Академический Документы
Профессиональный Документы
Культура Документы
Ethocel HP
Загружено:
abhijit_gothoskar6039Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Ethocel HP
Загружено:
abhijit_gothoskar6039Авторское право:
Доступные форматы
Dow Pharma & Food Solutions
ETHOCEL High Productivity (HP)
Safe. Fast. Efficient.
ETHOCEL HP
Improving Productivity Through Innovation
As our oldest brand, Dow continues to invest in and innovate
with ETHOCEL. Our constant pursuit of production excellence
has helped us out manufacture our competition by producing
ethylcellulose (EC) that is less fibrous, narrower in viscosity,
and tighter in ethoxyl functionality than other ethylcellulose
producers. And now, weve optimized our ETHOCEL offering
for a new dry powder layering process using rotor technology.
This rotor technology allows customers to coat multiparticulates, powders, even mini-tablets without the need
for solvent or aqueous-based polymer systems. Just enough
moisture is added to allow adherence of the polymer to
the substrate but the barrier membrane polymer is added
completely as a dry powder.
Our tailored ETHOCEL offering is ideal for this new coating
technology. Trials show that using our innovative ETHOCEL HP
(High Productivity) product in a rotor coater allows for improved
productivity and shorter coating times, while completely
eliminating the need for environmentally-harmful solvents.
This new technology can be a powerful solution for our
customers looking to move away from solvent-based systems,
without experiencing the long coating time and potential
stability issues of aqueous-based dispersions.
Figure 1. VFC-LAB 3 FLO-COATER with GXR-35 Rotor Insert.
By using ETHOCEL HP, your formulation can remain simple
and robust, avoiding concerns around tackiness and stability
often found with low Tg polymers such as methacrylate based
technology which requires not only significant amounts of talc
for the dry powder layering process and post processing coating,
but also additives overall to avoid agglomeration. Customers
looking for innovative solutions to their formulation challenges
can turn to our latest ETHOCEL HP product combined with
this rotor technology to provide alternative solutions in the
areas of controlled release and taste masking.
Through our CR Alliance with Colorcon, we offer this new
product with the backing of the application expertise our
partners bring to the industry. We have also collaborated with
Freund-Vector in the development of this product, as their
equipment knowledge has proven invaluable in ensuring our
new polymer offering brings the right productivity benefits to
our customers while processing cleanly.
Why Go With This Solvent-Less System?
ETHOCEL HP can help you:
Coat 40-60% faster than current MUPS coating technologies
Achieve 98 - 99% coating efficiency consistently
Improve sustainability (with the elimination of solvents)
Avoid high solvent costs including costs of disposal,
capital expenditure, and infrastructure modifications
Improve operational safety
Figure 2. GRANUREX GXR-35 Rotor.
ETHOCEL HP
Atomization of
Solution
Layer Formation
Final Film
Formation
Process repeated thousands of
times to form uniform film
ETHOCEL in organic solvent
or as aqueous dispersion
sprayed onto substrate
Droplet spreading
and evaporation
ETHOCEL Layer
Formed
Final Film
Formation
Figure 3. The spray coating process.
Figure 4. Cross sectional image (left) and magnified view (right) of ETHOCEL barrier membrane
formed from solvent solution.
Initial wetting and
powder feeding
Rotor Layering
(rolling snowball effect)
Final Film
Formation
Curing to induce
coalesence
ETHOCEL HP
Tacking Agent:
Water & Plasticizer
Repeated liquid and
solid bridging
Figure 5. The dry powder layer process.
How The Spray Coating Process Works
Typical spray coating systems of ethylcellulose rely on a
dissolving the polymer in a solvent or dispersing in an
aqueous medium to apply the coating onto the substrate. Film
formation process occurs by atomizing the solution into small
droplets that pelt the surface of the substrate at which point
the carrier medium (solvent or water) is quickly evaporated
and the ethylcellulose remains on the surface of the substrate.
This process is repeated thousands of times during the coating
process, resulting in many layers of ethylcellulose that deposit
on the surface of the substrate and coalescence into a solid,
homogenous film. The industry understands this system,
which allows for great film-formation, and is compatible with
both aqueous and solvent systems. Solvent-based coatings
provide stable, reproducible coatings but the use of solvents
can be problematic for customers who dont have solvent
capabilities at their sites.
