Академический Документы
Профессиональный Документы
Культура Документы
MISS. MODERATE. Superior Quanto Á Insulina. The Effect of Vigorous - Versus Moderate-Intensity Aerobic Exercise On Insulin Action. Mcgarrah2016
Загружено:
Paolo AltoéОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
MISS. MODERATE. Superior Quanto Á Insulina. The Effect of Vigorous - Versus Moderate-Intensity Aerobic Exercise On Insulin Action. Mcgarrah2016
Загружено:
Paolo AltoéАвторское право:
Доступные форматы
Curr Cardiol Rep (2016) 18:117
DOI 10.1007/s11886-016-0797-7
DIABETES AND CARDIOVASCULAR DISEASE (S MALIK, SECTION EDITOR)
The Effect of Vigorous- Versus Moderate-Intensity Aerobic
Exercise on Insulin Action
Robert W. McGarrah 1,2 & Cris A. Slentz 1 & William E. Kraus 1,2
# Springer Science+Business Media New York 2016
Abstract Due to the beneficial effects on a wide range of
modern medical conditions, most professional societies recommend regular aerobic exercise as part of a healthy lifestyle.
Many of the exercise-related health benefits exhibit a doseresponse relationship: Up to a point, more exercise is more
beneficial. However, recent studies have suggested that different exercise intensities may provide distinct health benefits,
independent of energy expenditure (i.e., exercise dose). One
of these benefits, primarily mediated by the skeletal muscle, is
exercise-related changes in insulin action and glucose homeostasis. Glucose uptake in the exercising muscle occurs through
insulin-independent mechanisms whose downstream signaling events ultimately converge with insulin-signaling pathways, a fact that may explain why exercise and insulin have
additive effect on skeletal muscle glucose uptake. Although
the existing evidence is somewhat conflicting, well-controlled
randomized studies suggest that, when controlled for total
energy expenditure, moderate-intensity aerobic exercise improves insulin sensitivity more than vigorous-intensity aerobic
This article is part of the Topical Collection on Diabetes and Cardiovascular
Disease
* Robert W. McGarrah
robert.mcgarrah@duke.edu
Cris A. Slentz
cris.slentz@duke.edu
William E. Kraus
william.kraus@duke.edu
1
Duke Molecular Physiology Institute, Duke University Medical
Center, 300 North Duke Street, Durham, NC 27701, USA
Division of Cardiology, Department of Medicine, Duke University
Medical Center, Durham NC, USA
exercise. The mechanisms underlying this difference are
largely unknown. One possible explanation involves enhanced metabolism of fatty acid stores in the skeletal muscle
by moderate-intensity exercise, which may directly improve
insulin sensitivity. Overall, new technologic and physiologic
investigative tools are beginning to shed light on the biology.
Further understanding of these mechanisms will lead to better
understanding of the clinical implications of a healthy lifestyle
and may ultimately offer new therapeutic targets for common
medical conditions such as insulin resistance and diabetes.
Keywords Exercise intensity . Aerobic training . Insulin
sensitivity . Insulin action
Introduction
Modern life begets a sedentary lifestyle. As people become
less and less active, not only do they expend less energy
through physical activity, leading to the development of obesity and consequent obesity-related chronic health conditions,
but also their overall physiology and metabolism changes [1].
To this point, sedentary behavior, independent of comorbid
risk factors, is associated with increased risk for cardiovascular disease (CVD), metabolic syndrome, diabetes, cancer, and
all-cause mortality [25]. Because of these well-recognized
risks of prolonged physical inactivity, most professional societies have issued formal activity guidelines in an attempt to
reduce the incidence of these increasingly more common
conditions. In this review, we will focus on the role of physical activity in glucose homeostasis. In particular, we will
address the effects of different aerobic exercise intensities
on insulin action.
