Академический Документы
Профессиональный Документы
Культура Документы
lct8 PDF
Загружено:
SeanMarxAdanzaИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
lct8 PDF
Загружено:
SeanMarxAdanzaАвторское право:
Доступные форматы
gjr-- 1
Unit One Part 8: stereochemistry
Describe the difference between stereoisomers & structural isomers
Nomenclature used for double bonds (cis-trans or E-Z)
Predict conformations of cyclohexanes
Define chirality and recognise stereocentres
Understand enantiomers and diastereoisomers
dr gareth rowlands; g.j.rowlands@massey.ac.nz; science tower a4.12
http://www.massey.ac.nz/~gjrowlan
gjr-- 2
Isomerism
different bond
pattern
structural
isomers
diastereomers
ISOMERS
stereoisomers
same
bond pattern
enantiomers
non-superimposable
mirror images
Structural isomers
OH
OH
(E)-pent-3-en-1-ol
C5H10O
O
HO H
O
cyclopentanol
C5H10O
4-methoxybut-1-ene
C5H10O
3-methylbutan-2-one
C5H10O
(S)-pent-1-en-3-ol
C5H10O
gjr-- 3
Configurational isomerism or stereoisomerism
The spatial arrangement of atoms in a molecule is its configuration
Configurational isomers have the same bonds
Configurational isomers can only be interconverted by breaking a bond
Easiest compounds to see this in are the alkenes...
A
MeO2C
Atom connectivity identical
But different configurations
Known as diastereoisomers
They are different compounds
H
CO2Me
dimethyl fumarate
trans (E)
mp 103C
bp 193C
less stable
steric crowding
E or trans
Z or cis
MeO2C
H
CO2Me
H
dimethyl maleate
cis (Z)
mp 19C
bp 202C
gjr-- 4
E and Z nomenclature
E-alkenes have the highest priority groups on the opposite sides
Z-alkenes have the highest priority groups on the same side
Priority rules
1. Rank atoms attached to alkene in order of decreasing atomic number
atom = Br>Cl>O>N>C>H
Atomic number = 35, 17, 8, 7, 6 & 1
2. If atoms are the same, move along substituent until a difference is found
H2
C
CH3
>
CH3
CH3
>
OH
.3. Multiple bonds are assumed to count as the same number of single bonds
C N
1 Cl
2
H3C
1 CH2CH3
C C
CH3
(Z)-2-chloro-3methylpent-2-ene
N C
1CHO
HO2C 1
C C 2
2
Ph
CH2OH
(Z)-3-hydroxymethyl-4-oxo-2phenylbut-2-enoic acid
C N
N C
N
1CH OH
C2
2
C C 2
1
Br
CH2CH3
(E)-2-bromo-3(hydroxymethyl)
pent-2-enenitrile
H3C
2
H3C
O
2 CH
3
C C
C6H5
(E)-3-methyl-4phenylpent-3-en-2-one
gjr-- 5
Cistrans isomerism in cyclohexanes
Cl
Cl
cis-1,2dichlorocylohexane
(syn)
Cl
Cl
Cl
trans-1,2dichlorocylohexane
(anti)
Cl
Cl
trans-1,2dichlorocylohexane
(anti)
Cl
cis-1,2dichlorocylohexane
(syn)
If two groups on a ring we must specify relative stereochemistry
Cis - both groups are on the same face
Trans - groups are on opposite faces
1Cl
2Cl
2Cl
H
H
both conformations have one axial
substituent & are identical
Me
2Cl
1Cl
2ClH
1Cl
H
bulky group equatorial
favoured
1Cl
one conformation is disfavoured as it has two
axial groups so maximum 1,3-diaxial interactions
H
tBu
Me
H
H
tBu
tert-butyl group
rarely is axial
gjr-- 6
Decalins
H
H
H
H
trans-decalin
H
equatorial, equatorial
ring fusion
H
cis-decalin
equatorial, axial
ring fusion
Another example of stereoisomers comes from fused bicyclic molecules
Like a double bond the substituents could be cis or trans
Like a double bond these two are not interconvertible unless a bond broken
gjr-- 7
Chirality
Mirror image
Left & right hands
Non-superimposable
The most confusing form of stereochemistry
A chiral structure is one that is non-superimposble on its mirror image
Occurs in nature (snails, honeysuckle etc), man-made objects (propellers,
corkscrews etc) and in molecules...
