Академический Документы
Профессиональный Документы
Культура Документы
ITS Undergraduate 16946 Paper 1404058
Загружено:
Stephy Florez MenesesАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
ITS Undergraduate 16946 Paper 1404058
Загружено:
Stephy Florez MenesesАвторское право:
Доступные форматы
DESIGN OF AUTO SWITCH PID ON pH NEUTRALIZATION PROCESS
(Syahrizal Ismail, Hendra Cordova)
Department of Engineering Physics FTI ITS Surabaya
ITS Keputih Sukolilo Surabaya 60111
Telp : +6231-5947188 Fax : +6231-5923626
E-mail : syahrizal_ismail@yahoo.com
Abstract
This paper presents result of design auto switch PID on pH neutralization process. Nonlinear
nature of pH neutralization become problem in designing control system. Thus, this research
builds pH control system at neutralization pH based on auto switch PID. Auto switch PID is a
PID controller that works based on pH range setpoint, and then tune in each setpoint region. pH
setpoint region obtained from titration curve acid-base experiment. Acid solution used is a strong
acid HCl 0,1M, while base solution is strong base NaOH 0,1M. Acid solution flow rate is
maintained constant, while the base flow rate is controlled. Dc pump 12 V used to drain solution.
Sensor used pH meter and pH electrode. The software used LabVIEW 2009 with DAQ as
acquisition data. Systems analysis is based on three criteria, maximum overshoot, time settling,
and error steady state. Result of distribution setpoint regions are 2,3-4 for region 1, 4-9,9 for
region 2, and 9,9-11,2 for region 3. Tuning results for each region obtained Kp 1, Ti 1, Td 0.002
for region 1, Kp 1, Ti 1, Td 0.1 for region 2, Kp 2, Ti 2, Td 0.005 for region 3. The lowest maximum
overshoot and the fastest settling time given by region 3 which are 1,90% and 169 seconds. The
smallest error steady state is region 1 by 0,66%. Beside that, system successfully handle load
which is addition of acid solution flow rate, as long as lower than base solution flow rate.
Key words : pH neutralization, auto switch PID, LabVIEW
by applying concept of auto switch PID in real
time process.
1.
Introduction
pH neutralization is a mixing process
between the acid solution and base solution. The
addition of one solution against pH values
resulting in mixing process has nonlinear
properties. It can be seen clearly on the titration
curves of acid and bases established process. The
addition of a little volume of one solution, pH
value can change siginificantly especially on the
way to neutral.
Hendra Cordova (2004), has been done
research on the design of auto switch PID in pH
neutralization process in CSTR (Continuous
Stirred Tank Reactor). In that concept, the
nonlinear titration curves is divided into several
linear regions. These regions are then performed
controller parameter tuning.
Fadloli Luthfi (2011), design an auto switch
PID on CIPM (Continuous Injection Pipe
Mixing). In that study has successfully
demonstrated how auto switch PID able to
handle the process PID control pH. However,
these studies are still limited to simulation.
Therefore, this research purposes to construct a
system of control at pH neutralization process,
2.
pH Theory
The concept of hydrogen ion exponent (pH)
was introduced by Sorrensen (1909) in order to
avoid the hassle of writing the number of
negative factor of 10[2]. The definition of the
concept of pH as in the equation below :
[
(1)
Based on the equation above, pH value
equal to negative logarithm of hydrogen ion or
the logarithm of the reciprocal of the
concentration of hydrogen ions. It is very easy to
write the level of acidity or alkalinity of a
solution with pH. value of pH 0-14. A molar
solution of strong acid changed a part, the pH of
the solution is 0 and 1 mole of a strong base of
monovalent has a pH of 14[5].
3.
Acid and Base Theory
Inorganic substances can be classified into
three main groups, namely: acids, alkalis and
salts. Arrhenius acid-base theory indicates that
electrolyte molecules always produce negative
and positive ions when dissolved in water. Acid,
expressed as a compound to dissolve in water
dissociates to produce hydrogen ion [H+] or
hydronium ions [H3O] as positive ions only[3].
An example solution is the acid HCl. HCl is an
acid, because the solution it can release
hydrogen ions [H+] by reaction :
Water (H2O) is a neutral solution, which is
located between the nature of acidic and alkaline
properties. Equilibrium reactions of dissociation
of water is as follows:
(7)
Water has a small constant value,
approximately 1,82x10-16 at 25C. This suggests
that the degree of dissociation of water to be
ignored and in practice can be considered
separate, so that the concentration of water can
be considered constant.
(2)
Base is defined as a substance to dissolve in
water will run into shape with the decoupling of
the hydroxyl ions, such as the ions only negative.
NaOH is an example of an alkaline solution.
NaOH is a basis because the water that can
release ion hydroxyl (OH)-according to the
reaction :
(8)
Equation 8 is the ion product of water (Kw)
at room temperature. The importance of product
ions of water lies in the fact that the value can be
considered constant. This means that, if the acid
is dissolved in water, hydrogen ion concentration
can be increased only with accompanying
reduction in the concentration of hydroxyl ions.
