Академический Документы
Профессиональный Документы
Культура Документы
SHC Latent Heat
Загружено:
Naillah SabaОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
SHC Latent Heat
Загружено:
Naillah SabaАвторское право:
Доступные форматы
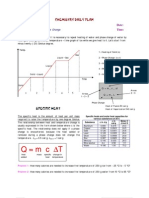
1(a) Define specific heat capacity of a solid.
(b) How much heat is needed to raise the temperature of a block of copper ( weighing 0.5 kg)
from 0C to 100 C ? (for copper, c = 386 J / kg oC)
(c) Explain why the latent heat of vaporization of water is 13 times its latent heat of fusion.
(d) Sketch a cooling curve of water labeling the condensation and freezing point.
2. An aluminium electric kettle has a 1200 E heater and is filled with 1.5 kg of tap water. After
the power is switched on for 10 minutes, the temperature of the water rises from 25 C to 100 C
and the power is automatically cut-off. Assume heat loss to the surroundings.
(a) Find the thermal energy supplied by the 1200 W heater to boil the water.
(b) Find the energy gained by the tap water (specific heat capacity of water is 4200 J/kg C).
(c) The principle of conservation of energy states that energy can only be converted from one
form to another. Give two reasons to account for the difference between the values (a) and (b).
(d) The aluminium kettle weighs 2kg and during the process, its temperature gained is 72 C.
If 117 900 J of energy is used to turn water into steam, find the specific heat capacity of
aluminium.
3. The diagram below shows a solar cooker which is used to heat food. A black cooking pot,
fitted with a lid and enclosed in a clear plastic bag, is placed in an L-shaped shell with silver
lining. The main features of the cooker are:
(1) the shiny interior wall of the cooker
(2) the black surface of the pot
(3) the L-shaped reflector shell.
(i)
Explain how these features help heat the food
efficiently.
(ii)
Explain why the lid on the pot helps to cook the
food more quickly.
(iii) The closed clear plastic bag traps a layer of air
around the pot. Explain how enclosing the cooker inside the
clear plastic bag aids faster cooking.
4 In terms of kinetic theory, explain briefly
(i) how evaporation takes place,
(ii) how evaporation causes a liquid to become cooler
(iii) why the rate of evaporation of a liquid increases when it is heated.
1(a) Define specific heat capacity of a solid.
(b) How much heat is needed to raise the temperature of a block of copper ( weighing 0.5 kg)
from 0C to 100 C ? (for copper, c = 386 J / kg oC)
(c) Explain why the latent heat of vaporization of water is 13 times its latent heat of fusion.
(d) Sketch a cooling curve of water labeling the condensation and freezing point.
2. An aluminium electric kettle has a 1200 E heater and is filled with 1.5 kg of tap water. After
the power is switched on for 10 minutes, the temperature of the water rises from 25 C to 100 C
and the power is automatically cut-off. Assume heat loss to the surroundings.
(a) Find the thermal energy supplied by the 1200 W heater to boil the water.
(b) Find the energy gained by the tap water (specific heat capacity of water is 4200 J/kg C).
(c) The principle of conservation of energy states that energy can only be converted from one
form to another. Give two reasons to account for the difference between the values (a) and (b).
(d) The aluminium kettle weighs 2kg and during the process, its temperature gained is 72 C.
If 117 900 J of energy is used to turn water into steam, find the specific heat capacity of
aluminium.
3. The diagram below shows a solar cooker which is used to heat food. A black cooking pot,
fitted with a lid and enclosed in a clear plastic bag, is placed in an L-shaped shell with silver
lining. The main features of the cooker are:
(1) the shiny interior wall of the cooker
(2) the black surface of the pot
(3) the L-shaped reflector shell.
(i)
Explain how these features help heat the food
efficiently.
(ii)
Explain why the lid on the pot helps to cook the
food more quickly.
(iii) The closed clear plastic bag traps a layer of air
around the pot. Explain how enclosing the cooker inside the
clear plastic bag aids faster cooking.
4 In terms of kinetic theory, explain briefly
(i) how evaporation takes place,
(ii) how evaporation causes a liquid to become cooler
(iii) why the rate of evaporation of a liquid increases when it is heated.