gjr-- 8
Enantiomers
This form of stereoisomerism arises from a lack of symmetry
The molecule & its mirror image are different
The molecule & its mirror image are known as enantiomers
Achiral
Chiral
Mirror
plane
Mirror
plane
rotate
For those of a sick & twisted disposition more info on chirality & stereochemistry
can be found at my website: http://www.massey.ac.nz/~gjrowlan/teaching.html
gjr-- 9
Enantiomers II
Most common form of chirality has a carbon atom with 4 different groups
attached & is called central chirality
Me
Me2N
H
Ph O
O
Et
darvon
painkiller
Me
N
H
O
N
O
H
(R)-thalidomide
(morning sickness)
CO2H
N
NH2
Me
nicotine
toxin / stimulant
L-alanine
mammalian amino acid
Many other forms of chirality including helical, axial & planar
Ph
PPh2
Ph2P
PPh2
(R)-(+)-BINAP
(R)-2-(diphenylphosphino)-1-(2-(diphenyl
phosphino)naphthalen-1-yl)naphthalene
M-[8]helicene
Fe
(R)-2-phenyl-1(diphenylphosphanyl)
ferrocene
gjr-- 10
Physical properties of enantiomers
Enantiomers have identical physical properties such as mp, bp etc
They only differ in their interactions with other chiral entities
H
OH
Ph
CO2H
(R)-(-)-mandelic acid
131-133C
23
[]D
153
light
source
light ()
polariser
sample
cell length l (dm)
plane
polarised light
reading
HO
Ph
CO2H
(S)-(+)-mandelic acid
131-134C
20 +154
[]D
Chiral compounds rotate the plane of polarised light (hence optical isomers)
As most biological systems are chiral, enantiomers can have different effects
O
H
(R)-carvone
spearmint
H
(S)-carvone
caraway
H
(S)-limonene
lemons
H
(R)-limonene
oranges
gjr-- 11
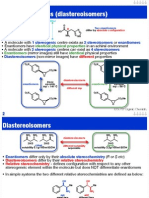
Two chiral centres (diastereoisomers)
Mirror
O
N
HN
O
OH
HO
NH2
NH2
NH
Two enantiomers
differ by absolute configuration
A molecule with 1 stereogenic centre exists as 2 enantiomers
Enantiomers have identical physical properties in an achiral environment
A molecule with 2 stereogenic centres can exist as 4 stereoisomers
Enantiomers (mirror images) still have identical physical properties
Diastereoisomers (non-mirror images) have different properties
O2N
O2N
CO2Me
CO2Me
enantiomers
trans
epoxide
mp = 141C
O2N
diastereoisomers
enantiomers
different mp
cis
epoxide
mp = 98C
O2N
CO2Me
O
CO2Me
O
gjr-- 12
Chirality in our bodies
HO2C
Me
NH2
alanine
HO2C
H
N
HO2C
NH2
phenylalanine
NH2
histidine
Common amino acids are all have the same stereochemistry (L or S)
Aspartame (sweetener) is a dipeptide (two amino acids) - two stereocentres
Potentially 4 compounds (n stereocentres can give 2n diastereoisomers)
O
H2N
N
H
CO2CH3
HO2C
Insulin is made of 2 peptide chains - 1x30 amino acids & 1x21 amino acids
Potentially 2.25 x 1015 diastereoisomers
A relatively small protein is ribonuclease at 124 amino acids or
Potentially 2.13 x 1037 diastereoisomers
gjr-- 13
Chiral drugs
Et
H
N
N
H
OH
OH
Et
OH
H
N
N
H
Et
NH
Me
()-propanolol
-blocker for heart disease
OH
(R,R)-enantiomer
causes blindness
Ethambutol
tuberculostatic (anti-TB)
OH
Me
Et
HN
Me
Me
OH
(+)-propanolol
contraceptive
O
N
O
OH
N
H
N
H
OH
N
S
(S)-timolol
high blood pressure
N
S
(R)-timolol
glaucoma
gjr-- 14
Overview
What have we learnt?
Molecules are three dimensional...and it makes a difference
The arrangement of atoms in space can form new isomers
Such isomers can only be interconverted by breaking bonds
Mirror images can have very different properties
Synthesis of single enantiomers is very important
What's next?