Conversely, if the base is dissolved in water, the
concentration of hydroxyl ions increases and
decreases in the concentration of hydrogen ions.
Acid and base theory also related to acids
and bases titration. Titration means mixing
between acid solution and base solution using. In
laboratorium scale, usually using burette for
titration process. There are several types of acids
and bases titration, in this research using strong
acid and strong base titration. Typical titration
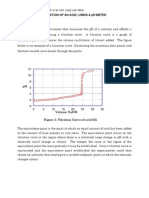
curve of strong acids and strong bases titration
shown in figure 1.
(3)
Preparation of acidic or alkaline solution
with the State of the Molarity (concentration)
can be done by mixing an acid or base with
aquades water distilled. He began to mix with
knowing the Molarity of the acid or base value
(
) with specific concentration levels
(
) and relative mass (
) by equation
below :
(4)
After that, use the similarity between the
volume of acid or base should be diluted (V1) is
multiplied by the molarity (M1) by the volume
of solution the desired acid or base (V2) with
molarity (M2) as below:
pH
(5)
Salt on the basis of the definition of
substance is the result of the reaction between
the acid and base neutralization reaction or the
substance of a solution of acid and alkaline
solutions are equivalent. The salt has no
distinctive features of an acid or alkaline.
14
12
10
8
6
4
2
0
1
10
25
75 150 250 330 350 450 500 650 1000
vol basa (mL)
Fig. 1. Titration curve of strong acids and
strong bases titration[2]
(6)
4.
pump, and plant is CSTR. Output process is
measured pH in CSTR.
pH Neutralization Process On CSTR
pH Setpoint
CSTR
pHT
DAQ
pHC
Pump
mv
CSTR
pH Output
PC
pHT
Motor
Driver
Fig. 3. Block diagram pH CSTR
5.
Acid Tank Acid Pump
Auto Switch PID
The use of PID controllers are usually
found in a linear process. The merger of the
three types of control elements shows the
advantages of each element and the deficit.
The weakness of the P controller can be
covered by combining with I. controller
controller I aims to eliminate the offset.
Weaknesses in control I is the delay that occurs
in the system response. Merging with a D
controller can speed up the response so that
weaknesses can be covered in my control.
Mathematical equations of the control
signal given by PID controller can formulated
below:
Drain Base Pump Base Tank
Fig. 2. pH neutralization on CSTR
Figure 2 is a process of neutralisation of pH
in continuous stirred tank reactor used in this
research. In the tank, there is an agitator for
mixing a rapid process. Acid flow rate remains
constant, while the basic rate of flow under
control. PC receives data about measured pH in
tank. Serial communication used to transfer data
from pH sensor. DAQ LabVIEW used to send
control signal from PC to drive dc pump. All
process parameters in this research can be seen
in table 1.
Table 1 Process Parameters
No
Parameters
1
Main Tank
Volume
2
Acid
TankVolume
3
Base Tank
Volume
4
Acid Flow Rate
5
Base Flow Rate
6
Acid
Concentration
7
Base
Concentration
u(t ) K p .e(t )
Kp
Ti
e(t )dt K T
0
p d
de(t )
(9)
dt
pH control which is a nonlinear process,
using a technique called PID automatic switches.
PID automatic switch is essentially a form of
division of the PID parameters Kp, Ti, Td, in
every region of the linear output. Furthermore, in
every region of linear PID tuning parameters in
accordance with design. Setpoint division shown
in figure 4.
Unit
11 L
13,8 L
13,8 L
0,047 L/min
0-0,2 L/min
0,1M
13 11 -
Model3, PID3
Model2, PID2
7-
0,1M
Model1, PID1
21-
Figure 3 is a block diagram of the process.
Input process is pH setpoint, actuator is dc
Flow rate Titration (ml/s)
Fig. 4. Setpoint division[1]
Figure 5 is a configuration of an auto
switch to the PID control block diagram. At the
time of the switch PID automated process will
work in accordance with the given input
setpoint. Setpoint determination depends on the
distribution of each linear region that has been
done before.
7.
Sensor Reading
Data retrieval for sensor readings
performed at 3 pH values pH 4, pH 7 and pH 10.
Solutions used to obtain a pH value of each pH
using a buffer solution.
Figure 7 is a result of sensor readings at a
pH of 4 is displayed on the LabVIEW software.
Errors resulting from a superficial reading of the
pH of the solution is as follows:
SETPOINT
RANGE 1
SETPOINT
+
-
PID 1
SETPOINT
RANGE 2
Error = pH solution output
PID 2
SETPOINT
RANGE 3
= 4-4 = 0
PID 3
(10)
Figure 8 and 9 are the readings for each pH
7 and pH 10. Using the same way as in equation
10 to determine the reading error in the reading
of pH 7 and pH 10. At the time of reading the
value at pH 10 increased the pH range pH 7 to
10. This is caused bulb sensor still reading rest
solution of pH 7. The overall results of sensor
readings contained in table 2.
