Академический Документы
Профессиональный Документы
Культура Документы
Discussion CSTR
Загружено:
illyzlОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Discussion CSTR
Загружено:
illyzlАвторское право:
Доступные форматы
8.
0 DISCUSSION
This experiment was conducted in order to determine the effect of step
change input on the concentration of the sodium chloride (NaCl) solution in
CSTR in series. In this experiment, it was expected that the conductivity
values of reactor 1 and reactor 3 will be equal as the NaCl solution was fed
into the reactors continuously throughout the experiment. The flowrate of the
feed was kept about 150 mL/min and the system was running isothermally
with each reactors temperature at around 29oC. The value of conductivity of
reactor 1 (QT1), reactor 2 (QT2) and reactor 3 (QT3) was recorded for every
3 minutes using the conductivity probe. The experiment was ended when the
conductivity of the reactor 1 and reactor 3 are equal and constant for the few
last readings.
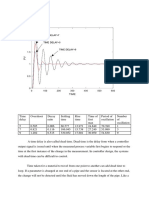
The first reading for each reactors at t 0 are as follows; QT1 is 12.61,
QT2 is 3.15, and QT3 is 0.756 mS/min. The results can be observed in the
results section of the report. As can be seen from the results obtained in the
experiment, the conductivity of the mixture in CSTR increases as time passes
on as more and more salt solution was fed into the reactors. At the 102 th
minute it can be observed that the conductivity of the three reactors are
slowly to be constant and finally about at 108 th minute, the conductivity
values of QT1 is 2.59, QT2 is 26.00, and QT3 is 26.00 mS/min.
The conductivity of the reaction mixture varies with conversion and
therefore the extent of the reaction can be observed by plotting the
conductivity with respect to time. (Reaction Engineering, n.d). From the
graph, it can be concluded that the conductivity values for each reactor
increase as time pass by. The conductivity of reactor 1 recorded at t0 was
12.61, this value is highest compared reactor 2 and reactor 3 which was 3.15
and 0.756 mS/min respectively.
As there were no valves or controllers to accurately regulate the flow
rate of deionized water, there were some fluctuations in the flow during the
experiments and the flow had to be adjusted manually during the
experiments. These fluctuations could have affected the results, hence the
graph obtained is not a smooth curve. (Sevrig, 2007).
The affect of the step change to the concentration are the same for all
three reactors. When the step change of solute concentration was introduced
at the feed of reactor 1, the tank will experience a transient behavior
(unsteady state) as shown in the result. The concentration of in the reactor
will increase in a period of time until it has reached a constant concentration.
For every reactor it has its own constant concentration. Reactor 1 reached the
constant concentration first followed by reactor 2 and reactor 3.
The reaction carried out in a series of CSTRs will eventually reach
steady state when a certain amount of conversion of the starting reagents has
taken place. The steady state conditions will be influenced by the
concentration of reagents, flow rate, volume of reactor and temperature of
reaction. (Engineering Reaction, n.d.).
The conversion for each reactor was calculated in order to investigate
whether CSTR in series will affect the conversion of NaCl solution in the
reactor. From the results obtained, reactor 1 has 95% of conversion while
reactor 2 has 99.7% of conversion and reactor 3, 99.8% conversion. The
CSTR in series have advantage in making the final product have higher
conversion than a single CSTR. This could be explained that the compound
NaCl have higher residence time in a series CSTR than in single CSTR.
A major weakness of a single CSTR is that all of the reaction takes
place at the low final reactant concentration and hence requires an
excessively large reactor hold-up. In order to overcome this weakness, in
CSTR series, only the last one will have a reaction rate governed by the final
reactant concentration. Besides, if one vessel in the cascade has to be put out
of commission, it may be by passed and production continued at a slightly
reduced rate whereas failure of a single CSTR will cause complete loss in
production. (Cooper, 1971).
Based on the results and the graph obtained, it is shown that the step
change input affects the concentration of NaCl solution in CSTR in series.
Reactor 3 has the highest conversion of 99.8% followed by reactor 2, 99.7%
and reactor 1, 95%.