117
Page 2 of 6
General Effects of Exercise on Glucose Homeostasis
While multiple organ systems are involved in the bodys response to exercise, skeletal muscle is one of the most important determinants of exercise-related changes in glucose homeostasis. Exercising muscle first consumes glucose within
the tissue, followed by muscle glycogen stores. As muscle
glycogen stores are depleted, skeletal muscle relies more for
an energy source on circulating glucose from hepatic glycogenolysis and gluconeogenesis. Glucose uptake in contracting
muscle undergoes complex regulation depending on several
factors: glucose delivery, glucose transport, and tissue-level
glucose metabolism [6]. During exercise, there is an up to
20-fold increase in blood flow enhancing glucose delivery to
exercising muscle [7]. Additionally, capillary recruitment to
exercising muscle increases the vascular surface area for glucose delivery.
Glucose transport into the exercising muscle primarily relies on the glucose transporter, GLUT4. The majority of
GLUT4 is stored in intracellular vesicles in resting muscle.
In other tissues, GLUT4 translocation to the cell surface occurs in response to insulin. However, due to the high demand
for substrate transport to exercising muscle, insulin secretion
is suppressed, preventing uptake of glucose by non-muscle
tissues. Therefore, skeletal muscle GLUT4 translocation in
exercising muscle occurs through insulin-independent mechanisms. Muscle contraction during exercise leads to translocation of GLUT4 to the sarcolemma and enhanced glucose uptake [6].
Though evidence is conflicting, there may be two intracellular pools of GLUT4 in skeletal muscle: one recruited by
insulin and the other by muscle contraction [8]. These two
pathways have distinct proximal signals but converge downstream, which may explain why exercise and insulin have an
additive effect on skeletal muscle glucose transport [911].
Trained muscle utilizes fuels more efficiently than the untrained muscle. Regular exercise enhances overall insulin- and
contraction-stimulated skeletal muscle glucose uptake. This
adaptation occurs, in large part, due to increased GLUT4 expression and cellular GLUT4 protein content. The subsequent
increase in GLUT4 tissue concentration leads to improved
insulin action, glucose disposal, and glycogen storage [6].
Additionally, training leads to expansion of muscle capillary
networks, increased size and number of mitochondria, and an
increased ability to utilize fatty acids, thus sparing glycogen
stores. Many of these metabolic changes correlate with the
switching of muscle fibers from the fast glycolytic (FG) type
(type IIX) to the fast oxidative glycolytic (FOG) type (type
IIA), which are more efficient at fat oxidization.
Physical inactivity leads to insulin resistance; conversely,
increased physical activity improves insulin sensitivity.
Indeed, this is a well-accepted phenomenon [12]. One difficulty in interpreting the effect of exercise per se on insulin
Curr Cardiol Rep (2016) 18:117
sensitivity is the other lifestyle interventions used in clinical
studies: diet, weight loss, and induced adiposity changes.
These interventions may have independent effects on insulin
action. Thus, although exercise enhances insulin action
through molecular changes in skeletal muscle, global changes
in insulin sensitivity may also be modified by non-skeletal
muscle mechanisms.
Exercise Intensity Effects on Insulin Action
What is the optimal exercise prescription to improve insulin
sensitivity? Professional society exercise guideline recommendations generally agree. The World Health Organization
and the 2008 Physical Activity Guidelines for Americans recommend at least 150 min of moderate intensity aerobic activity or 75 min of vigorous intensity activity weekly for all
adults [13]. Guidelines from the American Heart Association
and the American College of Sports Medicine are similar,
recommending either moderate-intensity exercise for 30 min
for a minimum of 5 days a week, or vigorous exercise for
20 min 3 days a week [14]. Underlying these guidelines is a
consistent message: the health benefits derived from exercise
are due to the total amount of energy expended, without respect to the type of exercise performed. However, are there
different effects with different exercise intensities?
The answer to this question is complicated and the evidence is conflicting. When reviewing and interpreting the data, four other questions must be asked:
1. Did the study control for the amount of exercise (energy
expenditure) among intensity groups? Energy expenditure is composed of three components: exercise intensity,
frequency, and duration per session. Exercise intensity is
usually determined as a percentage of VO 2 peak.
Guidelines and most studies define moderate-intensity exercise as an effort of 50 % VO2 peak (the equivalent of
brisk walking) and vigorous-intensity exercise as an effort
of 75 % VO2 peak (the equivalent of jogging). For studies
to compare exercise intensity effects on insulin sensitivity,
it is critical to control for total exercise energy expenditure, because increased energy expenditure per se can improve insulin sensitivity [15, 16, 17].
