Академический Документы
Профессиональный Документы
Культура Документы
03 Practice - Classification of The Elements Key PDF
Загружено:
jtОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
03 Practice - Classification of The Elements Key PDF
Загружено:
jtАвторское право:
Доступные форматы
T174
Name
Date
CHAPTER
Class
Name
STUDY GUIDE FOR CONTENT MASTERY
Date
CHAPTER
Class
STUDY GUIDE FOR CONTENT MASTERY
Chemistry: Matter and Change
Section 6.2 continued
Section 6.2
Classification of the Elements
In your textbook, read about s-, p-, d-, and f-block elements.
In your textbook, read about organizing the elements by electron configuration.
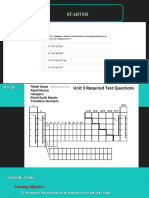
Use the periodic table on pages 156157 in your textbook and the periodic table below to
answer the following questions.
Use the periodic table on pages 156157 in your textbook to match each element in
Column A with the element in Column B that has the most similar chemical properties.
s2
2
He
s block
s1
Column A
Column B
1. arsenic (As)
a. boron (B)
1
H
s2
p1
p2
p block
p3
p4
p5
p6
4
Be
5
B
6
C
7
N
8
O
9
F
10
Ne
2. bromine (Br)
b. cesium (Cs)
3
Li
3. cadmium (Cd)
c. chromium (Cr)
11
Na
12
Mg
d1
d2
d3
d4
d block
d5
d6
d7
d8
d9
d10
13
Al
14
Si
15
P
16
S
17
Cl
18
Ar
19
20
Ca
21
Sc
22
Ti
23
V
24
Cr
25
Mn
26
Fe
27
Co
28
Ni
29
Cu
30
Zn
31
Ga
32
Ge
33
As
34
Se
35
Br
36
Kr
37
38
Sr
39
Y
40
Zr
41
Nb
42
Mo
43
Tc
44
Ru
45
Rh
46
Pd
47
Ag
48
Cd
49
In
50
Sn
51
Sb
52
Te
53
I
54
Xe
55
56
Ba
71
Lu
72
Hf
73
Ta
74
W
75
Re
76
Os
77
Ir
78
Pt
79
Au
80
Hg
81
Tl
82
Pb
83
Bi
84
Po
85
At
86
Rn
87
88
Ra
103
Lr
104
Rf
105
Db
106
Sg
107
Bh
108
Hs
109
Mt
110
Uun
111
Uuv
112
Uub
f11
f12
4. gallium (Ga)
d. cobalt (Co)
5. germanium (Ge)
e. hafnium (Hf)
6. iridium (Ir)
f.
7. magnesium (Mg)
g. iron (Fe)
8. neon (Ne)
h. nitrogen (N)
Rb
iodine (I)
Cs
Fr
f1
f2
f3
f4
f5
f6
f block
f7
f8
57
58
59
60
61
62
63
Study Guide for Content Mastery Answer Key
9. nickel (Ni)
i.
platinum (Pt)
La
10. osmium (Os)
j.
scandium (Sc)
Ac
11. sodium (Na)
k. silicon (Si)
12. tellurium (Te)
l.
13. tungsten (W)
m. sulfur (S)
14. yttrium (Y)
n. zinc (Z)
15. zirconium (Zr)
o. xenon (Xe)
89
Ce
90
Th
Pr
Nd
91
92
Pa
Pm
93
Np
Sm
94
Pu
Eu
95
Am
64
Gd
96
Cm
f9
65
Tb
97
Bk
f10
66
Dy
98
Cf
67
68
Ho
Er
99
100
Es
Fm
69
f14
70
Yb
101
102
Md
No
four
18. Into how many blocks is the periodic table divided?
strontium (Sr)
f13
Tm
19. What groups of elements does the s-block contain? groups 1A and 2A
20. Why does the s-block portion of the periodic table span two groups?
The s orbital holds a maximum of two electrons.
21. What groups of elements does the p-block contain? groups 3A through 8A
Answer the following questions.
22. Why are members of group 8A virtually unreactive?
group 8A elements have both their s orbitals and p orbitals completely filled with
16. Why do sodium and potassium, which belong to the same group in the periodic table,
have similar chemical properties?
electrons. This configuration is very stable, thus, the group 8A elements are very
Sodium and potassium have the same number of valence electrons.
unreactive.
23. How many d-block elements are there? 40
17. How is the energy level of an elements valence electrons related to its period on the
periodic table? Give an example.