How The Dry Powder Layering Process Works
The dry powder layering process applies the coating from a
dry state onto a substrate by first wetting the substrate with
a tacking agent such as a plasticizer/water combination. After
sufficient wetting, the dry polymer powder is applied to the
wet surface of the substrate upon which liquid bridging occurs
between the dry powder particles and the surface of the
substrate. The presence of the plasticizer lowers the Tg of the
polymer to levels for proper film formation. The wetting agent
and dry powder are continually sprayed on to the substrates
and polymer layers are formed similar to a snow ball forming
as it rolls down a hill due to the unique rotor insert design
and small gap for slit air flow. After sufficient coating, the dry
powder coated substrates are cured and stored. This dry process
reduces all environmental concerns faced with solvent systems.
It greatly improves efficiency as well, as the powder that goes
into the system finds itself on the substrate surface, and there
is no solvent or minimal liquid that needs to be driven off in
the process. The unit allows for flexibility in processing, where
one could perform API-layering, followed by a coating step, and
finished with a curing step all in the same piece of equipment.
Overall, processing is much faster.
Figure 6. Cross sectional image (left) and magnified view (right) of ETHOCEL HP barrier membrane
formed from dry powder rotary coating process.
ETHOCEL HP
How Does ETHOCEL HP Work In This Process?
ETHOCEL HP was specifically designed to be most efficient in a
dry powder coating setting. The mean particle size and particle
size distribution has been optimzed for best controlled release
performance.
These case studies demonstrate product attribute effects and
improvements over standard manufacturing technologies in
enhanced productivity, decreased coating times, and better
environmental impacts by removing solvent use.
Table 1. Particle size specification (in microns).
Particle size
specification
ETHOCEL Std
ETHOCEL FP
ETHOCEL HP
D10
N/A
N/A
<3
Mean
N/A
3-15
<8
D90
N/A
N/A
< 15
Maximum
N/A
100
N/A
Since ethylcellulose is applied from the dry state, a curing step is strongly recommended to ensure film stability. Curing can be
done in-situ in the equipment (referred to as dynamic curing) or in tray ovens (static curing).
Table 1 shows how we have tightened our particle size distribution to allow for best performance in a dry form. Considering
coating thickness can range from 40 - 100 microns, one can imagine how just one larger particle can harm the integrity of the
overall coating hence the importance of narrowing the particle size distribution.
Fast Coating Times and Weight Gain Influence
For a 200 kg batch size, Figure 7 illustrates how the dry powder delivery of ETHOCEL HP significantly reduces coating times
compared to aqueous ETHOCEL and organic ETHOCEL even when a 2 hour cure step is included for ETHOCEL HP and aqueous
ETHOCEL. For example, at 20% weight gain, ETHOCEL HP only takes 3.5 hours whereas organic ETHOCEL takes 9.8 hours and
aqueous ETHOCEL 7.2 hours.
Acetaminophen drug release was determined for ETHOCEL HP barrier coatings ranging from 5 - 30% weight gain. 25% dibutyl
sebacate plasticizer was used with respect to ETHOCEL HP weight and dissolved in water. No pore formers were used and samples
were statically cured for 2 hours at 60C. Figure 8 shows how drug release was predictably controlled by incremental weight gain.
16
12
Organic ETHOCEL
80
ETHOCEL HP
%APAP Released
Coating Time (hr)
14
100
Aqueous ETHOCEL
10
8
6
60
40
30% wg
2
10%
20%
Weight Gain
Figure 7. Equivalent performance coating times including curing.
5% wg
20
0%
5% wg
10% wg
15% wg
20% wg
25% wg
30% wg
30%
400
800
1200
1600
Time (min)
Figure 8. Influence of ETHOCEL HP weight gain on Acetaminophen drug release.
ETHOCEL HP
80
IPA/H20: 25/75
Acetaminophen drug release was determined for 20% weight
gain of ETHOCEL HP. 25% triethyl citrate plasticizer was used
with respect to ETHOCEL HP weight and dissolved in varying
concentrations of isopropyl alcohol and water. No pore formers
were used and samples were statically cured for 2 hours at 60C
70
IPA/H20: 75/25
60
IPA/H20: 0/100
Increased solvent provides improved film coalescence, but
solvent-free (purely aqueous) performance is the best (Figure
9). The fast evaporation rates of solvents compared to aqueous
based solutions does not provide enough time for liquid
bridging and particle to particle interaction to successfully form
a complete coalesced film (Figure 10).
%APAP Released
Solvent Influence
50
40
30
20
10
0
0
400
800
Time(min)
1200
1600
Figure 9. Influence of solvent levels on acetaminophen drug release using ETHOCEL HP.
Figures 10. ETHOCEL HP film formation for 25% isopropanol / 75% water (left), 75% isopropanol / 25% water (center), and 0% isopropanol / 100% water (right).
