Академический Документы
Профессиональный Документы
Культура Документы
Lab Report Lab 6
Загружено:
Milan KarkiОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Lab Report Lab 6
Загружено:
Milan KarkiАвторское право:
Доступные форматы
Microbiology Comment
Patatin-like proteins: a new
family of lipolytic enzymes
present in bacteria?
Patatins are a group of plant storage
glycoproteins that show lipid acyl
hydrolase activity (Andrews et al., 1988).
The patatin-associated lipolytic activity
may be a means of defence against plant
parasites (Strickland et al., 1995) and has
been shown to function in plant signal
transduction (Holk et al., 2002). Hirschberg
et al. (2001) found that potato patatin B2
and human cytosolic phospholipase A2
(cPLA2) share conserved domains of
protein homology and therefore predicted
that patatin B2, like cPLA2, contains an
active-site dyad instead of the more
common Ser-His-Asp (or Glu) triad of
lipolytic enzymes (Schrag & Cygler, 1997).
This was confirmed by the crystal structure
of the potato patatin isozyme Pat17 which
together with mutagenesis studies revealed
that the active site consists of a Ser-Asp
dyad (Rydel et al., 2003). Recently, Sato
et al. (2003) and Phillips et al. (2003)
have shown that ExoU, a type III
secreted cytotoxin and virulence factor
of Pseudomonas aeruginosa with lipase as
well as phospholipase A activity, contains
defined regions of protein homology to
potato patatin B2 and cPLA2 with a putative
catalytic Ser-Asp dyad. In animal models
and human infections, the toxicity of
ExoU has been linked to the development
of lung injury, sepsis and bacterial
dissemination (Allewelt et al., 2000;
Finck-Barbancon et al., 1997; Hauser et al.,
1998; Kurahashi et al., 1999). Thus, ExoU
is both an important virulence factor of
P. aeruginosa and the first patatin-like
bacterial hydrolase characterized. However,
it has yet to be determined whether
patatin-like proteins (PLPs) are found in
bacteria other than P. aeruginosa.
To find out whether PLPs exist in bacteria
other than P. aeruginosa, we searched (A) all
522
bacterial genomes available for patatin
B2 and P. aeruginosa ExoU homologues
by means of the BLAST algorithm
(http://www.ncbi.nlm.nih.gov/sutils/
genom_table.cgi) (Altschul et al., 1997)
and (B) we applied the key word patatin
to screen the 123 accessible completed
bacterial genomes using the text search tool
of the PEDANT server (http://pedant.gsf.de).
The PEDANT server provides extensive data
from computational analysis of genome
sequences by algorithms such as PROSITE,
PFAM, BLAST and others. For search (A),
the BLAST alignment, we only obtained a
minimal number of hits for PLPs in
bacteria (<20 for patatin B2 or ExoU,
BLAST expect value1). On the other
hand, we found a high number of hits
(>200) when we used the PEDANT server
text search. In 134 proteins (PFAM domain
expect value0?5), text search hits for
patatin originated from the detection of
the patatin domain by the PFAM algorithm
(Bateman et al., 2002), indicating that only
short amino acid sequences of homology
were conserved within related proteins.
This might also explain why the application
of the BLAST algorithm did not lead to
adequate results. Genes encoding PLPs
were detected in 55 of the 123 completed
bacterial genomes including a wide range
of Gram-positive and Gram-negative
bacteria, such as Bacillus sp., Brucella sp.,
Rickettsia sp., Staphylococcus aureus,
Yersinia pestis and many others.
Moreover, we observed that particularly
some animal pathogen and plant
pathogen/symbiont genomes contain
a high number of PLP genes, e.g.
Bradyrhizobium japonicum (n=8),
Leptospira interrogans (n=6),
Mesorhizobium loti (n=6), Mycobacterium
tuberculosis (n=8) and Ralstonia
solanacearum (n=5). We were interested
in whether pathogens/symbionts possess
more PLP genes than non-pathogens.