24. What groups of elements does the d-block contain? group B elements
The energy level indicates the period. For example, lithiums valence electron is in
25. Why does the f-block portion of the periodic table span 14 groups?
The seven f orbitals hold a maximum of 14 electrons.
the second energy level and lithium is found in period 2.
2
4
26. What is the electron configuration of the element in period 3, group 6A? [Ne]3s 3p
Study Guide for Content Mastery
Chemistry: Matter and Change Chapter 6
Copyright Glencoe/McGraw-Hill, a division of the McGraw-Hill Companies, Inc.
33
34
Chemistry: Matter and Change Chapter 6
Study Guide for Content Mastery
Вам также может понравиться
- 10 Science TP 5 1Документ5 страниц10 Science TP 5 1Nawaab PuneetОценок пока нет
- Honors Chemistry WKSHT Periodic Table IA ANSWERSДокумент10 страницHonors Chemistry WKSHT Periodic Table IA ANSWERSKaleb HuttoОценок пока нет
- Periodic TableДокумент4 страницыPeriodic Tablesohailhasmi984Оценок пока нет
- Cbse 2020 Boards MCQ ScienceДокумент5 страницCbse 2020 Boards MCQ ScienceAbuzar AzharОценок пока нет
- Chapter 5 and 6 TestДокумент9 страницChapter 5 and 6 Testshahinazs100% (1)
- SAT Chem 01 Atomic Structure & PeriodicityДокумент2 страницыSAT Chem 01 Atomic Structure & Periodicityarshad_bah100% (1)
- Cdu Unit Chemistry Informal Diagnostics: Grade Level: 9 Stream: ADV The Year 2023/2024Документ27 страницCdu Unit Chemistry Informal Diagnostics: Grade Level: 9 Stream: ADV The Year 2023/2024alshamsi5001010Оценок пока нет
- Exercise Solution of Periodic ElementsДокумент9 страницExercise Solution of Periodic ElementsiTutor Classes BapiОценок пока нет
- Xi Chem Chapt3 PEriodic Properties of Elements WorksheetДокумент10 страницXi Chem Chapt3 PEriodic Properties of Elements WorksheetNandini Classes,City Light ,Surat. Cell (9429090525Оценок пока нет
- 10 Science NcertSolutions Chapter 5 ExercisesДокумент4 страницы10 Science NcertSolutions Chapter 5 ExercisesContacts nilОценок пока нет
- Valence Electrons and Electron Configuration: Chemistry WorksheetДокумент2 страницыValence Electrons and Electron Configuration: Chemistry Worksheetrobertkani2004Оценок пока нет
- Chap 3 ModДокумент26 страницChap 3 ModM Zia DogarОценок пока нет
- Periodic Classification of ElementsДокумент6 страницPeriodic Classification of ElementsKumar AbhishantОценок пока нет
- Chemistry Chang 11th Edition Test Bank Full DownloadДокумент18 страницChemistry Chang 11th Edition Test Bank Full Downloadkatherineguzmanqrzncbmida100% (32)
- PeriodicДокумент16 страницPeriodicJoyel DsouzaОценок пока нет
- Lecture 1Документ23 страницыLecture 1Az-zahraa'Оценок пока нет
- ICSE Selina Solution For Class 9 Chemistry Chapter 5 Exercise QuestionsДокумент15 страницICSE Selina Solution For Class 9 Chemistry Chapter 5 Exercise QuestionsAnubrata SarkarОценок пока нет
- Page Number: 81: Subject-Chemistry DATE - 22/12/2020 Class - X TOPICS - Solutions of Textual Questions of Chapter 5Документ5 страницPage Number: 81: Subject-Chemistry DATE - 22/12/2020 Class - X TOPICS - Solutions of Textual Questions of Chapter 5Umar Aman VirkОценок пока нет
- By Pri Nce Sir: ChemistryДокумент37 страницBy Pri Nce Sir: ChemistryShantam AnandОценок пока нет
- Grace Pfenning - CHДокумент3 страницыGrace Pfenning - CHapi-526065196Оценок пока нет
- 1 Chapter 5: Periodic Classification of ElementsДокумент3 страницы1 Chapter 5: Periodic Classification of Elementsjoydeep17590Оценок пока нет
- Chapter 5 Periodic Classification of ElementsДокумент9 страницChapter 5 Periodic Classification of ElementsasuhassОценок пока нет
