Академический Документы
Профессиональный Документы
Культура Документы
Chapter 19 Summary
Загружено:
Charlotte0 оценок0% нашли этот документ полезным (0 голосов)
31 просмотров10 страницAlberts
Авторское право
© © All Rights Reserved
Доступные форматы
DOCX, PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документAlberts
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате DOCX, PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
31 просмотров10 страницChapter 19 Summary
Загружено:
CharlotteAlberts
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате DOCX, PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 10
Chapter 19 Summary
SCI 231 Molecular and Cell Biology
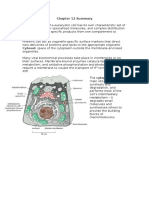
Cells may be linked by direct interactions, or held together in the
extracellular matrix, a network of proteins and polysaccharide
chains that the cells secrete.
Connective tissues (bones, tendons) are formed from an
extracellular matrix produced by cells that are distributed sparsely
in the matrix. The cell-matrix junctions, typically mediated by
integrins, link the cytoskeleton to the matrix, allowing the cells to
move through the matrix and monitor changes in its mechanical
properties.
In epithelial tissues (epidermal covering of skin, lining of gut),
cells are tightly bound together into epithelia sheets. The
extracellular matrix is less pronounced, consisting mainly of a thin
mat called the basal lamina underlying the sheet. Within the
epithelium, cells are attached to each other directly by cell-cell
junctions, where cytoskeletal filaments are anchored, transmitting
stresses across the interiors of the cells, from adhesion site to
adhesion site. Cell-cell junctions are typically mediated by
cadherins.
The tight junction,
adherens junction and
row of desmosomes
together form a
junctional complex. Each
of the four main
anchoring junction types
depends on
transmembrane
adhesion proteins that
span the plasma
membrane, with one end
linking to the
cytoskeleton inside the
cell and the other to the
structures outside it.
Cadherins depend on Ca2+ ions: removing calcium from the
extracellular medium causes adhesions mediated by cadherins to
come apart. All cadherin types have extracellular portions
containing multiple copies of the extracellular cadherin domain. In
the classical cadherins, which are more closely related, there are 5
of these domains, but nonclassical (more distantly related)
cadherins have more than 30. The intracellular portions are
more varied, reflecting interactions with a wide variety of
intracellular ligands, including signaling molecules and
adaptor proteins that connect the cadherin to the
cytoskeleton. Together, classical and nonclassical proteins
make up the cadherin superfamily.
Anchoring junctions between cells are usually symmetrical:
if the linkage is to actin in the cell on one side of the
junction, it will be to actin in the cell on the other side. The
binding between cadherins is homophilic: cadherin
molecules of a specific subtype on once cell bind to those
of the same or closely related subtype on adjacent cells.
The spacing between the cell membranes at an anchoring
junction is precisely defined and depends on the structure of the
participating cadherin molecules. Homophilic binding occurs at the
N-terminal tips of the cadherin molecules the domains that lie
furthers from the membrane. These domains each form a knob and
a nearby pocket, and the cadherin molecules protruding from
opposite cell membranes bind by insertion of the knob of one
domain into the pocket of another.
Desmosomes are structurally similar to
adherens junctions, but contain
specialized cadherins that link to
intermediate filaments instead of actin
filaments. Their main function is to
provide mechanical strength.
On the cytoplasmic surface of each
interacting plasma membrane is a dense
plaque composed of a mixture of
intracellular adaptor proteins. A bundle of
keratin intermediate filaments is attached
to the surface of each plaque.
Transmembrane nonclassical cadherins
bind to the plaques and interact through
their extracellular domains to hold the
adjacent membranes together.
Inside the cell, bundles of ropelike intermediate
filaments that are anchored to the desmosomes
form a structural framework of great tensile
strength, with linkage to similar bundles in
adjacent cells, creating a network that extends
throughout the tissue. The type of intermediate
filaments attached depends on the cell type:
they are keratin filaments in most epithelial cells, and desmin
filaments in heart and muscle cells.
Essentially all epithelia are anchored to other tissue on one side
the basal sideand free of such attachment on their opposite side
the apical side. A basal lamina lies at the interface with the
underlying tissue, mediating the attachment, while the apical
surface of the epithelium is generally bathed by extracellular fluid.
Thus, all epithelia are structurally polarized, and so are their
individual cells: the basal end of a cell, adherent to the basal lamina
below, differs from the apical end, exposed to the medium above.
All epithelia have at least one function in common: selective
permeability barriers, separating the fluid that permeates the tissue
on their basal side from fluid with a different chemical composition
on their apical side. This barrier function requires that the adjacent
cells be sealed together by tight junctions, so that molecules
cannot leak freely across the cell sheet. Tight junctions, besides
sealing the gaps between the cells, also function as fences that
help prevent apical or basolateral proteins from diffusing into the
wrong region.