Curing Influence
Due to the nature of the ETHOCEL HP and rotary coating process, a cure step is required to achieve full film coalesence
and assure stability and reproducible performance (Figure 11). The cure step for ETHOCEL HP coated substrates can be done
dynamically or statically. Static curing is defined as taking the ETHOCEL HP coated samples and placing them on a tray and
placing in an oven at a set temperature and time.
Dynamic curing allows the customer to keep the coated samples in the rotor processor and use forced hot air from above plus
the frictional movement of the product bed to induce curing of the film. Dynamic curing can allow customers for true one stop,
one pot, processing conditions where API drug layering, functional coating, and curing can be completed in the same piece
of equipment. Dynamic curing also enduces smooth and uniform film formation on the surface of the substrates coated with
ETHOCEL HP compared to static curing (Figure 12).
Experiments have demonstrated that static and/or dynamic curing can be sufficient for film formation, but deciding which type of
curing and degree of curing (length/time) is formulation dependent.
Figure 11 Cross-sectional images of uncured (left) and cured (right) samples coated with ETHOCEL HP
Figure 12: Surface morphology of static curing (left) and dynamic curing (right)
ETHOCEL HP
Equivalent Performance Of Highly Soluble Drug
The ETHOCEL HP applied at 20% weight gain has equivalent
performance to organic ETHOCEL applied at 10% weight gain
without any lag in drug release in the first 60 minutes (Figure
13). Although a higher amount of ETHOCEL HP was needed
in this example, total coating time only took 221 minutes
including the 2 hour cure step whereas organic ETHOCEL took
293 minutes. More time saving could be achieved by switching
to dynamic curing.
100
90
80
Dissolved (%)
Metoprolol tartrate drug release was determined for 20% weight
gain of ETHOCEL HP. 40% dibutyl sebacate plasticizer was used
with respect to ETHOCEL HP weight and dissolved in water.
No pore formers were used and samples were statically cured
for 2 hours at 60C. Metoprolol tartrate drug release was also
determined for 10% weight gain of ETHOCEL Std 10 Premium
which was dissolved in 90% isopropanol and 10% water. 10%
dibutyl sebacate plasticizer was used with respect to ETHOCEL
Std 10 premium weight and dissolved in the same solution. No
pore formers were used and samples were not cured.
70
60
50
40
ETHOCEL HP - uncured
30
ETHOCEL HP - static cured 60C - 2hrs
20
Organic EC-10%wg
10
0
200
400
600
800
Time(min)
1000
1200
Figure 13. Metoprolol tartrate drug release for ETHOCEL HP
rotary coatings or organic ETHOCEL spray coatings.
Improving Particle Size For Improved Film Formation
Acetaminophen drug release was determined for 20% weight gain for Dow ethylcellulose milled at various mean particle size and
particle size distributions (Figure 14). 25% dibutyl sebacate plasticizer was used with respect to ethylcellulose weight and dissolved
in water. No pore formers were used and all samples were statically cured for 2 hours at 60C. As mean particle size becomes
smaller and particle size distributions are tightened, film formation is improved. Larger mean particle sizes even after curing
resulted in highly porous films, whereas ETHOCEL HP after curing had a fully formed film and slowest drug release.
90
X : 50um
80
%APAP Released
70
60
X : 24um
50
X: 50um
40
X: 5um
30
X : 5um
20
Table 2. Particle size distributions of ethyl cellulose samples.
10
0
400
800
Time (min)
1200
Figure 14. Influence of particle size on film formation and drug release.
X: 24um
1600
Sample
D10 (m)
Mean (m)
D90 (m)
ETHOCEL HP
Medium Size
24
55
Large Size
50
108
ETHOCEL HP
Productivity Modeling Work
400
Days of Production
A case model using Venlafaxine HCl was used to determine
the productivity opportunities that would exist using the
ETHOCEL HP coating process versus the next best alternative
spray coatings. The productivity model evaluated companies
of various sizes including a large company which would
manufacture 400 million dosage units a year, a Medium
company with 100 million dosage units a year, and a Small
company with 30 million dosage units a year. Assumptions
employed included: using once piece of equipment for 24hr 7
days a week, 200 kg batch size, and equivalent performance at
10% weight gain Organic ETHOCEL to 20% weight gain Aqueous
ETHOCEL to 20% weight gain ETHOCEL HP. Processing
parameters and time elements of the model are tabled below.
400 MM Dosage Units
100 MM Dosage Units
30 MM Dosage Units
363
300
266
200
154
91
100
0
67
28
20
Aqueous
ETHOCEL
Organic
ETHOCEL
39
12
ETHOCEL
HP
Figure 15. Number of days of production for aqueous ETHOCEL or organic
ETHOCEL spray coatings and ETHOCEL HP dry powder rotary coatings
Table 3. Coating process batch size.