- CBSE Class 10 Science Chapter 5 NCERT Solutions 2022 - Free PDFДокумент7 страницCBSE Class 10 Science Chapter 5 NCERT Solutions 2022 - Free PDFMuzafar ahmadОценок пока нет
- 5.2 Electron Configuration and The Periodic TableДокумент21 страница5.2 Electron Configuration and The Periodic Tableapi-3863745Оценок пока нет
- Idk QPДокумент26 страницIdk QPalshamsi5001010Оценок пока нет
- Villegas.w3. Periodic Table and PeriodicityДокумент3 страницыVillegas.w3. Periodic Table and PeriodicityShivsОценок пока нет
- Periodic Table WorksheetДокумент4 страницыPeriodic Table Worksheettony zouОценок пока нет
- Concept-Skills Development - PerdДокумент65 страницConcept-Skills Development - Perdjnbp1Оценок пока нет
- Worksheet 1: Periodic Properties and Variation of PropertiesДокумент3 страницыWorksheet 1: Periodic Properties and Variation of Propertiessai hitheshОценок пока нет
- Periodic ClassificationДокумент4 страницыPeriodic ClassificationtusharОценок пока нет
- Periodic Classification of Elements: Chapter-5Документ98 страницPeriodic Classification of Elements: Chapter-5Throwaway AccountОценок пока нет
- Exercise Soln of Periodic ElementsДокумент13 страницExercise Soln of Periodic ElementsiTutor Classes BapiОценок пока нет
- ChemДокумент1 страницаChemsuhaanОценок пока нет
- Chapter 6 - Chang Test BankДокумент22 страницыChapter 6 - Chang Test BankDariusz MilewskiОценок пока нет
- Chemistry Worksheet Chapter 5 Periodic Classification of ElementsДокумент3 страницыChemistry Worksheet Chapter 5 Periodic Classification of ElementsRajesh SrinivasanОценок пока нет
- 03 Periodic Properties Formula Sheets Getmarks AppДокумент10 страниц03 Periodic Properties Formula Sheets Getmarks Appmusk7597Оценок пока нет
- Periodic Classification of Elements ExerciseДокумент11 страницPeriodic Classification of Elements ExerciseupsahuОценок пока нет
- Chapter 6 Assessment PDFДокумент17 страницChapter 6 Assessment PDFMichael Foster67% (3)
- 4a ANSWERS Periodic Patterns (2017)Документ2 страницы4a ANSWERS Periodic Patterns (2017)Karina LeungОценок пока нет
- Periodic Classification PYQsДокумент31 страницаPeriodic Classification PYQsa9758127118Оценок пока нет
- First 5 Chapters Chemistry XiiДокумент201 страницаFirst 5 Chapters Chemistry XiiIkram ali khan100% (2)
- Valence Shell Configuration Day 3Документ12 страницValence Shell Configuration Day 3Home ZamanОценок пока нет
- SS2 Chemistry 1st Term Lesson Note PDFДокумент73 страницыSS2 Chemistry 1st Term Lesson Note PDFAugustine AmaechiОценок пока нет
- Periodic Classification of Elements PDFДокумент8 страницPeriodic Classification of Elements PDFDeepak GH100% (1)
- First Term SS 2: ChemistryДокумент74 страницыFirst Term SS 2: Chemistryangus ogwucheОценок пока нет
- Chemistry Assignment 3 Class 11Документ4 страницыChemistry Assignment 3 Class 11Nayan ShahОценок пока нет
- Chang Problems Chapter 8Документ9 страницChang Problems Chapter 8ChaОценок пока нет
- Chemsitry X - Basic - Periodic Classification of ElementsДокумент3 страницыChemsitry X - Basic - Periodic Classification of ElementsAman9692Оценок пока нет
- Document (54) Idfk 1Документ14 страницDocument (54) Idfk 1Akshay ChakrabortyОценок пока нет
- CH - 4Документ5 страницCH - 4Phantom GamingОценок пока нет
- Periodic Table of Elements Task 1aДокумент7 страницPeriodic Table of Elements Task 1aOtgon OrgilОценок пока нет
- Clasification of Elements in The Periodic TableДокумент81 страницаClasification of Elements in The Periodic TableAZIAH ABUОценок пока нет
- Test Paper Class 10 Term 2 ChemistryДокумент3 страницыTest Paper Class 10 Term 2 ChemistryrudraprasadgiriОценок пока нет