Fig. 5. Auto switch PID
6.
Implementation of Auto Switch PID
Implementation of auto switch PID
controllers based on data from the experimental
titration curve in figure 6. Solution used strong
acid 0,1M HCL and strong base 0,1 NaOH. The
experiments were performed using a titration
burette. Experimental titration curves obtained
through the process of adding 59.4 ml of 0.1 M
NaOH in 25 ml of 0.1 M HCL Altogether there
are 30 experimental data from titration with
strong acids strong bases. Furthermore, the curve
formed by the experimental results are divided
into several linear regions. Based on the figure 6
then get the 3 pieces of the linear region, 2.3
pH <4.00, 4.00 pH <9.9, 9.9 pH <11.2. The
division of this area will be a guide in applying
the automatic switch controller PID, especially
in the process of tuning the PID parameters.
Table 2 Sensor reading results
pH Solution
Reading
Result
4
4
Error Reading
0
10
10
12
10
PID 3
PID 2
PID 1
Volume NaOH (mL)
Fig. 6. Titration experiment curve
59.4
54.9
52.9
47.9
42.9
41.4
38.4
35.4
33.4
32.4
31.4
30.9
30.4
29.9
29.4
28.9
28.4
27.9
25.6
25.2
25
23
20
15
10
pH
sustained oscillation response. Kp value
obtained when the system undergoes
sustained oscillations is Kp = 2. Oscillatory
response is shown in Figure 10.
4.5
4
3.5
pH
3
2.5
2
pH Setpoint
1.5
Table 3 Oscillation tuning table[9]
pH Output
Controller
P
PI
PID
0.5
0
1 3 5 7 9 11 13 15 17 19 21 23 25 27 29
Time (s)
Fig. 7. pH 4 reading
7
6
pH
5
pH Setpoint
pH Output
Reset
0.83 Tu
0.50 Tu
Rate
0.125 Tu
Then, find ultimate periode (Tu) which
is the distance from peak to peak
oscillations. Tu is represented by time in
seconds. Tu value obtained by 38 seconds,
but due to scaling, it becomes 3,8 seconds in
LabVIEWs graphic.
PB
2 PBu
2.22 PBu
1.67 PBu
1
0
1 3 5 7 9 11 13 15 17 19 21 23 25 27 29
Time (s)
8
7
Fig. 8. pH 7 reading
pH
12
5
4
pH Setpoint
10
pH Output
2
1
1
9
17
25
33
41
49
57
65
73
81
89
97
105
113
121
129
pH
8
pH Setpoint
pH Output
Time (s)
Fig. 10. Oscillatory response
0
1 3 5 7 9 11 13 15 17 19 21 23 25 27 29
Time (s)
Kp, Ti, Td are found by inserting
parameters controller into tuning oscillation
table in table 3. Thus, value Kp=1,19, Ti =19,
Td =0,475. After that, system response seen
by inserting each parameter to PID
controller. As for the system response as
shown in Figure 12.
Fig. 9. pH 10 reading
8.
Tuning PID Parameter
PID
parameter
adjustment
method
performed using an oscillating tuning and trial
and error. Oscillation Tuning early as the
determination of the PID parameter, if it feels
the results are still not meet the tuning is done by
modifying the oscillation by trial and error.
Oscillation tuning is done by inserting a
pH value of 7 as the setpoint. Oscillation
adjustment done in the area because of area
2 is the most sensitive area than other areas.
Thus, by tuning in region 2 in advance, will
allow tuning to another area.
After entering the setpoint value of pH
7 and then raise the value of Kp for a
Fig. 11. Ultimate periode (Tu)
5
Based on the result, it appears that the
system has been able to pursue the setpoint
and steady. However, the resulting response
is still not satisfactory, because the system
oscillates at the beginning of the process. In
addition, the time required to achieve stable
is also very slow. Thus, each parameter is
modified to get better results. For region 1
and region 3 tuned by trial and error.
Performance criteria of system respone is
describe below :
Fig. 13. Tuning response region 1
1. Maximum Overshoot
1%
9.2 Tuning Region 2 (4,0pH<9,9)
Tuning region 2 of the setpoint range
between 4.0 to 9.9 is done by entering a value of
7 as a setpoint. Through trial and error method
the values obtained for each of the control
parameters Kp=1, Ti =1, Td =0,1. As for tuning
the response graphs for area 2 as shown in
Figure 14 the response tuning of area 2.
2. Error Steady State
-0,5%
3. Settling Time
732 seconds
8
7
8
7
pH Setpoint
pH
pH
pH Output
5
4
pH Setpoint
pH Output
0
1
52
103
154
205
256
307
358
409
460
511
562
613
664
715
766
817
0
1
14
27
40
53
66
79
92
105
118
131
144
157
170
183
196
Waktu (s)
Fig. 12. Response oscillatory tuning
Time (s)
Fig. 14. Tuning response region 2
9.1 Tuning Region 1 (2,3 pH<4,0)
Tuning is done by inserting the pH value of
3 as the setpoint. Parameter values have been
obtained in tuning oscillation is converted to
lower value. Thus, parameter values are Kp=1,
Performance criteria of tuning response
graphs are as follows :
1. Maximum Overshoot
21,71%
Ti =1, Td =0,002.