A brief look at spectroscopy in many of its glorious forms
Starting with NMR or nuclear magnetic resonance spectroscopy
An idea of what information this tells us about protons in a molecule
dr gareth rowlands; g.j.rowlands@massey.ac.nz; science tower a4.12
http://www.massey.ac.nz/~gjrowlan
Вам также может понравиться
- Structure of Biological Macromolecules: ChiralityДокумент27 страницStructure of Biological Macromolecules: ChiralityArshaan ShaikhОценок пока нет
- CHM 102 (Stereochemistry)Документ15 страницCHM 102 (Stereochemistry)christdan75Оценок пока нет
- CY1101 Stereochemistry 290920Документ209 страницCY1101 Stereochemistry 290920Adarsh PriyaranjanОценок пока нет
- Stereochemistry Isomer OrganizationДокумент37 страницStereochemistry Isomer OrganizationJelica ŠutovićОценок пока нет
- Coordination Geometries and IsomersДокумент25 страницCoordination Geometries and IsomersAb IrizarryОценок пока нет
- CHEM 210 Chapter 5 Wrap-UpДокумент27 страницCHEM 210 Chapter 5 Wrap-UpTuan NguyenОценок пока нет
- Chm102a Oc-L4-SdДокумент42 страницыChm102a Oc-L4-SdDanish VasdevОценок пока нет
- CM2127 - Organic Chemistry: StereochemistryДокумент58 страницCM2127 - Organic Chemistry: StereochemistryLeslieLooОценок пока нет
- Stereochemistry Arrangement of Atoms in SpaceДокумент59 страницStereochemistry Arrangement of Atoms in SpaceNAGARAJUОценок пока нет
- S.SEETARAM SWAMY, M.Pharm.,: Asst. Professor, Dept. of Pharmaceutical Chemistry, Chilkur Balaji College of PharmacyДокумент46 страницS.SEETARAM SWAMY, M.Pharm.,: Asst. Professor, Dept. of Pharmaceutical Chemistry, Chilkur Balaji College of PharmacyAli Akbar JamshaidiОценок пока нет
- Stereochemistry & Chiral MoleculesДокумент76 страницStereochemistry & Chiral MoleculesDr. Tara WorkmanОценок пока нет
- Organic - Class 7Документ27 страницOrganic - Class 7Sajan Singh LUCKYОценок пока нет
- Stereoisomerism and Biological ActivityДокумент32 страницыStereoisomerism and Biological Activitybruno de jesus fontesОценок пока нет
- S.SEETARAM SWAMY, M.Pharm.,: Asst. Professor, Dept. of Pharmaceutical Chemistry, Chilkur Balaji College of PharmacyДокумент46 страницS.SEETARAM SWAMY, M.Pharm.,: Asst. Professor, Dept. of Pharmaceutical Chemistry, Chilkur Balaji College of PharmacyAVVARI AMMUОценок пока нет
- Ch. 4 - ChiralityДокумент118 страницCh. 4 - ChiralityNora AounОценок пока нет
- StereochemistryДокумент22 страницыStereochemistryVenkataramana KondepaiОценок пока нет
- Stereochemistry and Isomerism in Organic CompoundsДокумент45 страницStereochemistry and Isomerism in Organic Compoundsjustin rodrigoОценок пока нет
- 4 StereoisomersimДокумент65 страниц4 StereoisomersimRayonesh RayanaОценок пока нет
- Stereochemistry: Prof. Dr. Harno Dwi PranowoДокумент45 страницStereochemistry: Prof. Dr. Harno Dwi Pranowoyosita ruzОценок пока нет
- IsomerismДокумент31 страницаIsomerismShofwa AnnisaОценок пока нет
- Organic Chem U-3Документ51 страницаOrganic Chem U-3sinte beyuОценок пока нет
- Stereochemistry (CHEM 2121)Документ150 страницStereochemistry (CHEM 2121)Jasia TasnimОценок пока нет
- CHE275 Chapter7 SlidesДокумент24 страницыCHE275 Chapter7 SlidesAnastasia BudinskayaОценок пока нет
- 13 ChiralityДокумент33 страницы13 ChiralityKazel Lyca SarmientoОценок пока нет
- Chapter-4 StereochemistryДокумент54 страницыChapter-4 StereochemistrytuanijoshuaОценок пока нет
- ICT BHB Sem 2 2Документ59 страницICT BHB Sem 2 2Ayushmaan TripathiОценок пока нет
- CHE-502 (Stereochemistryof Organic Compounds)Документ36 страницCHE-502 (Stereochemistryof Organic Compounds)dasalways4uОценок пока нет
