Академический Документы
Профессиональный Документы
Культура Документы
Fetal Maturation WHO
Загружено:
Gerry IrawanАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Fetal Maturation WHO
Загружено:
Gerry IrawanАвторское право:
Доступные форматы
30/1/2015
WHO|Antenataladministrationofcorticosteroidsforwomenatriskofpretermbirth
HelpusimproveRHLtakethisshortsurvey
Antenataladministrationofcorticosteroids
forwomenatriskofpretermbirth
Theadministrationofcertaincorticosteroidstowomenatriskof
pretermbirthcausesaconsiderablereductionintherisksof
complicationsofprematuritysuchasrespiratorydistresssyndrome,
intraventricularhaemorrhageandperinataldeath.Corticosteroidtherapy
shouldbeincorporatedintocomprehensivematernalhealthcare
servicesandofficialguidelinesformaternitycare.
RHLCommentarybyHofmeyrGJ
1.INTRODUCTION
Pretermbirthisaleadingcauseofperinataldeathanddisabilityandis
animportantpublichealthproblemglobally(1).Pretermbirthoccurs
mostcommonlyineconomicallydisadvantagedcommunitiesandthose
withhighratesofurinaryandgenitaltractinfection.Theproblemof
pretermbirthinunderresourcedsettingsiscompoundedbyalackof
neonatalhealthcarefacilitiesandaccesstoexpensiveinterventions
suchassurfactanttherapy.Informationontheeffectivenessof
antenatalcorticosteroids,acomparativelyinexpensiveintervention,is
thusparticularlyrelevantforthesesettings.Thiscommentarycovers
threeCochranereviewsthatsoughtto:(i)"assesstheeffectsonfetal
andneonatalmorbidityandmortality,onmaternalmortalityand
morbidity,andonthechildinlaterlifeofadministeringcorticosteroidsto
themotherbeforeanticipatedpretermbirth"(2)"assessthe
effectivenessandsafetyofarepeatdose(s)ofprenatalcorticosteroids"
(3)and"assesstheeffectsofdifferentcorticosteroidregimensfor
womenatriskofpretermbirth"(4).
2.METHODS
AllthreeCochranereviewsusedappropriate,standardCochrane
methodology,includingacomprehensivesearchfortrials,inclusionof
trialsaccordingtopredefinedqualitycriteria,transparentdataextraction
andprespecifiedanalyses.
3.RESULTS
3.1Antenatalcorticosteroidsforacceleratingfetallung
maturationforwomenatriskofpretermbirth
ThisCochranereviewwasupdatedin2006,withtheinclusionof21
studiesinvolving3885womenand4269infants.Thereviewfoundthat
theadministrationofcertaincorticosteroidstowomenatriskofpreterm
deliveryproducesaconsiderablereductionintherisksofcomplications
ofprematuritysuchascombinedfetalandneonataldeath,respiratory
distresssyndrome,cerebroventricularhaemorrhage,necrotizing
enterocolitis,systemicinfectionsandchildhooddevelopmentaldelay.
http://apps.who.int/rhl/pregnancy_childbirth/complications/preterm_birth/cd004454_hofmeyrgj_com/en/index.html
1/5
30/1/2015
WHO|Antenataladministrationofcorticosteroidsforwomenatriskofpretermbirth
Benefitswerefoundwhentreatmentwascommencedbetween26and
35weeksofgestation,andforbabiesborn17daysaftercommencing
treatment,andalsoforsubsetsofwomenwithprematureruptureofthe
membranesandwithhypertensivedisorders.Combinedfetaland
neonataldeathswerereducedevenininfantsbornlessthan24hours
afteradministrationofthefirstdose.
Nobenefitsweredemonstratedfortreatmentcommenced,orinfants
born,before26weeksofgestation,norforthosebornmorethanseven
daysaftertreatment.Forbabiesbornafter36weekstherewasatrend
toincreasecombinedfetalandneonataldeath.
Birthweightwasreducedininfantsborn17days,andmorethanseven
daysafterthefirsttreatment.Onetrialthathadrecruitedwomenwith
severepreeclampsiasuggestedthatthetreatedwomenwereat
increasedriskofgestationaldiabetes.