The movement of ions and
other molecules between
epithelial cells is called
paracellular transport.
When tight junctions are
visualized by freezefracture electron
microscopy (B in below image), they are seen as a branching
network of sealing strands that completely encircles the apical end
of each cell in the epithelial sheet. In conventional electron
micrographs (C), the outer leaflets of the two interacting plasma
membranes are tightly apposed where sealing strands are present.
Each sealing strand is composed of a long row of transmembrane
homophilic adhesion proteins embedded in each of the two
interacting plasma membranes. The extracellular domains of these
proteins adhere directly to one another to occlude the intercellular
space.
The main transmembrane proteins forming these strands are
claudins, which are essential for tight-junction formation and
function. Different claudin types are expressed in different
combinations to confer particular permeability properties on the
epithelial sheet. They form paracellular pores selective channels
allowing specific ions to cross the tight-junctional barrier, from one
extracellular space to another. Normal tight junctions also contain a
second major transmembrane protein called occludin, which is not
essential for the assembly or structure of the tight junction but is
important for limiting junctional permeability. Tricellulin is required
to seal cell membranes together and prevent transepithelial leakage
at the points where three cells meet.
Gap junctions bridge gaps between adjacent cells, to create direct
channels from the cytoplasm of one to that of the other. The gap is
spanned by channel-forming proteins, of which there are two
families: connexins (mostly found in vertebrates) and innexins
(mostly found in invertebrates).
They have pores of about 1.4 nm, allowing the
exchange of inorganic ions and other small
water-soluble molecules, but not
macromolecules. Al electrical current injected
into one cell causes electrical disturbance in
neighboring cells, due to the flow of ions
carrying electric charge through the gap
junctions.
Connexins are four-pass transmembrane proteins, 6 of which
assemble to form a hemichannel, or connexon. When the
connexons in the plasma membranes of two cells in contact are
aligned, they form a continuous aqueous channel that connects the
two cell interiors. A gap junction consists of many connexon pairs in
parallel, forming a sieve.
Skin, blood vessels, and lungs need to be both strong and elastic in
order to function. A network of elastic fibers in the extracellular
matrix of these tissues gives them the resilience to recoil after
transient stretch. Long, inelastic collagen fibrils are interwoven with
the elastic fibers to limit the extent of stretching and prevent the
tissue from tearing. The main component is elastin, a highly
hydrophobic protein coming from the soluble precursor tropoelastin,
which is secreted into the extracellular space and assembled into
elastic fibers close to the plasma membrane.
The elastin protein is composed of two types of short segments that
alternate along the polypeptide chain: hydrophobic segments, which
are responsible for the elastic properties, and alanine-lysine rich ahelical segments, which are cross-linked to adjacent molecules by
covalent attachment of lysine residues. Each segment is encoded by
a separate exon. Parts of the elastin polypeptide chain adopt a loose
random coil
formation, which
cross-link into the
elastic fiber
network and
allows the
network to stretch
and recoil.
The basil lamina is a thin, tough and flexible sheet of matrix
molecules that underpins all epithelia. It separates cells and
epithelia from the underlying or surrounding connective tissue and
forms the mechanical connection between them. It can also lie
between two sheets and function as a selective filter.
They also are able to determine cell polarity, influence cell
metabolism, organize the proteins in adjacent plasma membranes,
promote cell survival, proliferation or differentiation, and serve as
highways for cell migration.
The basal lamina is synthetized by the cells on each side of it: the
epithelial cells contribute one set of basal lamina components, while
cells of the underlying bed of connective tissue contribute another
set.
Laminin is the primary organizer of the sheet structure. Type IV
collagen is a second essential component, which assembles
extracellularly into a flexible network that gives the basil lamina
tensile strength. Laminin and type IV collagen interact with other
basal lamina components, such as the glycoprotein nidogen and the
proteoglycan perlecan, resulting in a highly cross-linked network of
proteins and proteoglycans.