Performance criteria of the tuning response
graphs are as follows :
2. Error Steady State
0,85%
1. Maximum Overshoot
32,67%
3. Settling Time
163 seconds
2. Error Steady State
0,66%
9.3 Tuning Region 3 (9,9pH<11,2)
Tuning region 3 that for the pH ranges from
9.9 to 11.2 is done by inserting a pH value of 11
as a setpoint. The value of each parameter for
3. Settling Time
202 seconds
local control obtained Kp=2, Ti =2, Td =0,005.
As for tuning the response graphs for area 2 as
shown in Figure 15 tuning the response area 3.
adding just a little can cause significant changes
in pH.
Reviewing the performance of the three
tuning regions, obtained the lowest values
obtained maximum overshoot and settling time
of the fastest occurring during the tuning region
3, then the smallest steady state error is given
when performing tuning region 1.
Setpoint Tracking Test
Setpoint Tracking test done by raising and
lowering pH setpoint from one region to the
other region. Setpoint raising from pH 3 to pH
11, and pH 7 to pH 11. Meanwhile, lowering
setpoint from pH 11 to pH 9.
Figure 16 shown setpoint tracking test from
pH 3 to pH 11. Performance criteria of tuning
response graph are as follows :
1. pH 1,55 to pH 3
a. Maximum Overshoot
27,33%
b. Error Steady State
2%
c. Settling Time
196 seconds
9.
Fig. 15. Tuning response region 3
Performance criteria of the tuning response
region 3 graphs are as follows :
1. Maximum Overshoot
1,90%
2. Error Steady State
-0.9%
2. pH 3 to pH 11
a. Maximum Overshoot
0,81%
b. Error Steady State
-0,09%
c. Settling Time
125 seconds
3. Settling Time
141 seconds
Based on data from the previous
explanation of the value of each parameter for
each PID setpoint can be grouped in regions as
in Table 4 the value of the PID parameters.
Tabel 4 PID parameters values[13]
No
Setpoint
PID Parameter
Regions
Kp
Ti
Td
1
0,002
4,0-9,9
0,1
9,9-11,2
0,005
6
pH Setpoint
4
pH Output
2
0
1
27
53
79
105
131
157
183
209
235
261
287
313
339
365
391
2,3-4,0
10
pH
12
Time (s)
In tuning the first area, a very high spikes
occur at the response graph. This is caused by a
narrow distribution where the pH value of 3 is
used as a setpoint, while the area is the range of
pH 2 and pH values 4. Therefore, the actuator is
still receiving a signal from the controller when
Fig. 16. Setpoint tracking test pH 3 to pH 11
In figure 17 the setpoint tracking test pH 7
to pH 11, pH values from pH 1,65. pH value
raises, and until 126 seconds system respond to
state up to 116 seconds. The highest peak value
at pH 11.1. After that lowered the setpoint value
at pH 9. The system goes down slowly until it
rests at pH 7.25 to 216 second. Furthermore, the
system starts in pursuit of the setpoint pH 9
where there is a small oscillation in progress.
1. pH 1,76 to pH 11
a. Maximum Overshoot
0,9%
b. Error Steady State
0%
c. Settling Time
101 seconds
pH 7. Oscillation occurred until pH 8,52 and at
200 seconds, steady state reached at pH 6,75.
12
10
pH
8
6
Setpoint pH
4
Output pH
2
1
23
45
67
89
111
133
155
177
199
221
243
265
287
309
331
0
Waktu (s)
Fig. 17. Setpoint tracking test pH 7 to pH 11
2. pH 11 to 9
a. Maximum Overshoot
5,3%
b. Error Steady State
-0,1%
c. Settling Time
163 seconds
Performance criteria of tuning response
graph for setpoint tracking test pH 7 to pH 11 are
as follows :
1. pH 1,65 to pH 7
a. Maximum Overshoot
21,71%
b. Error Steady State
-3,57%
c. Settling Time
163 seconds
At setpoint tracking test, can be seen that
the dominant oscillations occur in region 2 (4.0
pH <9.9), both setpoint tracking up or down.
This caused by characteristic in titration curve,
that region 2 is the most sensitive area. Thus, it
needs an aggressive controller that marked by
larger Td value.
2. pH 7 to pH 11
a. Maximum Overshoot
1,6%
b. Error Steady State
-0,09%
c. Settling Time
103 seconds
10. Load Test
Load test done by increasing acid flow rate
when system has reached steady state. Flow rate
increased to 0,095 L/min and 0,7 L/min. This
caused due to reason response system when
testing load for acid flow rate lower than base
flow rate, and second time for acid flow rate
higher than base flow rate.