Вам также может понравиться
- CSTR Series Response to Pulse InputДокумент13 страницCSTR Series Response to Pulse InputKhairul Zakirin78% (9)
- Lab ReportДокумент12 страницLab ReportkaimanwatsoNОценок пока нет
- Analysis and Design of Multicell DC/DC Converters Using Vectorized ModelsОт EverandAnalysis and Design of Multicell DC/DC Converters Using Vectorized ModelsОценок пока нет
- BNBBHBHBHJBJHДокумент25 страницBNBBHBHBHJBJHZati TarhiziОценок пока нет
- TubularДокумент15 страницTubularSharing CaringОценок пока нет
- CSTR SeriesДокумент14 страницCSTR SeriesElina Nes100% (1)
- CKB 20104 Reaction Engineering UniKL MICET Experiment 2a Effect of RTD On The Reaction in CSTR Full Lab ReportДокумент29 страницCKB 20104 Reaction Engineering UniKL MICET Experiment 2a Effect of RTD On The Reaction in CSTR Full Lab ReportSiti Hajar Mohamed100% (6)
- TFR ReportДокумент23 страницыTFR ReportJessie Ctiffany EveОценок пока нет
- Modeling of The Residence Time Distribution and Application of The Continuous Two Impinging Streams Reactor in Liquid-Liquid ReactionsДокумент6 страницModeling of The Residence Time Distribution and Application of The Continuous Two Impinging Streams Reactor in Liquid-Liquid ReactionsNorma JenarezОценок пока нет
- CSTRДокумент15 страницCSTRbilisfreak100% (3)
- Discussion Conclusion RecommendationДокумент2 страницыDiscussion Conclusion RecommendationFaiz BasriОценок пока нет
- CKB 20104 Reaction Engineering UniKL MICET Experiment 4: Reactor Test Rig Full Lab ReportДокумент14 страницCKB 20104 Reaction Engineering UniKL MICET Experiment 4: Reactor Test Rig Full Lab ReportSiti Hajar Mohamed100% (10)
- Chemical Engineering Laboratory Ii: /DT Term Is Zero SinceДокумент9 страницChemical Engineering Laboratory Ii: /DT Term Is Zero SinceKayathre Raveendran100% (1)
- CSTR 40L LAB EXPERIMENTДокумент18 страницCSTR 40L LAB EXPERIMENTSaber Minato Azrul100% (2)
- Tutorial Exp 4Документ4 страницыTutorial Exp 4Faris Hamir100% (4)
- Final Report PFRДокумент12 страницFinal Report PFRmark_ancotОценок пока нет
- L9-Tubular Flow ReactorДокумент20 страницL9-Tubular Flow ReactorCik Tiem Ngagiman82% (11)
- Chemical Reactors: DC DT RДокумент8 страницChemical Reactors: DC DT ROsas Jessica UwoghirenОценок пока нет
- Tubular ReactorДокумент20 страницTubular ReactorMuhamad Hafifi AjwadОценок пока нет
- Experiment 1 - SampleДокумент20 страницExperiment 1 - SampleNasrul FirdausОценок пока нет
- Saponification Reaction of Sodium Hydroxide An Ethyl Acetate in A Continuous-Stirred Tank Reactor (CSTR)Документ21 страницаSaponification Reaction of Sodium Hydroxide An Ethyl Acetate in A Continuous-Stirred Tank Reactor (CSTR)drami94100% (13)
- Effect of Residence Time on Saponification Reaction in a Plug Flow ReactorДокумент21 страницаEffect of Residence Time on Saponification Reaction in a Plug Flow ReactorValentinoDullSatin100% (1)
- Continuous Stirred Tank Reactor CSTR in Series PDFДокумент15 страницContinuous Stirred Tank Reactor CSTR in Series PDFMuhamad Hafifi AjwadОценок пока нет
- Plug Flow Reactor ExperimentДокумент16 страницPlug Flow Reactor ExperimentN Afiqah RazakОценок пока нет
- Continuous Distillation Column Lab Report (40Документ27 страницContinuous Distillation Column Lab Report (40JimОценок пока нет
- PFR ReactorДокумент19 страницPFR Reactorkhairi100% (1)