Thus, we compared the number of PLP
genes present per 1000 open reading
frames (ORFs) in the two groups and
found that the genomes of the pathogens/
symbionts showed a significantly higher
number of PLP genes than the genomes of
non-pathogens, more precisely 0?800?50
and 0?530?24 (P0?01, Students t-test),
respectively. The number of PLP genes
also varied between different species of a
genus. For example, the genome of
Microbiology 150
Microbiology Comment
Fig. 1. Alignment of selected bacterial PLPs with conserved patatin domains. Sixty proteins with patatin-like domains (PFAM
domain expect value5?561019) were aligned by the CLUSTAL W method (Thompson et al., 1994) using the program
55
MEGALIGN (DNASTAR). Conserved protein domains of a selection of PLPs (PFAM domain expect values from 6610
to
2?661038) representing different bacterial genera and including proteins with different N- or C-terminal extensions are listed
in the order of increasing PFAM expect values. Residues which were conserved in more than 80 % of the 60 aligned proteins
are marked bold. An arrow designates the members of the catalytic dyad in patatin B2 (Rydel et al., 2003). D, The number of
amino acid residues present before and after the conserved blocks. Conserved regions of patatin B2 (modified from
Hirschberg et al., 2001) and of P. aeruginosa ExoU are shown for comparison.
http://mic.sgmjournals.org
523
Microbiology Comment
Mycobacterium leprae included only
one PLP gene, whereas the genome
of M. tuberculosis contained eight
corresponding genes. This finding is in
agreement with a previous report which
stated that M. leprae originated by a
process of reductive evolution resulting in
a minimal gene set sufficient for survival
in its highly specialized niche (Cole et al.,
2001). In contrast, 52 % of the proteome
of M. tuberculosis was derived from gene
duplication (Tekaia et al., 1999), leading to
a higher flexibility in utilizing nutrients.
Furthermore, the genome of pathogenic
Escherichia coli O157 : H7 encoded a higher
number of PLP genes (n=4) than the
genome of non-pathogenic strain E. coli
K-12 (n=2), which again implies a
correlation between the number of PLP
genes and virulence. Hence, we speculate
that a high number of PLP genes might
confer an advantage to the bacterium in
(i) interaction with the host, (ii) adaptation
to various environments and (iii)
competition with other micro-organisms.
To determine whether the bacterial PLPs
share all characteristic domains with their
eukaryotic counterparts, 60 bacterial PLPs
(PFAM domain expect value5?5610219)
were aligned by the CLUSTAL W method
(Thompson et al., 1994). Bacterial PLPs
were found to possess four conserved
domains (block IIV) similar to those in
potato patatin B2 (Fig. 1). Block I consists
of a glycine-rich region with a conserved
arginine or lysine residue which probably
serves as an oxyanion hole. Block II is
located in proximity to block I (about
1020 aa distance) and comprises the
hydrolase motif G-X-S-X-G with the
putative active-site serine (Schrag &
Cygler, 1997). Block III contains a
conserved serine, which may be an
important structural element as a potential
phosphorylation site or due to its capacity
to form hydrogen bonds. The adjacent
highly conserved proline residues (in
blocks III and IV) may be important for
the proper conformation of the protein.
In addition to many structural features
shared both by eukaryotic and bacterial
PLPs, we nevertheless observed that in
block III bacterial and eukaryotic PLPs
show different conserved motifs. While
bacterial PLPs possessed the conserved
sequence A-S-X-X-X-P, eukaryotic patatin
homologues contained the conserved
524
amino acids A-A-P, as seen by CLUSTAL W
alignment of 20 eukaryotic PLPs (Fig. 1,
and data not shown). Block IV includes the
putative active-site aspartate which is the
second member of the catalytic dyad in
patatin Pat17 (Rydel et al., 2003). Successive
to the domain with the active-site aspartate,
Hirschberg et al. (2001) have defined an
additional region of protein homology for
potato patatin B2 and human cPLA2
containing a conserved serine. We found
that this domain was present in at least 15
other eukaryotic patatin homologues (data
not shown) but was not conserved in the
respective prokaryotic proteins (Fig. 1).
We therefore conclude that eukaryotic
and bacterial PLPs share many but not all
structural features, suggesting that they
originated from a common ancestor and
were modified to support/expand the
specific lifestyle of an organism.
proteins. Some bacterial proteins with
N-terminal extensions showed a predicted
cyclic nucleotide-binding domain (e.g.
Caulobacter crescentus gi13423668,
L. interrogans gi24214875, M. tuberculosis
gi13883156) and therefore might be
regulated by cAMP or cGMP (McCue
et al., 2000). Several of the proteins with
C-terminal extensions possessed a putative
bacterial-surfaceantigen domain (e.g.