12
10
pH
8
6
Setpoint pH
4
Output pH
12
10
0
pH
1
20
39
58
77
96
115
134
153
172
191
210
229
248
267
286
Waktu (s)
6
pH Setpoint
Fig. 18. Setpoint tracking test pH 11 to pH 9
Figure 18 is a setpoint tracking test of the
pH value of 11 pH 9. When starting the process,
the pH at pH 1.76. This system takes about 95
seconds to reach the setpoint value and steady
pH Output
1
36
71
106
141
176
211
246
281
316
351
386
421
456
491
526
Time (s)
Fig. 19. Load test for acid flow rate 0,095 L/min
Figure 19 is a system response for load test
with acid flow rate 0,095 L/min. Acid flow rate
was increased at 231st seconds. The system
began to respond due to the addition of acid flow
rate after 40 seconds. pH value down until pH
6,63. However, system gives settling time by
since addition of flow rate is 229 seconds,
maximum overshoot by 0,9%, and error steady
state by -0,3%.
12. Recommendation
There are a few suggestions that can be
given for subsequent pH control research are:
1. Titrate the solution can be varied in the
titration of strong acid to weak base or
strong acid titrated with a weak base.
2. Build level control system for each
tank, thus mini plant robuster than
previous.
REFERENCES
12
[1] Cordova, H. 2004. PID Self-Tuning
Based On Auto Switch
Algorithm To Control pH.
Teknik Fisika, ITS : Surabaya
[2] Luthfi, Fadloli. 2010. Perancangan
Sistem Pengendalian pH pada
Continuous Pipe Mixing
(CIPM) dengan Metode
Pengendalian PID-Selftuning
Berbasis Auto Switch
Algorithm. Teknik Fisika, ITS :
Surabaya
[3] Zainuddin Hamidi, M. 2007;
Perancangan Sistem
Pengendalian pH dengan
Metode Input-Output
Linearization pada Plant
Saturator di PT. Petrokimia
Gresik. Teknik Fisika, ITS :
Surabaya
[4] Fitra Wijaya, Andry. 2004.
Perancangan Kontroler Neuro
PID Self Tuning Berbasis
Jaringan Syaraf Tiruan Pada
Proses Netralisasi pH di PT
Petrokimia Gresik. Teknik
Fisika, ITS : Surabaya.
[5] Chang, Raymond. 2005. Kimia Dasar
Edisi Ketiga Jilid 1. Jakarta :
Erlangga.
[6] Chang, Raymond. 2005. Kimia Dasar
Edisi Ketiga Jilid 2. Jakarta :
Erlangga.
[7] Ogata, Katsuhiko. 1997. Teknik
Kontrol Automatik. Jakarta :
Erlangga.
10
pH
8
6
pH Setpoint
4
pH Output
1
33
65
97
129
161
193
225
257
289
321
353
385
417
449
481
0
Time (s)
Fig. 20. Load test for acid flow rate 0,7 L/min
Figure 20 is a system response for load test
with acid flow rate 0,7 L/min, which is larger 3x
than base flow rate. Addition of acid flow rate
was done when system reach steady state for 69
seconds. The system began to respond due to
addition of acid flow rate after 62 seconds. Yet,
due to acid flow rate is larger than base flow
rate, system can not bring back to previous
condition. Even, pH value down until pH 2,06.
This caused by, the characteristic of titration
process that need acid flow rate smaller than
base flow rate. Thus, the problem in this research
was limited that, acid flow rate must not exceed
than base flow rate.
11. Conclusion
After doing research on the implementation
of auto switch PID in real time, it can be
concluded that auto switch PID have been
successfully implemented and integrated with NI
LabVIEW software. The results of performance
tests as a whole, the lowest maximum overshoot
and the fastest settling time given by the tuning
of the region 3, and the smallest steady state
error is given by region 1. Beside that, the
system is able to respond to the addition of a
specific flow rate of acid during the acid level is
lower than base flow rate.
[8] Peter YIen, Jean. 2001. Measuring,
Modelling And Controlling
The pH Value And The
Dynamic Chemical State.
Helsinsky University of
Technology.
[9] PID User manual control toolkit
Syahrizal Ismail was born in
Bekasi, 6-Nopember-1989.
Student
majoring
in
Engineering Physics FTI-ITS
2007 class. Active as a board
of HMTF period 2008-2009.
Internship
program
conducted at PT Astra
Daihatsu Motor to the theme
of the analysis of materials at
the electrode tip of welding
robots. Areas of interest are
tracked instrumentation and
control. His carrer path
continues in oil field services
company
Schlumberger,
which placed in Duri, Riau.