2. When was insulin sensitivity measured in relation to the
last bout of training? As discussed in the previous section,
exercise induces acute changes in glucose homeostasis
and insulin action. Therefore, in order to avoid capturing
this transient response to the acute exercise bout, insulin
sensitivity assessments are generally performed before the
next bout of training. However, it is often difficult to separate the effects on insulin action from the most recent
training bout versus the cumulative effects from an entire
training program. To illustrate this point, we assigned
Curr Cardiol Rep (2016) 18:117
overweight/obese individuals to 8 months of either lowvolume/moderate-intensity (equivalent to brisk walking
12 miles/week, 1200 kcal/week at 4055 % VO2 peak,
200 min exercise/week), low-volume/vigorous-intensity
(jogging 12 miles/week, 1200 kcal/week at 6580 %
VO2 peak, 125 min/week), or high-volume/vigorous-intensity (jogging 20 miles/week, 2000 kcal/week at 65
80 % VO2 peak, 200 min/week). Insulin sensitivity, as
measured by an intravenous glucose tolerance test, was
measured when subjects were sedentary and at 1624 h
and 15 days after the final training bout. All exercise
groups had improved insulin sensitivity at 1624 h after
the last bout of training compared with the sedentary condition. However, only the low-volume/moderate-intensity
and high-volume/vigorous-intensity groups had persistent
improvements in insulin sensitivity at the 15-day time
point, which may be attributed to changes in body composition rather than changes in skeletal muscle [16].
3. How was insulin sensitivity measured? The most common methods used in exercise intensity studies are the
oral glucose tolerance test (OGTT), intravenous glucose
tolerance test (IVGTT), and the hyperinsulinemiceuglycemic clamp. All of these methods reliably measure
insulin sensitivity; however, they differ slightly in what
organ systems they assess. For instance, IVGTTs and
hyperinsulinemic-euglycemic clamps primarily assess peripheral (i.e., skeletal muscle) insulin sensitivity while the
OGTT provides an integrated assessment of whole body
glucose disposal by taking into account gastric emptying,
hepatic first-pass metabolism, glucose sensing by the pancreas, and then glucose delivery and insulin-stimulated
glucose uptake by the skeletal muscle. Because of these
differences, studies may reflect slightly different exerciserelated effects on insulin action depending on what measure of insulin sensitivity was employed. As an example,
an OGTT would be able to assess the effect of exercise on
gastrointestinal hormones such as incretins whereas an
IVGTT would mainly assess changes in skeletal muscle
insulin sensitivity.
4. Can the changes in insulin sensitivity be explained by
changes in other metabolic parameters? Exercise provides
additional health benefits, such as weight loss and decrease in visceral adiposity, that can indirectly improve
insulin sensitivity. If possible, studies should make efforts
to take into account changes in these other factors when
drawing conclusions about the mechanisms mediating responses in insulin sensitivity.
Vigorous-Intensity Exercise Is Better
Several studies suggest that vigorous-intensity exercise improves insulin sensitivity to a greater extent than low- or
Page 3 of 6 117
moderate-intensity exercise. DiPietro et al. studied 25 older
women (mean age 73 years) in a 9-month training program
with vigorous-intensity (80 % of VO2 peak achieved by jogging, rowing, or step aerobics); moderate-intensity (65 % of
VO2 peak achieved by the same activities as the vigorousintensity group), or low-intensity (50 % of VO2 peak achieved
by stretching and strengthening exercises) exercise as a control; energy expenditure of the exercise regimens was the same
(300 kcal/session). Insulin sensitivity was measured by
hyperinsulinemic-euglycemic clamp 72 h after the last bout
of training. Only the vigorous-intensity group experienced a
training-induced increase in glucose disposal (21 %, P = 0.02)
compared with control. Glucose disposal improved in the
moderate-intensity (65 % of VO2 peak) group to a similar
degree compared with control; however, this difference was
not statistically significant (16 %, P = 0.17). There were no
changes in body composition in any of the groups [18]. With
only 25 individuals divided among three exercise conditions,
the study may have been underpowered to detect differences
among the groups. Also, at 65 % of VO2 peak, the Bmoderateintensity exercise^ was a greater intensity than more contemporary studies, which typically define Bmoderate^ as 50 % of
VO2 peak.