Pseudomonas putida gi26991199, Vibrio
cholerae gi9655035, Vibrio vulnificus
gi27361160), which is also present in
outer-membrane proteins such as D15
from Haemophilus influenzae (Loosmore
et al., 1997). Furthermore, P. aeruginosa
ExoU (gi2429143) contains a C-terminal
extension, which has been described to
be essential for its cytotoxic activity
(Finck-Barbancon & Frank, 2001; Hauser
et al., 1998).
Arpigny & Jaeger (1999) classified bacterial
esterases and lipases into eight groups based
on their amino acid sequence. Bacterial
PLPs do not show any homology to known
groups of bacterial lipases as evaluated by
BLAST (Altschul et al., 1997) or PFAM
domain search (Bateman et al., 2002) (data
not shown), indicating that the amino
acid sequences of PLPs and established
groups of lipolytic enzymes are not closely
related. Second, except for the G-X-S-X-G
lipase motif which is common even in
distinct lipase families, the conserved
domains presented here for bacterial PLPs
do not exhibit the same features as found
for other bacterial lipases (Fig. 1) (Arpigny
& Jaeger, 1999). Finally, the lipases classified
by Arpigny & Jaeger (1999) either possess
a catalytic Ser-Asp-His triad or a Ser-His
dyad which makes the putative Ser-Asp
catalytic dyad of patatin homologues
unique among bacterial lipases (Rydel et al.,
2003). Since the prokaryotic PLPs appear
to be more related to their eukaryotic
counterparts than to any other group of
bacterial lipases, we propose that they
comprise a new group of bacterial
lipolytic enzymes.
We have herein presented evidence that
genes of PLPs are found in many bacterial
genomes. Therefore, it is conceivable that
many bacteria possess as-yet-unidentified
lipolytic enzymes. Since these proteins
show catalytic domains and conserved
regions distinct from all groups of lipolytic
enzymes known so far in bacteria, we
believe that they comprise a new group
of hydrolytic enzymes. With the exception
of P. aeruginosa cytotoxin ExoU, none of
the bacterial PLPs have been characterized
at present. Consequently, the identification
of the patatin domain may be a valuable
clue for characterizing these putative
hydrolases and could help discover new
virulence factors.
Finally, while comparing the size of
bacterial PLPs, we noticed that several of
them possess a N- or C-terminal extension
(300 aa) preceding or successive to the
conserved blocks, respectively (Fig. 1). An
additional domain search revealed that
many PLPs are two- or multi-domain
Acknowledgements
We thank Winlet Sheba Shadrach for
helpful suggestions with regard to the
manuscript.
Sangeeta Banerji and Antje Flieger
Robert Koch-Institut, Nordufer 20,
D-13353 Berlin, Germany
Correspondence: Antje Flieger
(fliegera@rki.de)
Allewelt, M., Coleman, F. T., Grout, M.,
Priebe, G. P. & Pier, G. B. (2000). Acquisition
of expression of the Pseudomonas aeruginosa
ExoU cytotoxin leads to increased bacterial
virulence in a murine model of acute
pneumonia and systemic spread. Infect
Immun 68, 39984004.
Microbiology 150
Microbiology Comment
Altschul, S. F., Madden, T. L., Schaffer, A. A.,
Zhang, J., Zhang, Z., Miller, W. & Lipman, D. J.
(1997). Gapped BLAST and PSI-BLAST: a new
generation of protein database search programs.
Nucleic Acids Res 25, 33893402.
Andrews, D. L., Beames, B., Summers, M. D.
& Park, W. D. (1988). Characterization of the
lipid acyl hydrolase activity of the major potato
(Solanum tuberosum) tuber protein, patatin,
by cloning and abundant expression in a
baculovirus vector. Biochem J 252, 199206.
Arpigny, J. L. & Jaeger, K.-E. (1999). Bacterial
lipolytic enzymes: classification and properties.
Biochem J 343, 177183.
Bateman, A., Birney, E., Cerruti, L. & 7 other
authors (2002). The Pfam protein families
database. Nucleic Acids Res 30, 276280.
Cole, S. T., Eiglmeier, K., Parkhill, J. & 41
other authors (2001). Massive gene decay in
the leprosy bacillus. Nature 409, 10071011.
Finck-Barbancon, V., Goranson, J., Zhu, L.,
Sawa, T., Wiener-Kronish, J. P., Fleiszig, S. M.,
Wu, C., Mende-Mueller, L. & Frank, D. W.