10
Вам также может понравиться
- PH Measurement BasicsДокумент8 страницPH Measurement Basicsviralshukla85Оценок пока нет
- Potentiometric Titration Curve Determines Unknown Acid pKaДокумент3 страницыPotentiometric Titration Curve Determines Unknown Acid pKaDaniele Joseph HizonОценок пока нет
- Experiment 4 - Potentiometric TitrationДокумент11 страницExperiment 4 - Potentiometric TitrationJoemer Absalon Adorna100% (2)
- ANN Based PH Control ReportДокумент36 страницANN Based PH Control ReportSumit GuptaОценок пока нет
- ElectrochemicalAnalysis Manual Part 2Документ14 страницElectrochemicalAnalysis Manual Part 2estraj1954Оценок пока нет
- Maintaining Constant pH: Blood Buffer System and Factors Affecting Buffer CapacityДокумент31 страницаMaintaining Constant pH: Blood Buffer System and Factors Affecting Buffer CapacityJoyce Castil (Joyceee)Оценок пока нет
- Acid Base TitrationДокумент12 страницAcid Base TitrationMsfaeza HanafiОценок пока нет
- PH Control SimulationДокумент14 страницPH Control SimulationJohn Walter Ticona QuispeОценок пока нет
- Determine pH of samples using a pH meterДокумент5 страницDetermine pH of samples using a pH meterAjuba AbujaОценок пока нет
- Labexercise 2Документ7 страницLabexercise 2Ma Catherine MalanogОценок пока нет
- Expt.1 BiochemДокумент4 страницыExpt.1 BiochemMc de RamosОценок пока нет
- CPB 30103 Biochemical Engineering UniKL MICET Experiment 1: Preparation of Buffer Solution Full Lab ReportДокумент10 страницCPB 30103 Biochemical Engineering UniKL MICET Experiment 1: Preparation of Buffer Solution Full Lab ReportSiti Hajar Mohamed0% (1)
- Instrumentation and Control in Bio ReactorsДокумент4 страницыInstrumentation and Control in Bio ReactorsJonathan Arredondo50% (2)
- Team 2, Lab 1 - Determination of The Concentration of Ethanoic Acid in Commercial VinegarДокумент24 страницыTeam 2, Lab 1 - Determination of The Concentration of Ethanoic Acid in Commercial VinegarAlondra Fernández AcadémicoОценок пока нет
- Temperature Compensation in PH Meter-A Survey: April 2015Документ10 страницTemperature Compensation in PH Meter-A Survey: April 2015Deepakrao Bornare PatilОценок пока нет
- GA7 Potentio Titr Rev7 99Документ9 страницGA7 Potentio Titr Rev7 99Jerome SadudaquilОценок пока нет
- USP791 PHДокумент4 страницыUSP791 PHJoaquín Andrés Aravena PérezОценок пока нет
- Acid Rain IIДокумент3 страницыAcid Rain IIMaxWittОценок пока нет
- PH Theory Guide - EN - 230113 PDFДокумент98 страницPH Theory Guide - EN - 230113 PDFraiedОценок пока нет
- PH GuideДокумент57 страницPH GuideKshitij MehtaОценок пока нет
- PH USPДокумент4 страницыPH USPAhmad Abdalraheem AamerОценок пока нет
- Experiment 1 Preparation of Buffer SolutionsДокумент16 страницExperiment 1 Preparation of Buffer Solutionsmohamad ashaziq89% (56)
- AP Chemistry - Titration Curves of Strong and Weak Acids and BasesДокумент5 страницAP Chemistry - Titration Curves of Strong and Weak Acids and BasesJonathan Chen100% (2)
- Determination of pKa for Weak AcidДокумент5 страницDetermination of pKa for Weak AcidSonu DubeyОценок пока нет
- Potentiometric TitrationДокумент9 страницPotentiometric Titrationiah_guevarraОценок пока нет
- CSTR Behavior and Saponification Reaction KineticsДокумент7 страницCSTR Behavior and Saponification Reaction KineticsMohammad MdardasОценок пока нет
- PI Exp9 CompiledДокумент12 страницPI Exp9 CompiledZaid SalmanОценок пока нет
- 62 Experiment #5. Titration of An Acid Using A PH MeterДокумент7 страниц62 Experiment #5. Titration of An Acid Using A PH MeteryumnatehreemОценок пока нет
- Laporan Kimdal Percobaan Unit 1Документ7 страницLaporan Kimdal Percobaan Unit 1rulmadhaniОценок пока нет
- Oecd Guidelines For The Testing of ChemicalsДокумент6 страницOecd Guidelines For The Testing of ChemicalsNikita La CruzОценок пока нет
- Experiment 1: Determination of Total Acidity of Vinegar: Final Laboratory ReportДокумент13 страницExperiment 1: Determination of Total Acidity of Vinegar: Final Laboratory ReportEunice OpinioОценок пока нет