Coating process
Spray/Powder
Delivery Rate (g/min)
% ETHOCEL In Solution
Cure Time (min)
Additional Steps (pre-heat, solution prep)
ETHOCEL HP
450
100%
120
10
Aqueous ETHOCEL
900
15%
120
90
Organic ETHOCEL
1200
6%
90
ETHOCEL High Productivity is an innovative, new product for
the greater ETHOCEL portfolio which enables customers to
increase productivity while still maintaining the advantages
of Dow manufactured ETHOCEL such as tight viscosity,
narrow ethoxyl distribution, and reduced fiber content. Using
ETHOCEL HP could result in up to 60% reduction in coating
times versus aqueous ETHOCEL and 40% reduction versus
organic ETHOCEL spray coating systems. In addition to
the controlled release market, ETHOCEL HP and the rotor
technology can also be extended to taste masking to eliminate
the bitter taste of actives upon swallowing. Proven to be
most effective as a solvent-less coating, this new technology
enables customers to achieve processes which are sustainable,
environmentally friendly, and safe for their employees.
Amount Manufactured (Kg)
Modeling indicates any company of any size will experience a dramatic decrease in coating time using the ETHOCEL HP product.
Coating times decreased by 60% compared to aqueous systems and 40% compared to organic systems (Figure 15). But what does
that improvement really mean for you? It could yield increased production capability to make more product in a given year, or
free equipment to work on other products as well! For example, using the venlafaxine case study, we can estimate the additional
amount one can manufacture (kg) using the ETHOCEL HP technology in same amount of time it would take to use aqueous
ETHOCEL or organic ETHOCEL spray coatings (Figure 16).
100,000
75,000
50,000
25,000
0
67,600
36,488
ETHOCEL HP vs
Aqueous ETHOCEL
ETHOCEL HP vs
Organic ETHOCEL
Figure 16. Additional manufacturing amounts when switching to ETHOCEL HP
technology vs traditional aqueous ETHOCEL or organic ETHOCEL spray coatings.
Dow-Colorcon Alliance: UNIQUE TOGETHER
Dow Pharma & Food Solutions and Colorcon, together in the Controlled Release
Alliance, bring joint resources in polymer and formulation expertise to accelerate your
pharmaceutical product development efforts and reach markets throughout the world.
Colorcon has exclusive, global sales and distribution rights for Dow products used in
controlled release applications, including ETHOCEL.
Dow Pharma & Food Solutions
www.dowpharmaandfood.com
US
Toll Free 800 441 4DOW
989 832 1542
International
Europe / Middle East
+ 800 36 94 63 67
Italy
+ 800 783 825
Asia / Pacific
+ 800 77 76 77 76
+ 60 37 958 3392
South Africa
+ 800 99 5078
Dow requests that customers considering use of Dow products in medical applications notify Dow so that appropriate assessments may be conducted. Dow has a Corporate Medical Application Policy in place that
guides the use of Dow products in potential new pharmaceutical and medical device uses. Dow reviews all new applications/uses according to this Medical Application Policy to determine if the use is appropriate for
Dow materials. Dow does not endorse or claim suitability of its products for specific medical applications. It is the responsibility of the medical device or pharmaceutical manufacturer to determine that the Dow product
is safe, lawful, and technically suitable for the intended use. DOW MAKES NO WARRANTIES, EXPRESS OR IMPLIED, CONCERNING THE SUITABILITY OF ANY DOW PRODUCT FOR USE IN MEDICAL APPLICATIONS.
NOTICE: No freedom from infringement of any patent owned by Dow or others is to be inferred. Because use conditions and applicable laws may differ from one location to another and may change with time, Customer
is responsible for determining whether products and the information in this document are appropriate for Customers use and for ensuring that Customers workplace and disposal practices are in compliance with
applicable laws and other government enactments. The product shown in this literature may not be available for sale and/or available in all geographies where Dow is represented. The claims made may not have been
approved for use in all countries. Dow assumes no obligation or liability for the information in this document. References to Dow or the Company mean the Dow legal entity selling the products to Customer unless
otherwise expressly noted. NO WARRANTIES ARE GIVEN; ALL IMPLIED WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE ARE EXPRESSLY EXCLUDED.