Coker et al. also examined the effects of various degrees of
exercise intensity on insulin sensitivity in an elderly population
[19]. Twenty-one overweight (body mass index 29 1 kg/m2)
elderly individuals (age 74 1 years) were randomized to a 12week regimen, controlled for energy expenditure, of vigorousintensity exercise (75 % of VO2 peak, exact exercise regimen
not specified), moderate-intensity exercise (50 % of VO2 peak),
or sedentary controls. There were no changes in weight or body
fat composition in any of the groups. Glucose disposal, as measured by hyperinsulinemic-euglycemic clamp 72 h after the last
bout of training, improved only in the vigorous-intensity group.
Again, with only 21 individuals randomized to three groups, the
study may have been underpowered to detect differences
among the exercise regimens.
One of the largest studies to date to address the question of
exercise-intensity effect on insulin sensitivity enrolled 300
obese individuals (age 51 8 years, BMI 33 5 kg/m2) and
randomized them to a 24-week exercise regimen, controlled
for energy expenditure, of no-exercise control, low-amount/
low-intensity (50 % of VO2 peak achieved by brisk walking),
high-amount/low-intensity (50 % of VO2 peak achieved by
brisk walking), or high-amount/vigorous-intensity (75 % of
VO2 peak achieved by jogging) [20]. Insulin sensitivity was
measured by the 2-h glucose level after an oral glucose tolerance test performed 36-48 h after the last exercise bout at 16
and 24 weeks. All groups experienced a significant decrease
in waist circumference compared with the control group, but
there was no difference among the exercise groups. Only the
vigorous-intensity exercise group experienced a significant
change in 2-h glucose level compared with the control group.
117
Page 4 of 6
Curr Cardiol Rep (2016) 18:117
Our Studies of a Targeted Risk Reduction Intervention
through Defined Exercise (STRRIDE) study was a randomized controlled study of amount and intensity effects of exercise training on metabolic markers of cardiovascular risk.
Conducted during 19982003, we studied 260 men and women that were overweight or moderately obese (25 BMI (kg/
m2) 35) with atherogenic dyslipidemia (HDL 40 mg/dl for
men, 45 mg/l for women; 130 LDL (mg/dl) 190) [21].
Individuals were assigned to a 6-month no-exercise control
group or 8-month low-amount/moderate-intensity (1200 kcal;
800 MET-min at 4055 % VO2 peak; equivalent to brisk
walking 12 miles/week) per week, low-amount/vigorous-intensity (1200 kcal; 800 MET-min at 6075 % VO2 peak;
equivalent to jogging 12 miles/week) per week, or highamount/vigorous-intensity (2200 kcal; 1314 MET-min at
6075 % VO2 peak; equivalent to jogging 20 miles/week)
per week.
Compared with the no-exercise control group, all exercise
groups had beneficial effects on body weight and composition
[22], whole body insulin sensitivity [15], metabolic syndrome [23], and cardiorespiratory fitness variables [24].
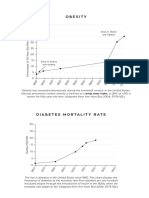
However, moderate-intensity exercise was three times as effective at improving insulin action (Si) of an IVGTT as was
vigorous-intensity exercise (Fig. 1) [15]. Additionally,
moderate-intensity exercise improved the disposition index
(a marker of pancreatic beta-cell function) more than the calorically equivalent vigorous-intensity regimen [25]. These
findings were independent of weight loss or changes in body
composition.
It is important to note that, although the participants in
STRRIDE were all overweight or obese, they were not
selected according to glycemic traits. Sixty-four of the
260 participants had fasting glucose from 100 to 125 mg/
dl and seven participants had fasting glucose >125 mg/dl.