Вам также может понравиться
- Practitioner 'S Guide To Social Network Analysis: Examining Physics Anxiety in An Active-Learning SettingДокумент18 страницPractitioner 'S Guide To Social Network Analysis: Examining Physics Anxiety in An Active-Learning SettingNaillah SabaОценок пока нет
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Female Students With A 'S Have Similar Physics Self-Efficacy As Male Students With C 'S in Introductory Courses: A Cause For Alarm?Документ17 страницFemale Students With A 'S Have Similar Physics Self-Efficacy As Male Students With C 'S in Introductory Courses: A Cause For Alarm?Naillah SabaОценок пока нет
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- An Investigation of The Influence of Emotional Factors On Learning in Physics InstructionДокумент20 страницAn Investigation of The Influence of Emotional Factors On Learning in Physics InstructionNaillah SabaОценок пока нет
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Effect of Self-Efficacy To Mathematical Anxiety On Junior High School Students of YDM Learning Guidance Course MakassarДокумент6 страницThe Effect of Self-Efficacy To Mathematical Anxiety On Junior High School Students of YDM Learning Guidance Course MakassarNaillah SabaОценок пока нет
- EASJPBS 15 79-83 CДокумент5 страницEASJPBS 15 79-83 CNaillah SabaОценок пока нет
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- Mathematics Anxiety and Self-Efficacy: A Phenomenological DimensionДокумент13 страницMathematics Anxiety and Self-Efficacy: A Phenomenological DimensionNaillah SabaОценок пока нет
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Usher Pajares 2008Документ47 страницUsher Pajares 2008Naillah SabaОценок пока нет
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Physics A Level DefinitionsДокумент12 страницPhysics A Level DefinitionsNaillah Saba100% (1)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- General Tips For Paper 4Документ4 страницыGeneral Tips For Paper 4Naillah SabaОценок пока нет
- Dowtherm A PropertiesДокумент2 страницыDowtherm A Propertiesflashiitm100% (1)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- Form 4 Chapter 4: Heat: Understanding Thermal EquilibriumДокумент10 страницForm 4 Chapter 4: Heat: Understanding Thermal EquilibriumbatrisyiaОценок пока нет
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Steam Flow Through Safety Valve Vent PipesДокумент11 страницSteam Flow Through Safety Valve Vent PipesminhphuongphamОценок пока нет
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Iso 80000-5Документ15 страницIso 80000-5Francesc PerezОценок пока нет
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- Class 12 - Physics (Complete Notes)Документ281 страницаClass 12 - Physics (Complete Notes)Sadiq HameedОценок пока нет
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- Technical Data Sheet R404A ENGLISH PDFДокумент4 страницыTechnical Data Sheet R404A ENGLISH PDFjane.yuchen8283100% (1)
- Thermodynamics II - LectureДокумент82 страницыThermodynamics II - LectureJohn Panopio100% (1)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Physics 5058 2009Документ23 страницыPhysics 5058 2009winwarrior100% (8)
- CH 101Документ2 страницыCH 101nagravОценок пока нет
- Heat by William ThomsonДокумент51 страницаHeat by William ThomsonAvanish KumarОценок пока нет
- PHYICSДокумент116 страницPHYICSDebayan BiswasОценок пока нет
- Arjuna For Physics Class-XI - Module-4Документ81 страницаArjuna For Physics Class-XI - Module-4kunal guptaОценок пока нет
- Thermal Physics TemperatureДокумент36 страницThermal Physics TemperatureJames SarkerОценок пока нет
- RAC Shit Notes 5Документ33 страницыRAC Shit Notes 5CERVANTES Adrian Christopher C.Оценок пока нет
- HVAC Quick Load Program LockedДокумент4 страницыHVAC Quick Load Program LockedameerОценок пока нет
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Important Questions Class 9 Science Chapter 1Документ17 страницImportant Questions Class 9 Science Chapter 1aayushchoudhary220Оценок пока нет
- Class X Physics Chapter 11 - Calorimetry Exercise 11 (A)Документ16 страницClass X Physics Chapter 11 - Calorimetry Exercise 11 (A)Isha PatelОценок пока нет
- Review of PCMДокумент19 страницReview of PCMamir khanОценок пока нет
- Heat PPT2Документ38 страницHeat PPT2anne ctОценок пока нет
- Bs 3 2019 Unit 01Документ65 страницBs 3 2019 Unit 01Saran T SОценок пока нет
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- Solution of The Non-Linear Process Model (NEWELL AND LEE Evaporator)Документ7 страницSolution of The Non-Linear Process Model (NEWELL AND LEE Evaporator)Harvey Spec100% (2)
- Heat Engines Lab PDFДокумент24 страницыHeat Engines Lab PDFAnonymous ee5dOjОценок пока нет
- PDFДокумент58 страницPDFSyed MuneebОценок пока нет
- Cameroon General Certificate of Education CompressДокумент41 страницаCameroon General Certificate of Education CompressClara MbouОценок пока нет
- AC 00-6B Aviation WeatherДокумент213 страницAC 00-6B Aviation WeatherSantiago HidalgoОценок пока нет
- Denizinglizce 735 LikişaretsizДокумент143 страницыDenizinglizce 735 LikişaretsizAlper ErenОценок пока нет
- Heat Revision MC TestДокумент28 страницHeat Revision MC TestCiv NortubОценок пока нет
- Change of State (3) BSEEДокумент5 страницChange of State (3) BSEEJoan BalendrezОценок пока нет
- Physics 1 - 1993Документ5 страницPhysics 1 - 1993Alhamdu Mussa SogoleОценок пока нет
- Concentration of Cane Sugar Syrup in An EvaporatorДокумент8 страницConcentration of Cane Sugar Syrup in An EvaporatorJohn Fritz FestejoОценок пока нет