Trademark of The Dow Chemical Company (Dow) or an affiliated company of Dow
Form No. 198-02324-1015CDP
Вам также может понравиться
- Christ and the Bible: A Marvelous UnityДокумент8 страницChrist and the Bible: A Marvelous Unityabhijit_gothoskar6039Оценок пока нет
- Damage Liability WaiverДокумент1 страницаDamage Liability WaiveritargetingОценок пока нет
- T.Shivakumar: Kottam Institute of Pharmacy Jntu, A.PДокумент45 страницT.Shivakumar: Kottam Institute of Pharmacy Jntu, A.PFree Escort ServiceОценок пока нет
- Design and Performance Diesel Fuel FiltersДокумент4 страницыDesign and Performance Diesel Fuel FiltersJaskirat Singh100% (1)
- The Purpose of The BibleДокумент4 страницыThe Purpose of The Bibleabhijit_gothoskar6039Оценок пока нет
- Tubular Vs Autoclave 1Документ2 страницыTubular Vs Autoclave 1bsarkar100% (2)
- UV Coatings For PlasticsДокумент5 страницUV Coatings For Plasticsrabbit22112Оценок пока нет
- Brochure DowДокумент8 страницBrochure DowLucas Bissoli100% (1)
- Rhoplex Ac 3001Документ12 страницRhoplex Ac 3001hongducxxxОценок пока нет
- Flexo and Gravure Inks FormulationДокумент14 страницFlexo and Gravure Inks FormulationMd Ali RazuОценок пока нет
- Carbopol Ultrez 21 Hoja Tecnica PDFДокумент4 страницыCarbopol Ultrez 21 Hoja Tecnica PDFAdrian Copa JОценок пока нет
- Waterborne Epoxy Curatives: High Performance. Low Emissions. Cost-EffectiveДокумент12 страницWaterborne Epoxy Curatives: High Performance. Low Emissions. Cost-EffectiverafchemОценок пока нет
- Polyurea Spray Coatings The Technology and Latest DevelopmentsДокумент16 страницPolyurea Spray Coatings The Technology and Latest DevelopmentsA MahmoodОценок пока нет
- Cellulose Ethers: Technical Overview and Product GuideДокумент8 страницCellulose Ethers: Technical Overview and Product GuideIdo RevelОценок пока нет
- Declaratory ReliefДокумент2 страницыDeclaratory ReliefChaОценок пока нет
- Motion For Leave Demurrer) MelgarДокумент11 страницMotion For Leave Demurrer) MelgarRichard Conrad Foronda Salango100% (2)
- Pulp and Paper Industry: Emerging Waste Water Treatment TechnologiesОт EverandPulp and Paper Industry: Emerging Waste Water Treatment TechnologiesРейтинг: 5 из 5 звезд5/5 (1)
- Aquaflow TechnicalДокумент11 страницAquaflow TechnicalCeratita ClarkОценок пока нет
- Easy IntentionsДокумент10 страницEasy IntentionsUmmahTechnology100% (1)
- XPU Flooring Balcony Case StudyДокумент12 страницXPU Flooring Balcony Case StudyyasafyОценок пока нет
- Coalesce RДокумент4 страницыCoalesce Rrieza_fОценок пока нет
- Spray Drying: A Primer on the Major Industrial Drying TechnologyДокумент7 страницSpray Drying: A Primer on the Major Industrial Drying TechnologyAnonymous 1zdRSWskhgОценок пока нет
- TIL 1897 Controller Connectivity and Stalled Io Live ValuesStandard - TIL 1897Документ13 страницTIL 1897 Controller Connectivity and Stalled Io Live ValuesStandard - TIL 1897bali abdelazizОценок пока нет
- Guide to Selecting Powder Coatings for SubstratesДокумент29 страницGuide to Selecting Powder Coatings for SubstratesThanh Nguyen100% (1)
- Ultrafiltration for Oily Industrial WaterДокумент13 страницUltrafiltration for Oily Industrial WaterböhmitОценок пока нет
- Tablet Coating With Its DefectsДокумент56 страницTablet Coating With Its Defectsjoshirohan100% (1)
- Enercon Uv Flexo Ink Adhesion For Flexible PackagingДокумент8 страницEnercon Uv Flexo Ink Adhesion For Flexible PackagingAndrei PaunОценок пока нет
- Hydrogel Porous Membrane PoretexДокумент9 страницHydrogel Porous Membrane PoretexAlek Lemkov100% (1)
- 4350 9644 1 PBДокумент65 страниц4350 9644 1 PBWan MuhamammadОценок пока нет
- Technical Tablet CoatingДокумент11 страницTechnical Tablet CoatinglaszlobeneszОценок пока нет
