
- Mechanism of Cytoplasmatic PH Regulation in Hypoxic Maize Root Tips and Its Role in Survival Under HypoxiaДокумент5 страницMechanism of Cytoplasmatic PH Regulation in Hypoxic Maize Root Tips and Its Role in Survival Under HypoxiaGabriel ZahariaОценок пока нет
- Lab ReportДокумент7 страницLab ReportJavier Fuentes100% (2)
- Aiba 1968 Kinetics of Product Inhibition in Alcohol FermentationДокумент20 страницAiba 1968 Kinetics of Product Inhibition in Alcohol Fermentationrishu2525Оценок пока нет
- Protein Synthesis in Avocado Fruit Tissue PDFДокумент4 страницыProtein Synthesis in Avocado Fruit Tissue PDFdr.sameer sainiОценок пока нет
- Lab 10Документ11 страницLab 10riskrulerОценок пока нет
- Cell MolecreportДокумент8 страницCell Molecreportapi-586722985Оценок пока нет
- 695 FullДокумент3 страницы695 FullZoya ShaikhОценок пока нет
- PROTOCOL Extraction and Determination of ProlineДокумент5 страницPROTOCOL Extraction and Determination of ProlineJorge GonzalezОценок пока нет
- Fluorescence Quenching of Albumin. A Spectrofluorimetric ExperimentДокумент3 страницыFluorescence Quenching of Albumin. A Spectrofluorimetric Experimentsujay85Оценок пока нет
- Investigation of Acetone-Butanol-Ethanol (ABE) Fermentation by FluorescenceДокумент4 страницыInvestigation of Acetone-Butanol-Ethanol (ABE) Fermentation by FluorescencechelogkОценок пока нет
- Journal of Chromatogmphy, 232 335-343 Biomedical Applkations ElsevierДокумент9 страницJournal of Chromatogmphy, 232 335-343 Biomedical Applkations ElsevierHuynh Thanh PhongОценок пока нет
- Protein Chromatography: Ion Exchange Chromatography AimsДокумент4 страницыProtein Chromatography: Ion Exchange Chromatography AimskapilphysioОценок пока нет
- PROTOCOL Extraction and Determination of ProlineДокумент4 страницыPROTOCOL Extraction and Determination of ProlineclventuriniОценок пока нет
- Jourraal of Chromatography, 421 (1988) 257-267 Biomedical ApplicationsДокумент11 страницJourraal of Chromatography, 421 (1988) 257-267 Biomedical Applicationsarunkumar76Оценок пока нет
- Cooperative Effects in Binding by Bovine Serum Albumin. The Binding 1 - Aniline-8-Naphthalenesulfonate. Fluorimetric TitrationsДокумент8 страницCooperative Effects in Binding by Bovine Serum Albumin. The Binding 1 - Aniline-8-Naphthalenesulfonate. Fluorimetric TitrationsThales FreireОценок пока нет
- Cloud-Point Extraction For The Determination of The Free Fraction of Antiepileptic Drugs in Blood Plasma and SalivaДокумент5 страницCloud-Point Extraction For The Determination of The Free Fraction of Antiepileptic Drugs in Blood Plasma and SalivaDan AndonieОценок пока нет
- Electrophoresis and Fractionation of Wheat GlutenДокумент14 страницElectrophoresis and Fractionation of Wheat GlutensecucaОценок пока нет
- Fodera Weert VestergaardДокумент7 страницFodera Weert VestergaardFatima Herranz TrilloОценок пока нет
- 813 FullДокумент11 страниц813 Fullmicrobiolgy16Оценок пока нет
- Comparison of Acetylcholinesterase by Michel and Ellman MethodsДокумент2 страницыComparison of Acetylcholinesterase by Michel and Ellman MethodschanОценок пока нет
- MillatДокумент22 страницыMillatMARIA DE LA PAZ GRAJEDA PINEDAОценок пока нет
- Catalasas 2Документ13 страницCatalasas 2Abel Franz Gutierrez EscaleraОценок пока нет
- Polyurethane Immobilization of Cells and Biomolecules: Medical and Environmental ApplicationsОт EverandPolyurethane Immobilization of Cells and Biomolecules: Medical and Environmental ApplicationsОценок пока нет
- Biochemical Factors Concerned in the Functional Activity of the Nervous System: First International Meeting of the International Society for Neurochemistry, Strasbourg, 1967От EverandBiochemical Factors Concerned in the Functional Activity of the Nervous System: First International Meeting of the International Society for Neurochemistry, Strasbourg, 1967D. RichterОценок пока нет