- Chapter 8: Periodic Relationships Among The ElementsДокумент14 страницChapter 8: Periodic Relationships Among The Elements216435964Оценок пока нет
- Chemistry: The Periodic Table and PeriodicityДокумент7 страницChemistry: The Periodic Table and PeriodicityAiden100% (1)
- Lakhmir Singh Solutions Class 10 Chemistry Chapter 5Документ23 страницыLakhmir Singh Solutions Class 10 Chemistry Chapter 5Karthikeya PuttaguntaОценок пока нет
- Ncert Solution Cbse Class 10 Science Chapter 5Документ9 страницNcert Solution Cbse Class 10 Science Chapter 5ANTONY DEV DОценок пока нет
- Continuum Mechanics: Concise Theory and ProblemsОт EverandContinuum Mechanics: Concise Theory and ProblemsРейтинг: 3.5 из 5 звезд3.5/5 (3)
- Biology M5 Cellular RespirationДокумент30 страницBiology M5 Cellular RespirationjtОценок пока нет
- Em WavesДокумент24 страницыEm WavesjtОценок пока нет
- 2.3 Shapes of Molecules and IonsДокумент33 страницы2.3 Shapes of Molecules and IonsjtОценок пока нет
- APEX EvolutionДокумент60 страницAPEX EvolutionjtОценок пока нет
- APEX Anatomy of GenesДокумент91 страницаAPEX Anatomy of Genesjt100% (1)
- Biology M4 PhotosynthesisДокумент28 страницBiology M4 Photosynthesisjt50% (2)
- Biology Module 14. Genetics - The Study of Inherited Traits2Документ34 страницыBiology Module 14. Genetics - The Study of Inherited Traits2Kameshvra92% (12)
- Revised Radar Form 1 2 TemplateДокумент2 страницыRevised Radar Form 1 2 Templatejt100% (4)
- Basic EndocrineДокумент7 страницBasic EndocrinejtОценок пока нет
- APEX The Organ SystemsДокумент171 страницаAPEX The Organ Systemsjt100% (2)
- RespirationДокумент33 страницыRespirationjtОценок пока нет
- BreathingДокумент7 страницBreathingjtОценок пока нет
- KS4 The Heart and Circulatory SystemДокумент49 страницKS4 The Heart and Circulatory SystemjtОценок пока нет
- KS4 Earth's StructureДокумент25 страницKS4 Earth's StructurejtОценок пока нет
- Family Feud 2016Документ16 страницFamily Feud 2016Ira MarianoОценок пока нет
- Worksheet - Evolution PacketДокумент10 страницWorksheet - Evolution PacketjtОценок пока нет
- Role of The SupervisorДокумент15 страницRole of The SupervisorThomas SilombaОценок пока нет
- Boardworks DiffusionДокумент8 страницBoardworks DiffusionjtОценок пока нет
- Biodiversity - Sample Exam Questions (Student Book)Документ5 страницBiodiversity - Sample Exam Questions (Student Book)GenОценок пока нет
- Weather and Climate-Cs VersionДокумент19 страницWeather and Climate-Cs VersionjtОценок пока нет
- PolynomialsДокумент12 страницPolynomialsjt100% (1)
- Noori SB - Proj Mot 1Документ52 страницыNoori SB - Proj Mot 1jtОценок пока нет
- Climate Change v2.0Документ48 страницClimate Change v2.0jtОценок пока нет
- Boardworks Exploring SpaceДокумент9 страницBoardworks Exploring SpacejtОценок пока нет
- 7L The Solar System and BeyondДокумент40 страниц7L The Solar System and BeyondjtОценок пока нет
- VolcanoesДокумент7 страницVolcanoesjtОценок пока нет
- Human ReproductionДокумент40 страницHuman Reproductionjt100% (2)
- Electrical Signals v2.0Документ42 страницыElectrical Signals v2.0jt100% (1)
- Basic EndocrineДокумент7 страницBasic EndocrinejtОценок пока нет
- DWATCHДокумент92 страницыDWATCHFawaz SayedОценок пока нет
- EVK203/EVK213/EVK223/EVK233/EVK253: Digital Thermostats For Ventilated Refrigerating UnitsДокумент2 страницыEVK203/EVK213/EVK223/EVK233/EVK253: Digital Thermostats For Ventilated Refrigerating UnitsMihai BordeianuОценок пока нет
- Supplementary Spec To API Specification 17D Subsea Wellhead and Tree Equipment With Justifications S 561Jv2022 11Документ81 страницаSupplementary Spec To API Specification 17D Subsea Wellhead and Tree Equipment With Justifications S 561Jv2022 11maximusala83Оценок пока нет