- Dia Stereo IsomerДокумент13 страницDia Stereo IsomerKhairunnisa FadhilahОценок пока нет
- Seven Star Health and Business College: StereochemistryДокумент65 страницSeven Star Health and Business College: StereochemistryHolly TadesseОценок пока нет
- Introduction To StereochemistryДокумент33 страницыIntroduction To StereochemistryApurba Sarker Apu100% (1)
- Stereochemistry PartiiДокумент23 страницыStereochemistry PartiiSharath PavanОценок пока нет
- Chapter5 R1 Compressed PDFДокумент57 страницChapter5 R1 Compressed PDFFran YОценок пока нет
- StereochemistryДокумент78 страницStereochemistryApurba Sarker Apu100% (10)
- NEPHAR 109 - Chapter 5Документ41 страницаNEPHAR 109 - Chapter 5Amirabbas SaffariОценок пока нет
- Importance of StereochemistryДокумент12 страницImportance of StereochemistrySiddarth PalletiОценок пока нет
- Stereo ChemistryДокумент57 страницStereo ChemistryAuliaОценок пока нет
- Stereochemistry Basic Concepts, Useful Notes For StudentsДокумент26 страницStereochemistry Basic Concepts, Useful Notes For StudentsATUL R BENDALE89% (45)
- StereochemisryДокумент34 страницыStereochemisryEluri YadaiahОценок пока нет
- Optical Isomers FINAL 1Документ310 страницOptical Isomers FINAL 1kinzaghaffar108Оценок пока нет
- Types of Isomerism: Structural, Geometric, and OpticalДокумент4 страницыTypes of Isomerism: Structural, Geometric, and OpticalAnonymous t5TDwdОценок пока нет
- Stereochemistry 21medДокумент70 страницStereochemistry 21med蔡秉宏Оценок пока нет
- Stereochemistry: Structural and Geometric Isomerism: Fabio Andrés Castellanos CastilloДокумент176 страницStereochemistry: Structural and Geometric Isomerism: Fabio Andrés Castellanos CastilloNicolasGarciaHernandezОценок пока нет
- STK 1233 Organic Chemistry 1: (Group 3)Документ37 страницSTK 1233 Organic Chemistry 1: (Group 3)Arllen Joy AlbertОценок пока нет
- StereochemistryДокумент44 страницыStereochemistryraghav79Оценок пока нет
- Isomer in Organic ChemistryДокумент111 страницIsomer in Organic ChemistryyenquynhОценок пока нет
- CH 05Документ31 страницаCH 05AbuОценок пока нет
- CH 105-2Документ67 страницCH 105-2Shubham KhokerОценок пока нет
- CH-105 - (2) StereochemistryДокумент66 страницCH-105 - (2) StereochemistryK T Prajwal PrathikshОценок пока нет
- Stereochemistry: If You Can't Find A Mirror Plane, It Doesn't Mean That There Isn't One. Compare Mirror Images!Документ2 страницыStereochemistry: If You Can't Find A Mirror Plane, It Doesn't Mean That There Isn't One. Compare Mirror Images!Sakshi BachhetyОценок пока нет
- Organic Chemistry: TopicsДокумент8 страницOrganic Chemistry: TopicsHritwick MannaОценок пока нет
- Stereochemistry Basic Concepts Useful Notes For StudentsДокумент26 страницStereochemistry Basic Concepts Useful Notes For StudentsReddappaОценок пока нет
- Unit 7 Stereochemistry Lecture NotesДокумент12 страницUnit 7 Stereochemistry Lecture NotesPUNISHERОценок пока нет
- ZXC ZCДокумент26 страницZXC ZCboiroyОценок пока нет
- Organic Chemistry Concepts ExplainedДокумент30 страницOrganic Chemistry Concepts ExplaineddhdfОценок пока нет
- StereokimiaДокумент63 страницыStereokimiaTabitaKristinaОценок пока нет
- Kimia Organik - 4Документ63 страницыKimia Organik - 4Gung AriОценок пока нет
- Stereochemistry: Chemistry in Three Dimensions (Chiral Compound)Документ54 страницыStereochemistry: Chemistry in Three Dimensions (Chiral Compound)yolandОценок пока нет
- Schaum's Easy Outline of Organic Chemistry, Second EditionОт EverandSchaum's Easy Outline of Organic Chemistry, Second EditionРейтинг: 3.5 из 5 звезд3.5/5 (2)