- Continuous Stirred Tank Reactor (40 L)Документ16 страницContinuous Stirred Tank Reactor (40 L)Mohd Zhariff75% (4)
- RTD Analysis of Tubular Flow ReactorДокумент19 страницRTD Analysis of Tubular Flow ReactorsueeumuraОценок пока нет
- Tubular Flow Reactor ReportДокумент19 страницTubular Flow Reactor ReportN Afiqah Razak100% (1)
- Examine Flow Reactor ExperimentsДокумент25 страницExamine Flow Reactor ExperimentsafiqahanuwarОценок пока нет
- Lab6-Tubular Flow ReactorДокумент11 страницLab6-Tubular Flow ReactorNurtasha Atikah100% (1)
- CSTR in SeriesДокумент17 страницCSTR in SeriesDhiyyah MardhiyyahОценок пока нет
- Result &calculationДокумент9 страницResult &calculationMohd Azman SuwandiОценок пока нет
- Student 4 Mini Project (Reaction Engineering)Документ7 страницStudent 4 Mini Project (Reaction Engineering)Muhammad KasyfiОценок пока нет
- 4 - (PFR BP101)Документ15 страниц4 - (PFR BP101)Aisyah Addia AzizanОценок пока нет
- Plug Flow ReactorДокумент16 страницPlug Flow Reactormirdza94Оценок пока нет
- CSTR 40 LДокумент20 страницCSTR 40 LMuhammad NasrulОценок пока нет
- TFR ExperimentДокумент25 страницTFR ExperimentSiti Norbaya100% (1)
- Full ReportДокумент27 страницFull ReportmnizamarzukiОценок пока нет
- LAB Plug FlowДокумент24 страницыLAB Plug FlowZalina SamsuddinОценок пока нет
- CSTR 40LДокумент11 страницCSTR 40LSeiji Kyousei100% (1)
- CKB 20104 Reaction Engineering UniKL MICET Experiment 3a: Effect of Residence Time On The Reaction in A PFR Full Lab ReportДокумент20 страницCKB 20104 Reaction Engineering UniKL MICET Experiment 3a: Effect of Residence Time On The Reaction in A PFR Full Lab ReportSiti Hajar MohamedОценок пока нет
- Lab ManualДокумент24 страницыLab ManualAasia FarrukhОценок пока нет
- Sample Lab ReportljДокумент10 страницSample Lab ReportljDavid DavisОценок пока нет
- CSTRsДокумент10 страницCSTRsAchini NawarathnaОценок пока нет
- Table of ContentДокумент17 страницTable of ContentOh DausОценок пока нет
- CSTR Saponification Reaction Rate StudyДокумент12 страницCSTR Saponification Reaction Rate StudyMohamad SyamilОценок пока нет
- Understanding CSTR Dynamics with a Step Change Input ExperimentДокумент16 страницUnderstanding CSTR Dynamics with a Step Change Input ExperimentAhmadAzriMohamad50% (2)
- CSTR Experiment: Effects of Process Variables on Reaction RateДокумент17 страницCSTR Experiment: Effects of Process Variables on Reaction RateAllyssa BadilloОценок пока нет
- Stirred Tank ReactorДокумент32 страницыStirred Tank ReactorChristopher Emeka Ominyi100% (1)
- CSTRДокумент25 страницCSTRAinul Mardhiah Abdul Rahim100% (1)
- CSTR Dynamics ExperimentДокумент14 страницCSTR Dynamics ExperimentBhinitha ChandrasagaranОценок пока нет
- Procedures: Safety Googles, Gloves and Lab Coat Were Worn When Handling These Chemicals)Документ4 страницыProcedures: Safety Googles, Gloves and Lab Coat Were Worn When Handling These Chemicals)JxinLeeОценок пока нет
- FullДокумент33 страницыFullEja RotiKeju100% (2)
- Conclusion & Recommendation (Test Rig)Документ1 страницаConclusion & Recommendation (Test Rig)Noor FatihahОценок пока нет
- 1.1 AbstractДокумент25 страниц1.1 AbstractZati TarhiziОценок пока нет
- Stirred Tank Reactor Series Experiment ReportДокумент20 страницStirred Tank Reactor Series Experiment ReportEmonbeifo EfosasereОценок пока нет