Evidencefromepidemiologicalandanimalstudiessuggeststhatthere
maybelongtermadverseeffectsofprenatalcorticosteroidexposure,
includingimpairedglucosetoleranceandhypertension.Animalstudies
havealsosuggestedimpairmentofbraingrowth.
Followupoftheoffspringofonetrialatage30yearsfoundanincrease
ininsulinreleaseinresponsetoa75gglucoseload,butnoother
morbidity.
Corticosteroidregimensshowntobeeffectiveinclude:betamethasone
12mgintramuscularly,2doses24hoursapartordexamethasone6mg
intramuscularly4doses12hourly.Studiesusingmethylprednisolone
wereexcludedbecausethissteroidhasbeenshowntohavealtered
placentaltransfer.Thereviewfoundindirectevidencesuggestinga
greaterreductionofrespiratorydistresssyndromewithbetamethasone
thanwithdexamethasonetreatment,anddexamethasonesignificantly
increasedtheriskofpuerperalsepsis(seeiiibelow).
3.2Repeatdosesofprenatalcorticosteroidsforwomenat
riskofpretermbirthforpreventingneonatalrespiratory
disease
Thisreviewincludesfivetrialsinvolving2028women.Inallfivetrials,
whichwereofhighmethodologicalquality,repeatcorticosteroidtherapy
wasgiventowomenwhohadreceivedasinglecourseofcorticosteroids
sevenormoredayspreviously.Fourtrialsusedtwodosesof
betamethasone12mgandoneusedsingledoses,allrepeatedweekly.
Oneormorerepeatcoursesofcorticosteroidswereassociatedwith
reducedseverelungdisease[relativerisk(RR)0.6095%confidence
interval(CI)0.480.75]seriousinfantmorbidity(RR0.7995%CI0.67
0.93).Ontheotherhand,inonetrialtherewasareductioninbirth
weightZscore(RR0.13,95%CI0.260.00)andintwotrialsthere
wasanincreasedriskofbeingsmallforgestationalageatbirth(RR
1.63,95%CI1.122.37)Nootheroutcomeswerestatistically
significant.However,availabledatafromthestudiesdidnotgivethe
reviewsufficientstatisticalpowertoassessimportantrareoutcomes
suchasperinatal/infantdeath(RR0.5395%CI:0.181.57).Inonetrial,
http://apps.who.int/rhl/pregnancy_childbirth/complications/preterm_birth/cd004454_hofmeyrgj_com/en/index.html
2/5
30/1/2015
WHO|Antenataladministrationofcorticosteroidsforwomenatriskofpretermbirth
in160womenwithpretermprematureruptureofthemembranes,there
wasnosignificantreductioninrespiratorydistress,andmaternal
chorioamnionitiswasincreased(RR1.56,95%CI1.052.31).Onetrial
reporteddataonasubgroupofwomenwhoreceivefourormorerepeat
coursesofcorticosteroids:birthweightwassignificantlyreduced(WMD
161,95%CI29131).Benefitsintermsofrespiratorydistressand
evidenceofgrowthimpairmentwereseenbothintrialswithasingle
doseandthosewithtwodoses,allrepeatedweekly.
Theauthorsconcludethat,whiletherewasevidenceofshortterm
respiratorybenefitsfromrepeatedcoursesofcorticosteroids,therewas
insufficientevidenceregardingthepotentialrisksandlongtermeffects
tojustifytheuseofrepeateddosesofcorticosteroidsinclinical
practice,andthatfurtherresultswereneeded(severaltrialsare
continuinglongtermfollowup).