- IsomersДокумент3 страницыIsomersjaiОценок пока нет
- 5b Stereochemistry PostДокумент41 страница5b Stereochemistry Postapi-3767370100% (1)
- Nitrogen Compounds Optical IsomerismДокумент3 страницыNitrogen Compounds Optical IsomerismRaja GaneshОценок пока нет
- Impurity and Stability StudiesДокумент40 страницImpurity and Stability StudiesPreethi PR67% (9)
- 3 SKO3023 Student Note - STEREOCHEMISTRYДокумент94 страницы3 SKO3023 Student Note - STEREOCHEMISTRYKHISHALINNI A/P M.MEGANATHANОценок пока нет
- Pre Ph.D. Exam 2019-20: 9009-Ruhs PHD Exam (Pharmaceutical Chemistry) Question Paper With Answer Key Common SectionДокумент20 страницPre Ph.D. Exam 2019-20: 9009-Ruhs PHD Exam (Pharmaceutical Chemistry) Question Paper With Answer Key Common SectionFarhadz Sailama BarahamaОценок пока нет
- Stereochemistry 22Документ21 страницаStereochemistry 22Ahmed SideegОценок пока нет
- Coordination Chemistry IsomersДокумент22 страницыCoordination Chemistry IsomersAnuragJainОценок пока нет
- E4 StereoisomersДокумент6 страницE4 StereoisomersShaun Martel BantuganОценок пока нет
- Coordination Compound MKAДокумент44 страницыCoordination Compound MKAAubal ShaubalОценок пока нет
- Isomerism One Shot BouncebackДокумент196 страницIsomerism One Shot BouncebackHarishОценок пока нет
- IsomeríasДокумент11 страницIsomeríasVictor Manuel Hernández EstebanОценок пока нет
- Organic Chemistry OpticalДокумент14 страницOrganic Chemistry OpticalPadirikuppam PavithraОценок пока нет
- Stereochemical Aspects of Nucleophilic Substitution ReactionsДокумент20 страницStereochemical Aspects of Nucleophilic Substitution ReactionsKalpa DihingiaОценок пока нет
- CHEM 160 Module 3 Resource 1Документ9 страницCHEM 160 Module 3 Resource 1meyaОценок пока нет
- Tutorial Problems, 2013/2014: Che 153 Organic Chemistry For EngineersДокумент21 страницаTutorial Problems, 2013/2014: Che 153 Organic Chemistry For EngineersIng Rashid BawahОценок пока нет
- Stereochemistry Chapter 5 SummaryДокумент37 страницStereochemistry Chapter 5 SummarySankar AdhikariОценок пока нет
- Smith Ch27 Lecture Edit-AMINOACIDOS 6TAДокумент85 страницSmith Ch27 Lecture Edit-AMINOACIDOS 6TAfabiana perez ruizОценок пока нет
- Problems in IsomerismДокумент5 страницProblems in IsomerismAt Tanwi100% (1)
- 03-12-20 OI A5 EnglishДокумент24 страницы03-12-20 OI A5 EnglishPrabhakar BandaruОценок пока нет
- Stereo PresentationДокумент24 страницыStereo PresentationSuman BalyaniОценок пока нет
- MC Murry 9Документ61 страницаMC Murry 9JoseОценок пока нет
- Homework Booklet (B)Документ34 страницыHomework Booklet (B)Pa GesОценок пока нет
- Full Download Principles of Biochemistry 5th Edition Moran Test BankДокумент21 страницаFull Download Principles of Biochemistry 5th Edition Moran Test Bankzerbaenayatc100% (34)
- 10 20 Organic Chemistry PPT PDFДокумент160 страниц10 20 Organic Chemistry PPT PDFGahyun (Jessica) HanОценок пока нет
- Gamsat Chemistry Sample QuestionsДокумент6 страницGamsat Chemistry Sample QuestionsM S Rahman100% (1)
- GABA Barbiturates2002Документ15 страницGABA Barbiturates2002biqilaadengОценок пока нет
- StereokimiaДокумент32 страницыStereokimiaBrenda GloriaОценок пока нет
- Organic Chemistry Chapter 5 - StereochemistryДокумент89 страницOrganic Chemistry Chapter 5 - StereochemistryGian BanaresОценок пока нет
- Full download book Loose Leaf For Organic Chemistry With Biological Topics Pdf pdfДокумент41 страницаFull download book Loose Leaf For Organic Chemistry With Biological Topics Pdf pdfannie.tetreault806100% (13)