(1997). ExoU expression by Pseudomonas
aeruginosa correlates with acute cytotoxicity
and epithelial injury. Mol Microbiol 25,
547557.
Finck-Barbancon, V. & Frank, D. W. (2001).
in Mycobacterium tuberculosis. Genome Res 10,
204219.
Phillips, R. M., Six, D. A., Dennis, E. A.
& Ghosh, P. (2003). In vivo phospholipase
activity of the Pseudomonas aeruginosa
cytotoxin ExoU and protection of mammalian
cells with phospholipase A2 inhibitors. J Biol
Chem 278, 4132641332.
Rydel, T. J., Williams, J. M., Krieger, E.,
Moshiri, F., Stallings, W. C., Brown, S. M.,
Pershing, J. C., Purcell, J. P. & Alibhai, M. F.
(2003). The crystal structure, mutagenesis,
and activity studies reveal that patatin is a lipid
acyl hydrolase with a Ser-Asp catalytic dyad.
Biochemistry 42, 66966708.
Sato, H., Frank, D. W., Hillard, C. J. & 9 other
authors (2003). The mechanism of action of
the Pseudomonas aeruginosa-encoded type III
cytotoxin, ExoU. EMBO J 22, 29592969.
Schrag, J. D. & Cygler, M. (1997). Lipases and
alpha/beta hydrolase fold. Methods Enzymol
284, 85107.
Strickland, J. A., Orr, G. L. & Walsh, T. A.
(1995). Inhibition of Diabrotica larval growth
by patatin, the lipid acyl hydrolase from potato
tubers. Plant Physiol 109, 667674.
Tekaia, F., Gordon, S. V., Garnier, T.,
Brosch, R., Barrell, B. G. & Cole, S. T.
(1999). Analysis of the proteome of
Multiple domains are required for the toxic
activity of Pseudomonas aeruginosa ExoU.
J Bacteriol 183, 43304344.
Mycobacterium tuberculosis in silico. Tuber
Lung Dis 79, 329342.
Hauser, A. R., Kang, P. J. & Engel, J. N. (1998).
Thompson, J. D., Higgins, D. G. & Gibson, T. J.
(1994). CLUSTAL W: improving the sensitivity
PepA, a secreted protein of Pseudomonas
aeruginosa, is necessary for cytotoxicity and
virulence. Mol Microbiol 27, 807818.
Hirschberg, H. J. H. B., Simons, J.-W. F. A.,
Dekker, N. & Egmond, M. R. (2001). Cloning,
expression, purification and characterization of
patatin, a novel phospholipase A. Eur J Biochem
268, 50375044.
of progressive multiple sequence alignment
through sequence weighting, position-specific
gap penalties and weight matrix choice. Nucleic
Acids Res 22, 46734680.
DOI 10.1099/mic.0.26957-0
Holk, A., Rietz, S., Zahn, M., Quader, H.
& Scherer, G. F. E. (2002). Molecular
identification of cytosolic, patatin-related
phospholipases A from Arabidopsis with
potential functions in plant signal transduction.
Plant Physiol 130, 90101.
Kurahashi, K., Kajikawa, O., Sawa, T.,
Ohara, M., Gropper, M. A., Frank, D. W.,
Martin, T. R. & Wiener-Kronish, J. P. (1999).
Pathogenesis of septic shock in Pseudomonas
aeruginosa pneumonia. J Clin Invest 104,
743750.
Loosmore, S. M., Yang, Y.-P., Coleman,
D. C., Shortreed, J. M., England, D. M.
& Klein, M. H. (1997). Outer membrane
protein D15 is conserved among Haemophilus
influenzae species and may represent a universal
protective antigen against invasive disease. Infect
Immun 65, 37013707.