3.3Differentcorticosteroidsandregimensforaccelerating
fetallungmaturationforwomenatriskofpretermbirth
Thisreview,publishedin2008,includestentrialsinvolving1089women
and1161infants.Thereviewfoundthatdexamethasonedecreasedthe
incidenceofintraventricularhaemorrhagecomparedwithbetamethasone
(RR0.44,95%CI0.210.92fourtrials,549infants).Nostatistically
significantdifferenceswereseenforotherprimaryoutcomes,including
respiratorydistresssyndrome,bronchopulmonarydysplasia,severe
intraventricularhaemorrhage,periventricularleukomalacia,perinatal
death,ormeanbirthweight.Resultsforbiophysicalparameterswere
inconsistent,butmostlynoimportantdifferenceswereseenforthoseor
anyothersecondaryoutcomeexceptthat,inonetrialof105infants,
significantlymoreinfantsinthedexamethasonegroupwereadmittedto
aneonatalintensivecareunitcomparedwiththebetamethasonegroup
(RR3.83,95%CI1.2411.87).
Oraldexamethasonecomparedwithintramusculardexamethasone
increasedtheincidenceofneonatalsepsis(RR8.48,95%CI1.11
64.93)inonetrialof183infants.Nostatisticallysignificantdifferences
wereseenforotheroutcomesreported.Inonesmalltrialof69infants,
whichhadcomparedbetamethasoneacetateandphosphatewith
betamethasonephosphate,nodifferenceswereseenforanyofthe
outcomesreported.
4.DISCUSSION
4.1.APPLICABILITYOFTHERESULTS
Mostofthestudiesreviewedwereconductedinindustrializedcountries.
Theriskofrespiratorydistresssyndromeatspecificgestationalages
maydifferindifferentsettings.Itmaythusbenecessarytouselocal
datatoidentifygestationalagesatwhichbabiesareatriskof
respiratorydistresssyndromeorperiventricularhaemorrhage.Thereis
nobiologicalreasontoexpectthatcorticosteroidtherapywillnotbe
equallyeffectiveinallsettingsinimprovingtheoutcomeforfetuses
identifiedasatriskofrespiratorydistressandothercomplicationsof
prematurebirthindifferentpopulations(personalopinion).
http://apps.who.int/rhl/pregnancy_childbirth/complications/preterm_birth/cd004454_hofmeyrgj_com/en/index.html
3/5
30/1/2015
WHO|Antenataladministrationofcorticosteroidsforwomenatriskofpretermbirth
4.2.IMPLEMENTATIONOFTHEINTERVENTION
Corticosteroidtherapyisrelativelyinexpensive.Itisfeasibleto
implementcorticosteroidtherapyatprimaryhealthcarelevelprovided
skilledhealthcareprovidersareavailabletoidentifywomenatriskof
pretermbirthandadministerintramuscularinjections.Givena
reasonablehealthserviceinfrastructure,nospecificorganizational
changesareneeded.Corticosteroidtherapyshouldbeincorporatedinto
comprehensivematernalhealthcareservices,andincludedinofficial
guidelinesformaternitycare.Dexamethasoneorbetamethasoneshould
beavailableinallmaternityfacilitiesandshouldbeincludedinnational
essentialdrugslists.OurexperienceinSouthAfricaisthathealthcare
providershavereadilytakenuptheuseofantenatalcorticosteroid
therapy,asisthecaseinotherlowincomecountriessuchasThailand
(5).
Themajorbarriertoimplementationofcorticosteroidtherapyisthe
difficultyofidentifyingwomenatriskofpretermdeliveryintimeto
administercorticosteroids.Thisrequiresaneffectiveandwellutilized
antenatalservice.Successfulimplementationofthisinterventionwould
involve:educationofhealthcareprovidersregardingtheeffectiveness
andimplementationofcorticosteroidtherapyintroductionofprotocols
foritsuseidentificationofwomenatrisk,includingeffectiveantenatal
screeningforhypertensionandproteinuria,aspreeclampsiaisan
importantcauseofpretermdeliveryinlowincomecountriesand
providinginformationtopregnantwomen.Theinformationtopregnant
womenwouldneedtofocusonearlyreportingtoahealthfacilityatthe
firstsignsofpregnancycomplicationssuchaspretermuterine
contractions,pretermruptureofmembranesandsymptomsofpre
eclampsia.