- Dry Powder Coating A New Trend in Coating TechnologyДокумент11 страницDry Powder Coating A New Trend in Coating TechnologyTimir PatelОценок пока нет
- Degasification of Polymer in The Vacuum EvaporatorДокумент4 страницыDegasification of Polymer in The Vacuum EvaporatorUlricОценок пока нет
- Continuous masterbatch fiber production processДокумент2 страницыContinuous masterbatch fiber production processkeyur1109Оценок пока нет
- Epikure 8537-w-60Документ7 страницEpikure 8537-w-60Cloudy DayОценок пока нет
- Complex Coacervation & HydrogelДокумент2 страницыComplex Coacervation & HydrogelkmorendhaОценок пока нет
- Son Enteric Film-CoatingДокумент2 страницыSon Enteric Film-CoatingLina Tadeo FabiánОценок пока нет
- Article - Novel Nano-Dispersion - PCI MagДокумент7 страницArticle - Novel Nano-Dispersion - PCI MagMaximiliano MackeviciusОценок пока нет
- Recycling of Condensation Plastics: GPEC 2004 Paper Abstract #52Документ8 страницRecycling of Condensation Plastics: GPEC 2004 Paper Abstract #52Anjum ParkarОценок пока нет
- Chemoxy International LTD Coalescing RangeДокумент8 страницChemoxy International LTD Coalescing RangeIliass MeskiОценок пока нет
- Tech - Topic: Introduction To PolyethyleneДокумент3 страницыTech - Topic: Introduction To PolyethyleneSulistyo RabonoОценок пока нет
- Influence of Film Coating Formulations On Process Time and Co2 Emissions - Chemistry TodayДокумент3 страницыInfluence of Film Coating Formulations On Process Time and Co2 Emissions - Chemistry TodayDgek LondonОценок пока нет
- Processes: Novel Technique For Coating of Fine Particles Using Fluidized Bed and Aerosol AtomizerДокумент18 страницProcesses: Novel Technique For Coating of Fine Particles Using Fluidized Bed and Aerosol AtomizerDenis Crispin IrazabalОценок пока нет
- HPMC CelluloseДокумент32 страницыHPMC Cellulosemailtorubal2573Оценок пока нет
- Materiales Alta Barrera para EmpaquesДокумент72 страницыMateriales Alta Barrera para EmpaquesLiliana RuizОценок пока нет
- Better Business in A Better IrelandДокумент4 страницыBetter Business in A Better Irelandapi-286562658Оценок пока нет
- Group No. 6 EVOH AssignmentДокумент31 страницаGroup No. 6 EVOH AssignmentGursevak SinghОценок пока нет
- TZ 21 PDFДокумент16 страницTZ 21 PDFAlmir MachadoОценок пока нет
- A Primer On Spray Drying Chemical Engineering Nov09Документ7 страницA Primer On Spray Drying Chemical Engineering Nov09Hikmah Triana HadiОценок пока нет
- Concrete Flooring Brochure PDFДокумент12 страницConcrete Flooring Brochure PDFKalyanasundaram ThirugnanasambandamОценок пока нет
- Presented By: M. Pharm Dr. B.C.Roy College of Pharmacy & AhsДокумент49 страницPresented By: M. Pharm Dr. B.C.Roy College of Pharmacy & AhsHafizur RahmanОценок пока нет
- Psi-MIX Inline Disperser eДокумент7 страницPsi-MIX Inline Disperser eKhamis KhamisОценок пока нет
- Voc Report - MembersДокумент40 страницVoc Report - MembersmehoОценок пока нет
- Dow Construction ChemcalДокумент7 страницDow Construction ChemcalchayanunОценок пока нет
- Problem StatementДокумент3 страницыProblem StatementJatin KanojiaОценок пока нет
- Mucell: Proven Lightweighting Technology For Automotive ApplicationsДокумент8 страницMucell: Proven Lightweighting Technology For Automotive ApplicationsRidha FaturachmiОценок пока нет
- Rhoplex WL 100Документ5 страницRhoplex WL 100fato85Оценок пока нет
- Microcellular Shoes Ole SystemsДокумент10 страницMicrocellular Shoes Ole SystemsA Mahmood100% (1)
- Equistar HDPEДокумент1 страницаEquistar HDPECalОценок пока нет
- TechNote 2 UKДокумент4 страницыTechNote 2 UKStéphane BlanchardОценок пока нет
- Assoziativepolyurethanverdickerfuerwaessrigebeschichtungen Neu enДокумент6 страницAssoziativepolyurethanverdickerfuerwaessrigebeschichtungen Neu enNguyen Ho Hoang HaiОценок пока нет