- 1019 ADI CFTL Industrial Water Quality Measurement WhitepaperДокумент10 страниц1019 ADI CFTL Industrial Water Quality Measurement WhitepaperGhraib Rady ObaidОценок пока нет
- Measure pH and Prepare BuffersДокумент6 страницMeasure pH and Prepare BuffersSheena PasionОценок пока нет
- Experiment 1 Experiment 1 Experiment 1 Experiment 1 Experiment 1Документ6 страницExperiment 1 Experiment 1 Experiment 1 Experiment 1 Experiment 1Shailesh GhildiyalОценок пока нет
- Automated PH Controller System For HydroponicДокумент5 страницAutomated PH Controller System For HydroponicChaitanya Sunkara50% (2)
- Determination Acetic AcidДокумент21 страницаDetermination Acetic Acidameyakem100% (1)
- CH142Exp5Titration PDFДокумент7 страницCH142Exp5Titration PDFSako RasheedОценок пока нет
- PH in Blood Experimental Model of A Blood Buffer and Its BufДокумент5 страницPH in Blood Experimental Model of A Blood Buffer and Its Bufbashar.adamat101Оценок пока нет
- Chem 18.1 Experiment 6 Formal ReportДокумент5 страницChem 18.1 Experiment 6 Formal Reportlouize_1496Оценок пока нет
- 〈791〉 pHДокумент4 страницы〈791〉 pHThu PhamОценок пока нет
- Weak Acid Strong Base Titration LabДокумент8 страницWeak Acid Strong Base Titration Labapi-265089380100% (1)
- Sub-Physical Pharmacy, B. Pharm, 3 Semester Unit - V: PH, Buffers and Isotonic SolutionsДокумент21 страницаSub-Physical Pharmacy, B. Pharm, 3 Semester Unit - V: PH, Buffers and Isotonic Solutionssatheeshpharma6Оценок пока нет
- PH MEASUREMENT AND BUFFER PREPARATIONДокумент3 страницыPH MEASUREMENT AND BUFFER PREPARATIONJuan Carlos100% (1)
- Exp 3 - Acid Daffa Madri AthaДокумент6 страницExp 3 - Acid Daffa Madri Athadaffa MadriОценок пока нет
- Lab Report DETERMINATION OF THE CONCENTRATION OF ACETIC ACID IN VINEGARДокумент27 страницLab Report DETERMINATION OF THE CONCENTRATION OF ACETIC ACID IN VINEGARمحمد ازوادي100% (1)
- Lab 1 Determination of Acetic Acid in VinegarДокумент17 страницLab 1 Determination of Acetic Acid in Vinegarieja03100% (4)
- P HmetryДокумент4 страницыP Hmetrydhungelsubhash8154Оценок пока нет
- Potentiometric Titration Ex17Документ10 страницPotentiometric Titration Ex17Tien HaminhОценок пока нет
- PH and PH MeterДокумент15 страницPH and PH MeterMuhammad Arshad Ali100% (1)
- PH, Buffers and Isotonic SolutionsДокумент44 страницыPH, Buffers and Isotonic SolutionsNEEMASUBIN100% (1)
- LAB REPORT - Determination of Concentration Acetic Acid in VinegarДокумент12 страницLAB REPORT - Determination of Concentration Acetic Acid in Vinegarhisham100% (3)
- Universidad de Las Américas PueblaДокумент8 страницUniversidad de Las Américas PueblaJesus Alfredo LMОценок пока нет
- Mixture of Carbonate BicarbonateДокумент9 страницMixture of Carbonate BicarbonateIan Justine SanchezОценок пока нет
- Carbonate-Bicarbonate Mixture Anal Chem Post LabДокумент7 страницCarbonate-Bicarbonate Mixture Anal Chem Post LabKennedy OrtegaОценок пока нет
- Acid/Base Titration LabДокумент5 страницAcid/Base Titration LabDavid GrahamОценок пока нет
- Preparative Chromatography for Separation of ProteinsОт EverandPreparative Chromatography for Separation of ProteinsArne StabyОценок пока нет
- Advanced Pharmaceutical analysisОт EverandAdvanced Pharmaceutical analysisРейтинг: 4.5 из 5 звезд4.5/5 (2)
- Enzyme Kinetics: Rapid-Equilibrium Applications of MathematicaОт EverandEnzyme Kinetics: Rapid-Equilibrium Applications of MathematicaОценок пока нет
- L4dxvFAQ1yt15J4cj4jo - PH Control Presentation7Документ5 страницL4dxvFAQ1yt15J4cj4jo - PH Control Presentation7Stephy Florez MenesesОценок пока нет
- Residential Photovoltaic Energy Storage SystemДокумент10 страницResidential Photovoltaic Energy Storage SystemStephy Florez MenesesОценок пока нет
- SimonaДокумент15 страницSimonaStephy Florez MenesesОценок пока нет
- LEDs and Solid-State Lighting History, Benefits, and FundamentalsДокумент82 страницыLEDs and Solid-State Lighting History, Benefits, and FundamentalsOrhan BayhanОценок пока нет
- Texas OptoelectronicsTheoryPracticeДокумент464 страницыTexas OptoelectronicsTheoryPracticeStephy Florez Meneses100% (1)