Currentevidencesupportstheuseofdexamethasoneasthefirstchoice
oftreatment:four6mgdosesgiven12hourly.However,if
dexamethosoneisnotavailable,betamethasonemaybeused:two12
mgdosesgiven24hourly.
4.3.IMPLICATIONSFORRESEARCH
Furthertrialsontheeffectivenessofantenatalcorticosteroidtreatment
arenotjustified.Researchshouldinsteadbedirectedtowardsmethods
ofachievingeffectiveimplementationoftheinterventionindifferent
settings.Furtherresearchisalsoneededonthebenefitsandrisksof
repeatedcoursesofcorticosteroidtherapy,andtheoptimaldrug,dose
androuteofadministration.
Sourcesofsupport:EasternCapeDepartmentofHealth,Universitiesof
theWitwatersrandandFortHare,SouthAfricaWHOLongterm
InstitutionalDevelopmentGrant.
References
1. SaigalS,DoyleLW.Anoverviewofmortalityandsequelaeof
pretermbirthfrominfancytoadulthood.TheLancet
2007371(9608):2619.
2. RobertsD,DalzielSR.Antenatalcorticosteroidsforaccelerating
fetallungmaturationforwomenatriskofpretermbirth.Cochrane
http://apps.who.int/rhl/pregnancy_childbirth/complications/preterm_birth/cd004454_hofmeyrgj_com/en/index.html
4/5
30/1/2015
WHO|Antenataladministrationofcorticosteroidsforwomenatriskofpretermbirth
DatabaseofSystematicReviews2006Issue3.Art.No.:
CD004454DOI:10.1002/14651858.CD004454.pub2.
3. CrowtherCA,HardingJE.Repeatdosesofprenatalcorticosteroids
forwomenatriskofpretermbirthforpreventingneonatalrespiratory
disease.CochraneDatabaseofSystematicReviews2007Issue3.
Art.No.:CD003935DOI:10.1002/14651858.CD003935.pub2.
4. BrownfootFC,CrowtherCA,MiddletonP.Differentcorticosteroids
andregimensforacceleratingfetallungmaturationforwomenat
riskofpretermbirth.CochraneDatabaseofSystematicReviews
2008Issue4.Art.No.:CD006764DOI:
10.1002/14651858.CD006764.pub2.
5. SaengwareeP,LiabsuetrakulT.Changingpracticeon
corticosteroids.JournaloftheMedicalAssociationofThailand
200588:30713.
Thisdocumentshouldbecitedas:HofmeyrGJ.Antenatal
corticosteroidsforwomenatriskofpretermbirth:RHLcommentary(last
revised:2February2009).TheWHOReproductiveHealthLibrary
Geneva:WorldHealthOrganization.
Relateddocuments
CochraneReview1
CochraneReview2
CochraneReview3
Abouttheauthor
HofmeyrGJ
http://apps.who.int/rhl/pregnancy_childbirth/complications/preterm_birth/cd004454_hofmeyrgj_com/en/index.html
5/5
Вам также может понравиться
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- 4 6019193414606653039Документ914 страниц4 6019193414606653039venkat mu100% (1)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Family Nursing Care Plan ModuleДокумент16 страницFamily Nursing Care Plan ModuleHarlene Joyce ReyОценок пока нет
- Overview of Postpartum Hemorrhage - UpToDateДокумент48 страницOverview of Postpartum Hemorrhage - UpToDatewendy100% (1)
- Preterm LabourДокумент3 страницыPreterm Labourcgao30Оценок пока нет
- Nursing QuestionsДокумент23 страницыNursing QuestionsTaSha Kalil MОценок пока нет
- Incompetent CervixДокумент20 страницIncompetent CervixTiffany AdriasОценок пока нет
- Case Pre EditedДокумент112 страницCase Pre EditedZanesville Lymont L. SubidoОценок пока нет
- Lesson Plan of Lab Demo Face PresentationДокумент17 страницLesson Plan of Lab Demo Face PresentationShalu Bohra Manola100% (1)