- Amine chemistries for high-solids isocyanate coatingsДокумент6 страницAmine chemistries for high-solids isocyanate coatingsCoraba JohnОценок пока нет
- Hempaxane BrochureДокумент12 страницHempaxane Brochuresorion61Оценок пока нет
- UTECH 2000, Additives For Pentane Lamination Rigid FoamsДокумент12 страницUTECH 2000, Additives For Pentane Lamination Rigid Foamszhangp6Оценок пока нет
- Water Borne CoatingsДокумент4 страницыWater Borne CoatingsAnil yucebasОценок пока нет
- Acusol 505nДокумент9 страницAcusol 505nNikesh ShahОценок пока нет
- Processing Techniques For Reinforced Thermosetting Urethane CompositesДокумент9 страницProcessing Techniques For Reinforced Thermosetting Urethane CompositesA MahmoodОценок пока нет
- torrent_pharmaceuticals_ltd._gujarat_india_12.11.23_483Документ6 страницtorrent_pharmaceuticals_ltd._gujarat_india_12.11.23_483abhijit_gothoskar6039Оценок пока нет
- Equine SkeletonДокумент1 страницаEquine Skeletonabhijit_gothoskar6039Оценок пока нет
- Sun Pharmaceutical Industries Inc., Mohali, Punjab, India 8.12.22 483Документ8 страницSun Pharmaceutical Industries Inc., Mohali, Punjab, India 8.12.22 483abhijit_gothoskar6039Оценок пока нет
- Centaur 06_05_2023 _ FDAДокумент5 страницCentaur 06_05_2023 _ FDAabhijit_gothoskar6039Оценок пока нет
- SB Printed DocumentДокумент203 страницыSB Printed Documentabhijit_gothoskar6039Оценок пока нет
- Atrigel A Potential Parenteral Controlled Drug Delivery SystemДокумент8 страницAtrigel A Potential Parenteral Controlled Drug Delivery SystemMaria RoswitaОценок пока нет
- The Three-Fold Formation of The BibleДокумент5 страницThe Three-Fold Formation of The Bibleabhijit_gothoskar6039Оценок пока нет
- Statements From Famous Personalities Concerning The BibleДокумент5 страницStatements From Famous Personalities Concerning The Bibleabhijit_gothoskar6039Оценок пока нет
- Human PhysiologyДокумент13 страницHuman Physiologyramakant chobheОценок пока нет
- An Introduction to the Bible's Authors, Books, Chapters and VersesДокумент5 страницAn Introduction to the Bible's Authors, Books, Chapters and Versesabhijit_gothoskar6039Оценок пока нет
- Computational Fluid Dynamics Modeling of The Paddle Dissolution Apparatus: Agitation Rate, Mixing Patterns, and Fluid VelocitiesДокумент10 страницComputational Fluid Dynamics Modeling of The Paddle Dissolution Apparatus: Agitation Rate, Mixing Patterns, and Fluid Velocitiesabhijit_gothoskar6039Оценок пока нет
- Amrophosu PharmaДокумент11 страницAmrophosu Pharmaabhijit_gothoskar6039Оценок пока нет
- Gobi Dhaba Style RecipeДокумент6 страницGobi Dhaba Style Recipeabhijit_gothoskar6039Оценок пока нет
- Cell Free Drug Permeability TestingДокумент15 страницCell Free Drug Permeability Testingabhijit_gothoskar6039Оценок пока нет
- Psalm 19Документ8 страницPsalm 19abhijit_gothoskar6039Оценок пока нет
- Commercializing Radical InnovationДокумент13 страницCommercializing Radical Innovationabhijit_gothoskar6039Оценок пока нет
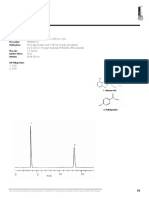
- Betamethasone + Lignocaine HPLCДокумент8 страницBetamethasone + Lignocaine HPLCabhijit_gothoskar6039Оценок пока нет
- Lidocaine HPLCДокумент1 страницаLidocaine HPLCabhijit_gothoskar6039Оценок пока нет
- Denamarin Chewable TabletsДокумент5 страницDenamarin Chewable Tabletsabhijit_gothoskar6039Оценок пока нет
- Itraconazole E100Документ10 страницItraconazole E100abhijit_gothoskar6039100% (1)
- JPRHC: Research ArticleДокумент9 страницJPRHC: Research Articleabhijit_gothoskar6039Оценок пока нет
- Betamethasone HPLCДокумент10 страницBetamethasone HPLCabhijit_gothoskar6039Оценок пока нет
- 222 Fluidized Bed Processing For MultiparticulatesДокумент4 страницы222 Fluidized Bed Processing For Multiparticulatesabhijit_gothoskar6039Оценок пока нет
- Kolliphor ELДокумент6 страницKolliphor ELabhijit_gothoskar6039Оценок пока нет