- FSK Demodulator/ Tone Decoder: ... The Analog Plus CompanyДокумент24 страницыFSK Demodulator/ Tone Decoder: ... The Analog Plus CompanyLuis Fernando RojasОценок пока нет
- ArubaДокумент13 страницArubaStephy Florez MenesesОценок пока нет
- At-3 1Документ72 страницыAt-3 1Javier RobletoОценок пока нет
- Pipe Welding Electrodes 1Документ5 страницPipe Welding Electrodes 1Adura OgunnuОценок пока нет
- Oxidations With Cerium Sulphate SolutionsДокумент4 страницыOxidations With Cerium Sulphate Solutionsliz_hobbs79Оценок пока нет
- G S Earth WireДокумент10 страницG S Earth WiresaratОценок пока нет
- Lime Neutralization Wastewater TreatmentДокумент15 страницLime Neutralization Wastewater TreatmentMehrdad AminkazemiОценок пока нет
- NA To Sls en 1993-5Документ14 страницNA To Sls en 1993-5Shan Sandaruwan AbeywardeneОценок пока нет
- Chapter 20-The First Law of Thermodynamics: Multiple ChoiceДокумент13 страницChapter 20-The First Law of Thermodynamics: Multiple ChoiceJhajha AlboniaОценок пока нет
- EOC c20 Ionic EquilibriaДокумент3 страницыEOC c20 Ionic EquilibriaKenneth KnightОценок пока нет
- Chemistry Assignment and Project 2Документ13 страницChemistry Assignment and Project 2Guru SrinivaasОценок пока нет
- Estudio Antifouling PDFДокумент12 страницEstudio Antifouling PDFJuanIgnacioIzquierdoОценок пока нет
- Eni Blasia 220Документ2 страницыEni Blasia 220hamadaОценок пока нет
- Day 1 Mock Board Exam QuestionsДокумент9 страницDay 1 Mock Board Exam QuestionsXZ Louise Pauleen PalmaresОценок пока нет
- IOGCA 2019 Conference Proceedings PDFДокумент295 страницIOGCA 2019 Conference Proceedings PDFadityamduttaОценок пока нет
- Rectangular Cooling Water Tank Design CalculationsДокумент43 страницыRectangular Cooling Water Tank Design CalculationsNguyễn Quốc PhượngОценок пока нет
- (Version 3.0) Effectiveness of Banana (Musa Paradisiaca) Peel As An Alternative Floor WaxДокумент2 страницы(Version 3.0) Effectiveness of Banana (Musa Paradisiaca) Peel As An Alternative Floor WaxAlexis Barquilla0% (1)
- Weld Inspection Check ListДокумент3 страницыWeld Inspection Check ListBGRLОценок пока нет
- Chapter 27 Static Fluids Chapter 27 Static Fluids ............................................................... 2Документ17 страницChapter 27 Static Fluids Chapter 27 Static Fluids ............................................................... 2Fernando ArrudaОценок пока нет
- Sulphuric Acid MSDSДокумент1 страницаSulphuric Acid MSDSGermán Cárdenas AlvarezОценок пока нет
- Groovy ICP ETCHER SST G Vinogradov - Apr05Документ7 страницGroovy ICP ETCHER SST G Vinogradov - Apr05Peter-sagami100% (1)
- Mole Funsheet 2Документ1 страницаMole Funsheet 2Sk Aul DherОценок пока нет
- Tankguard Storage: Technical Data Sheet Application GuideДокумент14 страницTankguard Storage: Technical Data Sheet Application GuideEngTamerОценок пока нет
- Ah-Polypropylene Case Pressure GaugeДокумент3 страницыAh-Polypropylene Case Pressure Gaugepankaj doshiОценок пока нет
- Mark Scheme (Results) January 2023Документ28 страницMark Scheme (Results) January 2023Rohee TariqОценок пока нет
- Ueditor PHP Upload File 20201124 1606185891368047Документ4 страницыUeditor PHP Upload File 20201124 1606185891368047Banse El-RahmanОценок пока нет
- Fosroc Solvent 102 resin cleaning solventДокумент2 страницыFosroc Solvent 102 resin cleaning solventShaikhRizwanОценок пока нет
- Revised NEQSДокумент10 страницRevised NEQSKhalid Masood GhaniОценок пока нет
- The Chemical Accidents (Emergency Planning, Preparedness and Response) Rules, 1996Документ44 страницыThe Chemical Accidents (Emergency Planning, Preparedness and Response) Rules, 1996erbhaveshparmarОценок пока нет
- Physical Electronics PDFДокумент4 страницыPhysical Electronics PDFSharath Poikayil SatheeshОценок пока нет
- Alamat Perusahaan Perusahaan Di CilegonДокумент19 страницAlamat Perusahaan Perusahaan Di CilegonSri RahayuОценок пока нет
- Production Report November 2021Документ9 страницProduction Report November 2021nasir ahmedОценок пока нет
- Indian Pharmacopoeia 2020 - Vol. 1 (PART 2)Документ242 страницыIndian Pharmacopoeia 2020 - Vol. 1 (PART 2)the reader100% (1)