McCue, L. A., McDonough, K. A. & Lawrence,
C. E. (2000). Functional classification of
cNMP-binding proteins and nucleotide cyclases
with implications for novel regulatory pathways
http://mic.sgmjournals.org
525
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5784)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- The Social BrainДокумент8 страницThe Social BrainZeljko LekovicОценок пока нет
- Organic Molecules: Chapter 2-3Документ33 страницыOrganic Molecules: Chapter 2-3Gissele AbolucionОценок пока нет
- Who Needs Bypass SurgeryДокумент70 страницWho Needs Bypass SurgerymikikiОценок пока нет
- Evaluation of Preoperative Pulmonary RiskДокумент19 страницEvaluation of Preoperative Pulmonary RiskCharlieBrown_QBОценок пока нет
- Limbic System (Behaviour and Emotion)Документ19 страницLimbic System (Behaviour and Emotion)Patterson MachariaОценок пока нет
- 100 Benefits of ExerciseДокумент3 страницы100 Benefits of ExercisePaul RavenWolf JuniorОценок пока нет
- Nutrients: Energy Metabolism and Intermittent Fasting: The Ramadan PerspectiveДокумент16 страницNutrients: Energy Metabolism and Intermittent Fasting: The Ramadan PerspectiveAlejandro Estrada RiosОценок пока нет
- Nursing Care Plan AmoebiasisДокумент2 страницыNursing Care Plan Amoebiasisderic97% (35)
- Nursing DictionaryДокумент353 страницыNursing DictionaryEmmanuel BoadОценок пока нет
- Leading Causes of Mortality - Department of Health WebsiteДокумент14 страницLeading Causes of Mortality - Department of Health WebsiteRippie RifdahОценок пока нет
- Physiology Objectives DetailedДокумент35 страницPhysiology Objectives DetailedSOOOS94Оценок пока нет
- PsychSim 5 Quizzing - Chapter 6-Sensation and PerceptionДокумент5 страницPsychSim 5 Quizzing - Chapter 6-Sensation and Perceptionneecee126100% (1)
- Autonomic Nervous System For MBBSДокумент20 страницAutonomic Nervous System For MBBSjacobsОценок пока нет
- Neurology Crossword Puzzle Answer KeyДокумент1 страницаNeurology Crossword Puzzle Answer KeyRavinaAhujaОценок пока нет
- Characterizing The Subtype of Anhedonia in Major Depressive DisorderДокумент9 страницCharacterizing The Subtype of Anhedonia in Major Depressive DisorderGkani ChrysoulaОценок пока нет
- Bioactive Lipid Mediators - (2015)Документ424 страницыBioactive Lipid Mediators - (2015)MohammedAjebliОценок пока нет
- Pregnant woman with painful swollen legДокумент12 страницPregnant woman with painful swollen legjimmojonesОценок пока нет
- From: Rapid Interpretation of EKG'sДокумент14 страницFrom: Rapid Interpretation of EKG'sLucija Kljaić100% (1)
- Analysis - Bio - NEET 2023Документ5 страницAnalysis - Bio - NEET 2023oniichanОценок пока нет
- Paediatric Emergency Drug DosageДокумент4 страницыPaediatric Emergency Drug DosageSamir SkejicОценок пока нет
- Nursing Care Plans for Acute Pain, Hyperthermia, Activity Intolerance, and Infection RiskДокумент20 страницNursing Care Plans for Acute Pain, Hyperthermia, Activity Intolerance, and Infection RiskNursidar Pascual Mukattil80% (5)
- Arab Board Orthopedic Exam June 2013Документ35 страницArab Board Orthopedic Exam June 2013Nasser AlbaddaiОценок пока нет
- Vocal AnatomyДокумент8 страницVocal AnatomyfunktotumОценок пока нет
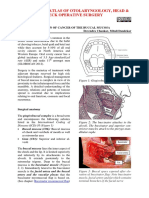
- Surgical Resection of Cancer of The Buccal MucosaДокумент21 страницаSurgical Resection of Cancer of The Buccal MucosapradeepОценок пока нет
- RECALLS - Key points from Preboard 2015 Clinical ChemistryДокумент2 страницыRECALLS - Key points from Preboard 2015 Clinical ChemistrySheanalene CastroОценок пока нет
- OSCHMANДокумент7 страницOSCHMANapi-3708784100% (1)
- HBCJ Npev Vedw T60B PDFДокумент20 страницHBCJ Npev Vedw T60B PDFAnonymous fPQaCe8Оценок пока нет
- Hyperlipedemia PPДокумент51 страницаHyperlipedemia PPrabarОценок пока нет
- The Roots Value of Each Branch Is Given in BracketsДокумент8 страницThe Roots Value of Each Branch Is Given in BracketsAneela PervezОценок пока нет
- Figure 1 Human Nervous System Source:courses - Lumenlearning.c Om/microbiology/chapter/anato My-Of-The-Nervous-SystemДокумент14 страницFigure 1 Human Nervous System Source:courses - Lumenlearning.c Om/microbiology/chapter/anato My-Of-The-Nervous-SystemShekaina Faith Cuizon LozadaОценок пока нет