- Diltizem MicrosphereДокумент11 страницDiltizem MicrospheredoddadineshОценок пока нет
- AsianJPharm4292-8280695 230006 PDFДокумент10 страницAsianJPharm4292-8280695 230006 PDFabhijit_gothoskar6039Оценок пока нет
- 222 Fluidized Bed Processing For MultiparticulatesДокумент4 страницы222 Fluidized Bed Processing For MultiparticulatesNaresh YechuriОценок пока нет
- In Vitro in Vivo Correlation Linking Drug Release To Clinical Performance QiuДокумент11 страницIn Vitro in Vivo Correlation Linking Drug Release To Clinical Performance Qiuabhijit_gothoskar6039Оценок пока нет
- Immanuel Sangcate ResumeДокумент2 страницыImmanuel Sangcate ResumeNonjTreborTendenillaToltolОценок пока нет
- AGAG v. Alpha FinancingДокумент5 страницAGAG v. Alpha Financinghermione_granger10Оценок пока нет
- Guimba, Nueva EcijaДокумент2 страницыGuimba, Nueva EcijaSunStar Philippine NewsОценок пока нет
- Lecture Powerpoints: Physics For Scientists and Engineers, With Modern Physics, 4 EditionДокумент29 страницLecture Powerpoints: Physics For Scientists and Engineers, With Modern Physics, 4 EditionKhawla MustafaОценок пока нет
- Salary Loan Application FormДокумент2 страницыSalary Loan Application FormRyan PangaluanОценок пока нет
- 5 Years Program Detailed Curriculum 2014 PDFДокумент106 страниц5 Years Program Detailed Curriculum 2014 PDFInder singhОценок пока нет
- Avr42783: Using Usart To Wake Up Atmega328Pb From Sleep ModeДокумент12 страницAvr42783: Using Usart To Wake Up Atmega328Pb From Sleep ModeMartin Alonso Cifuentes DazaОценок пока нет
- Case Study 1 StudДокумент11 страницCase Study 1 StudLucОценок пока нет
- Wolters History of Philippine Taxation SystemДокумент28 страницWolters History of Philippine Taxation SystemRonald FloresОценок пока нет
- United States Bankruptcy Court Southern District of New YorkДокумент21 страницаUnited States Bankruptcy Court Southern District of New YorkChapter 11 DocketsОценок пока нет
- Bank and Types of BanksДокумент9 страницBank and Types of Banksanju skariaОценок пока нет
- Law CV Template 3Документ2 страницыLaw CV Template 3jaslinОценок пока нет
- The People in The Trees: Also by Hanya YanagiharaДокумент5 страницThe People in The Trees: Also by Hanya YanagiharaMirОценок пока нет
- Sing To The Dawn 2Документ4 страницыSing To The Dawn 2Nur Nabilah80% (5)
- Loan Classification Rescheduling AmendmentДокумент8 страницLoan Classification Rescheduling AmendmentConnorLokmanОценок пока нет
- Diplomacy settings and interactionsДокумент31 страницаDiplomacy settings and interactionscaerani429Оценок пока нет
- Amazon Order 19-1-22Документ4 страницыAmazon Order 19-1-22arunmafiaОценок пока нет
- REC Infra Bond Application FormДокумент2 страницыREC Infra Bond Application FormPrajna CapitalОценок пока нет
- Constraint Manager User's Manual: Release PADS VX.2.7Документ228 страницConstraint Manager User's Manual: Release PADS VX.2.7Mokorily PalmerstonОценок пока нет
- Zweber v. Credit River Township, No. A14-0893 (Mn. July 27, 2016)Документ26 страницZweber v. Credit River Township, No. A14-0893 (Mn. July 27, 2016)RHTОценок пока нет
- 1 Air India Statutory Corporation vs. United Labour Union, AIR 1997 SC 645Документ2 страницы1 Air India Statutory Corporation vs. United Labour Union, AIR 1997 SC 645BaViОценок пока нет
- Companies View SampleДокумент4 страницыCompanies View Samplesuresh6265Оценок пока нет
- NC LiabilitiesДокумент12 страницNC LiabilitiesErin LumogdangОценок пока нет
- Rape Conviction Upheld Despite Victim's Inconsistent TestimonyДокумент5 страницRape Conviction Upheld Despite Victim's Inconsistent TestimonyAlvin NiñoОценок пока нет
- Oracle Applications EBS - Accounting EntriesДокумент43 страницыOracle Applications EBS - Accounting EntriesUdayraj SinghОценок пока нет