Вам также может понравиться
- Chapter 17 SummaryДокумент22 страницыChapter 17 SummaryCharlotteОценок пока нет
- Chapter 16 SummaryДокумент11 страницChapter 16 SummaryCharlotteОценок пока нет
- Chapter 20 SummaryyДокумент21 страницаChapter 20 SummaryyCharlotteОценок пока нет
- Chapter 8 SummaryДокумент25 страницChapter 8 SummaryCharlotteОценок пока нет
- Chapter 12 SummaryДокумент7 страницChapter 12 SummaryCharlotteОценок пока нет
- Chapter 13 SummaryДокумент5 страницChapter 13 SummaryCharlotteОценок пока нет
- Chapter 15 SummaryДокумент27 страницChapter 15 SummaryCharlotteОценок пока нет
- Chapter 18 SummaryДокумент7 страницChapter 18 SummaryCharlotteОценок пока нет
- Chapter 7 SummaryДокумент34 страницыChapter 7 SummaryCharlotteОценок пока нет
- Chapter 4 SummaryДокумент8 страницChapter 4 SummaryCharlotteОценок пока нет
- Chapter 3 SummaryДокумент10 страницChapter 3 SummaryCharlotteОценок пока нет
- Chapter 2 SummaryДокумент3 страницыChapter 2 SummaryCharlotteОценок пока нет
- Chapter 6 SummaryДокумент28 страницChapter 6 SummaryCharlotteОценок пока нет
- Chapter 1 SummaryДокумент4 страницыChapter 1 SummaryCharlotteОценок пока нет
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- The Impact of School Facilities On The Learning EnvironmentДокумент174 страницыThe Impact of School Facilities On The Learning EnvironmentEnrry Sebastian71% (31)
- Sarvali On DigbalaДокумент14 страницSarvali On DigbalapiyushОценок пока нет
- Os PPT-1Документ12 страницOs PPT-1Dhanush MudigereОценок пока нет
- Orc & Goblins VII - 2000pts - New ABДокумент1 страницаOrc & Goblins VII - 2000pts - New ABDave KnattОценок пока нет
- Bluetooth TutorialДокумент349 страницBluetooth Tutorialjohn bougsОценок пока нет
- Skuld List of CorrespondentДокумент351 страницаSkuld List of CorrespondentKASHANОценок пока нет
- Borello-Bolted Steel Slip-Critical Connections With Fillers I. PerformanceДокумент10 страницBorello-Bolted Steel Slip-Critical Connections With Fillers I. PerformanceaykutОценок пока нет
- If V2 would/wouldn't V1Документ2 страницыIf V2 would/wouldn't V1Honey ThinОценок пока нет
- DECA IMP GuidelinesДокумент6 страницDECA IMP GuidelinesVuNguyen313Оценок пока нет
- DLL - The Firm and Its EnvironmentДокумент5 страницDLL - The Firm and Its Environmentfrances_peña_7100% (2)
- ERP Complete Cycle of ERP From Order To DispatchДокумент316 страницERP Complete Cycle of ERP From Order To DispatchgynxОценок пока нет
- Mercedes BenzДокумент56 страницMercedes BenzRoland Joldis100% (1)
- Lifespan Development Canadian 6th Edition Boyd Test BankДокумент57 страницLifespan Development Canadian 6th Edition Boyd Test Bankshamekascoles2528zОценок пока нет
- Ujian Madrasah Kelas VIДокумент6 страницUjian Madrasah Kelas VIrahniez faurizkaОценок пока нет
- Account Statement From 30 Jul 2018 To 30 Jan 2019Документ8 страницAccount Statement From 30 Jul 2018 To 30 Jan 2019Bojpuri OfficialОценок пока нет
- Interpretation of Arterial Blood Gases (ABGs)Документ6 страницInterpretation of Arterial Blood Gases (ABGs)afalfitraОценок пока нет
- TheEconomist 2023 04 01Документ297 страницTheEconomist 2023 04 01Sh FОценок пока нет
- SEG Newsletter 65 2006 AprilДокумент48 страницSEG Newsletter 65 2006 AprilMilton Agustin GonzagaОценок пока нет
- Chennai Metro Rail BoQ for Tunnel WorksДокумент6 страницChennai Metro Rail BoQ for Tunnel WorksDEBASIS BARMANОценок пока нет
- NewspaperДокумент11 страницNewspaperКристина ОрёлОценок пока нет
- Case Study Hotel The OrchidДокумент5 страницCase Study Hotel The Orchidkkarankapoor100% (4)
- LM1011 Global ReverseLogДокумент4 страницыLM1011 Global ReverseLogJustinus HerdianОценок пока нет
- Petty Cash Vouchers:: Accountability Accounted ForДокумент3 страницыPetty Cash Vouchers:: Accountability Accounted ForCrizhae OconОценок пока нет
- Axe Case Study - Call Me NowДокумент6 страницAxe Case Study - Call Me NowvirgoashishОценок пока нет
- #### # ## E232 0010 Qba - 0Документ9 страниц#### # ## E232 0010 Qba - 0MARCOОценок пока нет
- Planning A Real Estate ProjectДокумент81 страницаPlanning A Real Estate ProjectHaile SilasieОценок пока нет
- AZ-900T00 Microsoft Azure Fundamentals-01Документ21 страницаAZ-900T00 Microsoft Azure Fundamentals-01MgminLukaLayОценок пока нет
- BIBLIO Eric SwyngedowДокумент34 страницыBIBLIO Eric Swyngedowadriank1975291Оценок пока нет
- DELcraFT Works CleanEra ProjectДокумент31 страницаDELcraFT Works CleanEra Projectenrico_britaiОценок пока нет
- Sharp Ar5731 BrochureДокумент4 страницыSharp Ar5731 Brochureanakraja11Оценок пока нет