- Lab 4Документ3 страницыLab 4illyzlОценок пока нет
- ETR300 NewДокумент88 страницETR300 NewillyzlОценок пока нет
- Introduction To Human Resource ManagementДокумент10 страницIntroduction To Human Resource ManagementillyzlОценок пока нет
- Pickling Process Effects on Food QualityДокумент2 страницыPickling Process Effects on Food QualityillyzlОценок пока нет
- Stream NumberДокумент1 страницаStream NumberillyzlОценок пока нет
- Discussion CSTRДокумент2 страницыDiscussion CSTRillyzl100% (1)
- Lab GDCДокумент19 страницLab GDCillyzlОценок пока нет
- Project Food 2017Документ1 страницаProject Food 2017illyzlОценок пока нет
- Abstract Conclusion Reference Lab 9Документ2 страницыAbstract Conclusion Reference Lab 9illyzlОценок пока нет
- Sheer Protein Business PlanДокумент44 страницыSheer Protein Business PlanillyzlОценок пока нет
- Growth Kinetics of E. coli in Shake Flask ExperimentДокумент14 страницGrowth Kinetics of E. coli in Shake Flask ExperimentillyzlОценок пока нет
- Stream NumberДокумент1 страницаStream NumberillyzlОценок пока нет
- IntroductionДокумент7 страницIntroductionillyzlОценок пока нет
- Lab Gas AbsorptionДокумент4 страницыLab Gas AbsorptionillyzlОценок пока нет
- Gas AbsorptionДокумент11 страницGas AbsorptionillyzlОценок пока нет
- Issue On SuicideДокумент4 страницыIssue On SuicideillyzlОценок пока нет
- Tubular ReactorДокумент19 страницTubular ReactorillyzlОценок пока нет
- LATESTTTTTTTTTTTДокумент6 страницLATESTTTTTTTTTTTillyzlОценок пока нет
- Views On SuicideДокумент6 страницViews On SuicideillyzlОценок пока нет
- Pressure Drop Vs Air Flow Rate: Flowra Te (L/Min) Pressure Drop (MMH O)Документ4 страницыPressure Drop Vs Air Flow Rate: Flowra Te (L/Min) Pressure Drop (MMH O)illyzlОценок пока нет
- Transport PhenomenaДокумент1 страницаTransport PhenomenaillyzlОценок пока нет
- Stream NumberДокумент1 страницаStream NumberillyzlОценок пока нет
- Abstract Conclusion Reference Lab 9Документ2 страницыAbstract Conclusion Reference Lab 9illyzlОценок пока нет
- SuicideДокумент4 страницыSuicideillyzlОценок пока нет
- Lab 3 Parameters (Using Constant) Simulation Time 600Документ4 страницыLab 3 Parameters (Using Constant) Simulation Time 600illyzlОценок пока нет
- Osha Chemical DescriptionДокумент3 страницыOsha Chemical DescriptionillyzlОценок пока нет
- Lab 1 DiscussionДокумент11 страницLab 1 DiscussionillyzlОценок пока нет
- Results Calculations CSTR 40LДокумент7 страницResults Calculations CSTR 40LillyzlОценок пока нет
- Script Mandarin Level 2 PinyinДокумент6 страницScript Mandarin Level 2 PinyinillyzlОценок пока нет
- Fehling's Test: Adlawan - Cainoy - Lawagon - Pascua - Rodriguez - Tarnate - UdalbeДокумент16 страницFehling's Test: Adlawan - Cainoy - Lawagon - Pascua - Rodriguez - Tarnate - UdalbeRocen Azleen TarnateОценок пока нет
- Oral Solid Dosage Forms GuideДокумент58 страницOral Solid Dosage Forms GuideMitul ShahОценок пока нет
- B.sc. (Chemistry)Документ41 страницаB.sc. (Chemistry)RahulОценок пока нет
- Biology U2 2020-09-30Документ2 страницыBiology U2 2020-09-30Daniel RoopchandОценок пока нет
- Allyl AДокумент5 страницAllyl AAhmed AliОценок пока нет
- Frac List of Fungicide Common Names (2016v2) PDFДокумент6 страницFrac List of Fungicide Common Names (2016v2) PDFFederico Posada GarcesОценок пока нет