- Preboard Nursing ExamsДокумент91 страницаPreboard Nursing ExamsJimmalyn Santos100% (1)
- Unit X - Abnormalities During Postnatal Period Assessment and Management of Women With Postnatal Complications Total: 4 HoursДокумент73 страницыUnit X - Abnormalities During Postnatal Period Assessment and Management of Women With Postnatal Complications Total: 4 Hourssoumya satheshОценок пока нет
- ObstetricsДокумент8 страницObstetricsrevathidadam55555100% (1)
- Tugas 1Документ20 страницTugas 1lastia meilinaОценок пока нет
- NCP - Delivery RoomДокумент8 страницNCP - Delivery RoomAUBREY MARIE . GUERREROОценок пока нет
- RLE-level-2-packet-week-12-requirement (SANAANI, NUR-FATIMA, M.)Документ26 страницRLE-level-2-packet-week-12-requirement (SANAANI, NUR-FATIMA, M.)Nur SanaaniОценок пока нет
- Ok Fer Ger 15.1.19Документ12 страницOk Fer Ger 15.1.19Gerry IrawanОценок пока нет
- Sonographic Large Fetal Head Circumference and Risk of Cesarean DeliveryДокумент3 страницыSonographic Large Fetal Head Circumference and Risk of Cesarean DeliveryGerry IrawanОценок пока нет
- Duty Ger 20.07.2018Документ25 страницDuty Ger 20.07.2018Gerry IrawanОценок пока нет
- 5-1-18 Konkli Er GerДокумент8 страниц5-1-18 Konkli Er GerGerry IrawanОценок пока нет
- Lembar JawabanДокумент6 страницLembar JawabanGerry IrawanОценок пока нет
- Soal Blok 14Документ8 страницSoal Blok 14blinkbumbumОценок пока нет
- Neet PG 2023 Imp TopicsДокумент21 страницаNeet PG 2023 Imp TopicsAslesh AnandОценок пока нет
- Session 17 HIV OverviewДокумент20 страницSession 17 HIV Overviewapi-19824701Оценок пока нет
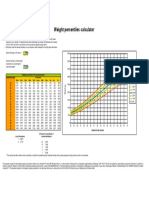
- Weight Percentiles CalculatorДокумент1 страницаWeight Percentiles CalculatorAqila MumtazОценок пока нет
- Postpartum Depression EssayДокумент10 страницPostpartum Depression Essayapi-549230626Оценок пока нет
- English Case Iufd+covid-19 Fuad SaddamДокумент57 страницEnglish Case Iufd+covid-19 Fuad SaddamanggatrifiandaprimaОценок пока нет
- Value Nurses Community April 2003 eДокумент42 страницыValue Nurses Community April 2003 eCarlos RobertoОценок пока нет
- Teenage Preg UpДокумент22 страницыTeenage Preg UpGift MesaОценок пока нет
- High Risk PregnancyДокумент4 страницыHigh Risk PregnancyForky DorkyОценок пока нет
- CHN Lecture Note For Set 24 Student NursesДокумент37 страницCHN Lecture Note For Set 24 Student NursesYahya Ahmad SulaimamОценок пока нет
- Midwifery in SpainДокумент14 страницMidwifery in SpainCarmen G GuerreroОценок пока нет
- Obstetric History & Examination SheetДокумент8 страницObstetric History & Examination SheetVaibhav BhatiaОценок пока нет
- Obstetrics Cards 19 30 PDFДокумент81 страницаObstetrics Cards 19 30 PDFNavid BabluОценок пока нет
- Meconium Stained Amniotic FluidДокумент21 страницаMeconium Stained Amniotic FluidPoonam RanaОценок пока нет
- MEHT130379 Postnatal Exercises 2.0Документ7 страницMEHT130379 Postnatal Exercises 2.0rachellycОценок пока нет
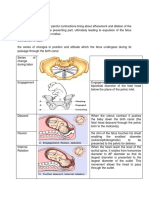
- Mechanism of LaborДокумент2 страницыMechanism of Laborgerold m chuaОценок пока нет
- Parturition or Child BirthДокумент21 страницаParturition or Child BirthInnoclazz AcademyОценок пока нет