- All Electricity Worksheets For Midterm 22Документ22 страницыAll Electricity Worksheets For Midterm 22Maryam AlkaabiОценок пока нет
- Jokes and Their Relation To The Unconscious: Laurence HenkelmanДокумент3 страницыJokes and Their Relation To The Unconscious: Laurence HenkelmanMilos VisnjicОценок пока нет
- Fitting in and Fighting Back: Stigma Management Strategies Among Homeless KidsДокумент24 страницыFitting in and Fighting Back: Stigma Management Strategies Among Homeless KidsIrisha AnandОценок пока нет
- Parenting Styles and Social Interaction of Senior Secondary School Students in Imo State, NigeriaДокумент10 страницParenting Styles and Social Interaction of Senior Secondary School Students in Imo State, NigeriaInternational Educational Applied Scientific Research Journal (IEASRJ)Оценок пока нет
- Ducted Exhaust Ventilation Fans: Low Noise, High Performance Air and Moisture ExtractionДокумент4 страницыDucted Exhaust Ventilation Fans: Low Noise, High Performance Air and Moisture ExtractionNicolas BaquedanoОценок пока нет
- Ashin Tejaniya: Questions & Answers WithДокумент241 страницаAshin Tejaniya: Questions & Answers WithharioОценок пока нет
- Guest-302344325-Quantitative Process AnalysisДокумент4 страницыGuest-302344325-Quantitative Process AnalysisAhmedОценок пока нет
- BIS Ventilation Brochure enДокумент16 страницBIS Ventilation Brochure enBruno SantosОценок пока нет
- Presentation 2Документ70 страницPresentation 2Vivek LathОценок пока нет
- Assignment PSДокумент2 страницыAssignment PSMohsin Islam RifatОценок пока нет
- Vertical Transportation in BuildingsДокумент46 страницVertical Transportation in BuildingsHIMA MiniОценок пока нет
- Plant Based Plan White PaperДокумент24 страницыPlant Based Plan White PaperSara AdemovicОценок пока нет
- Afcat Question Paper 01-2014 PDFДокумент10 страницAfcat Question Paper 01-2014 PDFTuhin AzadОценок пока нет
- English 1 Reading (CVC)Документ27 страницEnglish 1 Reading (CVC)Angelica ArcangelОценок пока нет
- Bycatch Reduction Devices - PresentationДокумент92 страницыBycatch Reduction Devices - PresentationSujit Shandilya0% (1)
- Michigan Clinic 2008 NotesДокумент10 страницMichigan Clinic 2008 NotesCoach Brown100% (3)
- Challenges in The Functional Diagnosis of Thyroid Nodules Before Surgery For TSH-producing Pituitary AdenomaДокумент5 страницChallenges in The Functional Diagnosis of Thyroid Nodules Before Surgery For TSH-producing Pituitary AdenomaAthul IgnatiusОценок пока нет
- Jurnal Kasus Etikolegal Dalam Praktik KebidananДокумент13 страницJurnal Kasus Etikolegal Dalam Praktik KebidananErni AnggieОценок пока нет
- Rapid Review Hematology PDFДокумент155 страницRapid Review Hematology PDFBaguma Michael100% (3)
- MSDS Industrial MargarineДокумент3 страницыMSDS Industrial MargarineAeropaulo14Оценок пока нет
- Penelitian-Amanda Desviani PДокумент89 страницPenelitian-Amanda Desviani PvantopelОценок пока нет
- Surveys For Maint'Ce ClassДокумент7 страницSurveys For Maint'Ce ClassSuhe EndraОценок пока нет
- Cocktail Making Techniques 13.3.11Документ3 страницыCocktail Making Techniques 13.3.11Ryan MenezesОценок пока нет
- 01 Head and Neck JMДокумент16 страниц01 Head and Neck JMJowi SalОценок пока нет
- Polycystic Ovary Syndrome (PCOS) - Symptoms, Causes, and TreatmentДокумент19 страницPolycystic Ovary Syndrome (PCOS) - Symptoms, Causes, and TreatmentAkshay HarekarОценок пока нет
- Psicologia BuenisimoДокумент6 страницPsicologia BuenisimoSophieОценок пока нет
- Presented By: Agamjeet Singh Batra, VI - NДокумент27 страницPresented By: Agamjeet Singh Batra, VI - NlovleshrubyОценок пока нет