- IIT Chemistry: EnthuseДокумент48 страницIIT Chemistry: EnthuseArjun SabnisОценок пока нет
- Bonding and Mixtures Answer Key GuideДокумент10 страницBonding and Mixtures Answer Key GuidemichaelalangcasОценок пока нет
- Investigatory Project On FertilizersДокумент5 страницInvestigatory Project On FertilizersMayankJain100% (2)
- Aqa CHM2 W QP Jun08Документ18 страницAqa CHM2 W QP Jun08Yashoda AmarasekeraОценок пока нет
- C-C Bond ActivationДокумент265 страницC-C Bond Activationnombre panchoОценок пока нет
- AS/CHM420 Equilibrium Constants and Reaction EffectsДокумент2 страницыAS/CHM420 Equilibrium Constants and Reaction EffectssyazaОценок пока нет
- 1100 Unlocking Corrosion MythДокумент76 страниц1100 Unlocking Corrosion MythAbdul Maabood Hassan AlviОценок пока нет
- P-Block Board Youtube Part-2Документ53 страницыP-Block Board Youtube Part-2vaibhav sainiОценок пока нет
- TDS - Ark 172Документ3 страницыTDS - Ark 172Santosh MhetreОценок пока нет
- II is the lie. Masking agents are used to mask fragrances, not keep ingredients active or maintain pH balance. Builders and solvents statements are trueДокумент56 страницII is the lie. Masking agents are used to mask fragrances, not keep ingredients active or maintain pH balance. Builders and solvents statements are trueCamille PasionОценок пока нет
- Crystallization 2013Документ2 страницыCrystallization 2013Peterter Paul100% (1)
- CIC CatalogueДокумент1 страницаCIC Catalogueterrance27Оценок пока нет
- Engineering Characteristics of Lime-Treated SoilДокумент16 страницEngineering Characteristics of Lime-Treated SoilRafi Mahmoud SulaimanОценок пока нет
- Types Chemical Reactions StudentДокумент3 страницыTypes Chemical Reactions StudentirzfileОценок пока нет
- PolymersДокумент72 страницыPolymersThe Private DetectiveОценок пока нет
- Exp6 chm361 PDFДокумент11 страницExp6 chm361 PDFShafiqahFazyaziqahОценок пока нет
- Canada Cordage Inc-RopeДокумент7 страницCanada Cordage Inc-RopeTagor SianiparОценок пока нет
- The Palms To Provide GurДокумент3 страницыThe Palms To Provide GurDr.Eswara Reddy SiddareddyОценок пока нет
- Boron DopingДокумент23 страницыBoron Dopingdbinod281Оценок пока нет
- ChemДокумент5 страницChemshishir kafleОценок пока нет
- Advanced Biological Processes For Wastewater TreatmentДокумент301 страницаAdvanced Biological Processes For Wastewater TreatmentEmiliano Rodriguez Tellez100% (1)
- Band Theory of ConductorsДокумент29 страницBand Theory of ConductorsSivakumar PonnusamyОценок пока нет
- Types of Reactions Practice WorksheetДокумент3 страницыTypes of Reactions Practice WorksheetKathryn Warner - Central Peel SS (2522)Оценок пока нет
- Case Study - Fuel OilДокумент34 страницыCase Study - Fuel OilironiteОценок пока нет
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaОт EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaОценок пока нет
- Faster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestОт EverandFaster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestРейтинг: 4 из 5 звезд4/5 (28)
- The Fabric of Civilization: How Textiles Made the WorldОт EverandThe Fabric of Civilization: How Textiles Made the WorldРейтинг: 4.5 из 5 звезд4.5/5 (56)
- A Place of My Own: The Architecture of DaydreamsОт EverandA Place of My Own: The Architecture of DaydreamsРейтинг: 4 из 5 звезд4/5 (241)