- Journal of Water Process Engineering: SciencedirectДокумент8 страницJournal of Water Process Engineering: SciencedirectRoxana ElenaОценок пока нет
- Whole PDFДокумент279 страницWhole PDFRoxana ElenaОценок пока нет
- The Effect of Protein Protein and Protei PDFДокумент12 страницThe Effect of Protein Protein and Protei PDFRoxana ElenaОценок пока нет
- Ultrafiltration: Boon or Bane?: R, Vol 51, NR 2, 2009, P 613-625Документ13 страницUltrafiltration: Boon or Bane?: R, Vol 51, NR 2, 2009, P 613-625Roxana ElenaОценок пока нет
- Dupa Ce Ne-Am Indragostit - Todd Anna - PDДокумент18 страницDupa Ce Ne-Am Indragostit - Todd Anna - PDRoxana ElenaОценок пока нет
- 1602 07467Документ54 страницы1602 07467Roxana ElenaОценок пока нет
- Chemical Engineering Science: Mylène Wang, Sourav Mondal, Ian M. GriffithsДокумент8 страницChemical Engineering Science: Mylène Wang, Sourav Mondal, Ian M. GriffithsRoxana ElenaОценок пока нет
- Study On The Stabilisation of Collagen With Vegetable Tannins in The Presence of Acrylic PolymerДокумент7 страницStudy On The Stabilisation of Collagen With Vegetable Tannins in The Presence of Acrylic PolymerRoxana ElenaОценок пока нет
- High Performance Dialysis GuideДокумент28 страницHigh Performance Dialysis GuideRoxana ElenaОценок пока нет
- Characteristics and Uses of CollagenДокумент6 страницCharacteristics and Uses of CollagenRoxana ElenaОценок пока нет
- Dialysis Tubing A. Procedure: Stockinger LabДокумент1 страницаDialysis Tubing A. Procedure: Stockinger LabRoxana ElenaОценок пока нет
- Estimarea Procesului de Poliare Termica in CanadaДокумент5 страницEstimarea Procesului de Poliare Termica in CanadaRoxana ElenaОценок пока нет
- ShoppingmallДокумент102 страницыShoppingmallNiro ThakurОценок пока нет
- Astm e 1131 TgaДокумент6 страницAstm e 1131 TgaClaudia Barrera100% (1)
- Pencinta Alam: June 2011Документ18 страницPencinta Alam: June 2011Timothy LimОценок пока нет
- Hydrology Analysis and Modelling For Klang River BДокумент7 страницHydrology Analysis and Modelling For Klang River BWhen2Meets2Оценок пока нет
- RET Problem SolvingДокумент13 страницRET Problem SolvingJayanath Nuwan SameeraОценок пока нет
- Zimbabwe Water PumpДокумент39 страницZimbabwe Water PumpTiago RamosОценок пока нет
- Md-89 Offshore Windwills 1Документ14 страницMd-89 Offshore Windwills 1Roger Hazim100% (1)
- Corsi 2001Документ8 страницCorsi 2001CostelCosОценок пока нет
- Indar Generadores PDFДокумент20 страницIndar Generadores PDFSridhar TholasingamОценок пока нет
- CE 333-05-Septic Tank SystemДокумент41 страницаCE 333-05-Septic Tank SystemEngr.kawsar ahmedОценок пока нет
- ARCHITECTURAL THESIS-Khushi RathodДокумент2 страницыARCHITECTURAL THESIS-Khushi RathodKhushi RathodОценок пока нет
- Transportation Engineering-II: DR RawidДокумент55 страницTransportation Engineering-II: DR RawidJunaid MahsudОценок пока нет
- Sources of Water: By: Aarya VoraДокумент11 страницSources of Water: By: Aarya VorahiteshvОценок пока нет
- NSTPДокумент2 страницыNSTPJed AbadОценок пока нет
- A Guide To The Investigation of Fish KillsДокумент10 страницA Guide To The Investigation of Fish KillsJoselito CortesОценок пока нет
- Automated Hydroponics Greenhouse: Regulation of PH and NutrientsДокумент49 страницAutomated Hydroponics Greenhouse: Regulation of PH and NutrientsarnuОценок пока нет
- AdaptationsДокумент4 страницыAdaptationsJack BarkerОценок пока нет
- Introduction:-: UnitsДокумент8 страницIntroduction:-: UnitsSANJAYОценок пока нет
- VBATДокумент23 страницыVBATIsaias NigthОценок пока нет
- Mock Test 907Документ4 страницыMock Test 907KIMTHOAОценок пока нет