- Dirt to Soil: One Family’s Journey into Regenerative AgricultureОт EverandDirt to Soil: One Family’s Journey into Regenerative AgricultureРейтинг: 5 из 5 звезд5/5 (124)
- Pale Blue Dot: A Vision of the Human Future in SpaceОт EverandPale Blue Dot: A Vision of the Human Future in SpaceРейтинг: 4.5 из 5 звезд4.5/5 (586)
- 35 Miles From Shore: The Ditching and Rescue of ALM Flight 980От Everand35 Miles From Shore: The Ditching and Rescue of ALM Flight 980Рейтинг: 4 из 5 звезд4/5 (21)
- The Technology Trap: Capital, Labor, and Power in the Age of AutomationОт EverandThe Technology Trap: Capital, Labor, and Power in the Age of AutomationРейтинг: 4.5 из 5 звезд4.5/5 (46)
- Sully: The Untold Story Behind the Miracle on the HudsonОт EverandSully: The Untold Story Behind the Miracle on the HudsonРейтинг: 4 из 5 звезд4/5 (101)
- Recording Unhinged: Creative and Unconventional Music Recording TechniquesОт EverandRecording Unhinged: Creative and Unconventional Music Recording TechniquesОценок пока нет
- Data-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElseОт EverandData-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElseРейтинг: 3.5 из 5 звезд3.5/5 (12)
- The Future of Geography: How the Competition in Space Will Change Our WorldОт EverandThe Future of Geography: How the Competition in Space Will Change Our WorldРейтинг: 4.5 из 5 звезд4.5/5 (4)
- The Big, Bad Book of Botany: The World's Most Fascinating FloraОт EverandThe Big, Bad Book of Botany: The World's Most Fascinating FloraРейтинг: 3 из 5 звезд3/5 (10)
- Packing for Mars: The Curious Science of Life in the VoidОт EverandPacking for Mars: The Curious Science of Life in the VoidРейтинг: 4 из 5 звезд4/5 (1395)
- A Garden of Marvels: How We Discovered that Flowers Have Sex, Leaves Eat Air, and Other Secrets of PlantsОт EverandA Garden of Marvels: How We Discovered that Flowers Have Sex, Leaves Eat Air, and Other Secrets of PlantsОценок пока нет
- Reality+: Virtual Worlds and the Problems of PhilosophyОт EverandReality+: Virtual Worlds and the Problems of PhilosophyРейтинг: 4 из 5 звезд4/5 (24)
- The End of Craving: Recovering the Lost Wisdom of Eating WellОт EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellРейтинг: 4.5 из 5 звезд4.5/5 (80)
- The Mushroom at the End of the World: On the Possibility of Life in Capitalist RuinsОт EverandThe Mushroom at the End of the World: On the Possibility of Life in Capitalist RuinsРейтинг: 4 из 5 звезд4/5 (139)
- The Things We Make: The Unknown History of Invention from Cathedrals to Soda CansОт EverandThe Things We Make: The Unknown History of Invention from Cathedrals to Soda CansОценок пока нет
- Across the Airless Wilds: The Lunar Rover and the Triumph of the Final Moon LandingsОт EverandAcross the Airless Wilds: The Lunar Rover and the Triumph of the Final Moon LandingsОценок пока нет
- Inventor of the Future: The Visionary Life of Buckminster FullerОт EverandInventor of the Future: The Visionary Life of Buckminster FullerРейтинг: 4 из 5 звезд4/5 (10)
- ChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindОт EverandChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindОценок пока нет
- Artificial Intelligence: A Guide for Thinking HumansОт EverandArtificial Intelligence: A Guide for Thinking HumansРейтинг: 4.5 из 5 звезд4.5/5 (30)