In STRRIDE-PD (ClinicalTrials.gov NCT00962962), we
randomized 195 sedentary 45- to 75-year-old adults with
body mass indices 25 to 35 kg/m2 and fasting plasma glucose between 95 and 125 mg/dl to 6-month exercise regimens of the following: (1) low-amount/moderate-intensity
exercise: exercise energy expenditure of 10 kcal per kg of
body weight per week (10 KKW) at 50 % VO 2 peak
(equivalent to walking 8.6 miles/week); (2) highamount/moderate-intensity exercise: 16 KKW at 50 %
VO2 peak (equivalent to walking 13.8 miles/week); (3)
high-amount/vigorous-intensity exercise: 16 KKW at 75 %
VO2 peak (equivalent to jogging 13.8 miles/week); (4)
clinical lifestyle intervention (diet + exercise): 10 KKW
at 50 % VO2 peak plus a calorically restricted diet designed to reduce body weight by 7 % over 6 months
[17]. For improvement in glucose tolerance (glucose area
under the curve of an oral glucose tolerance test measured
1624 h after the last exercise training bout), moderateintensity exercise was about four times as potent as was
vigorous-intensity exercise in these pre-diabetic subjects
(Fig. 1).
Additionally, moderate-intensity exercise in STRRIDE
improved metabolic syndrome variables compared with inactive controls while the same amount of vigorousintensity exercise had no effect on these variables [23].
The changes in the metabolic syndrome paralleled the
changes in insulin sensitivity seen with moderate- versus
vigorous-intensity exercise.
Regardless of the conflicting data presented above, one
conclusion is shared: compared to no exercise, either
moderate- or vigorous-intensity exercise improves markers
of insulin sensitivity.
Fig. 1 Effect of moderate- and vigorous-intensity aerobic exercise on
insulin sensitivity. a In STRRIDE, with the same amount of energy
expenditure (1200 kcal; 800 MET-min), moderate-intensity exercise
(4055 % VO2 peak) improved insulin sensitivity (Si) as measured by
IVGTT more than vigorous-intensity exercise (6075 % VO2 peak),
#
P < 0.05 versus low-dose vigorous-intensity exercise, *P < 0.05 versus
inactive controls [15]. Of note, too, is the dose effect of exercise: highdose (2200 kcal; 1314 MET-min) vigorous-intensity exercise improved Si
more than low-dose vigorous-intensity exercise. b In STRRIDE-PD,
moderate-intensity exercise improved insulin sensitivity as measured by
OGTT compared with calorically equivalent vigorous-intensity exercise
in pre-diabetic individuals, **P < 0.05 [17]
Moderate-Intensity Exercise Is Better
Curr Cardiol Rep (2016) 18:117
Page 5 of 6 117
Possible Mechanisms for Moderate Intensity?
Compliance with Ethical Standards
Based on the STRRIDE studies findingscalorically equivalent moderate-intensity exercise improves insulin action
more than vigorous-intensity exercisethe next logical step
is to find the mechanisms underlying this difference.
Understanding these mechanisms is important not only for
understanding the clinical implications of lifestylespecifically exercisefor preventing type 2 diabetes; this understanding also is important for the possible development of
pharmacologic exercise analogues and biochemical targets
for new drug therapies. Given the complexity of systemic
glucose homeostasis, which involves the interplay of multiple
organ systems, the search for mechanisms will not be easy. It
will require a comprehensive and integrative approach to simultaneously investigate whole body and skeletal metabolism
on both physiologic and molecular levels. To begin this endeavor, we used targeted metabolomics in skeletal muscle of
STRRIDE participants in an attempt to identify molecular
signatures that could differentiate exercise intensity groups
and relate to changes in insulin sensitivity [26]. Moderateintensity exercise of the same amount as vigorous-intensity
exercise resulted in significantly greater elevations of skeletal
muscle medium chain acylcarnitines and succinate; this implicates a differential metabolic response in muscle by exercise
intensity when controlling for exercise amount. Moreover,
these changes in metabolites were significantly related to
change in insulin sensitivity in response to exercise. These
findings suggest that moderate-intensity exercise may enhance the metabolism of fatty acid stores within skeletal muscle. Because lipid accumulation in skeletal muscle is associated with insulin resistance [27], moderate-intensity exercise
may, in part, improve insulin sensitivity by reducing lipid
stores in muscle.
Conflict of Interest Robert W. McGarrah, Cris A. Slentz, and William
E. Kraus declare that no relevant conflicts of interest exist.
Human and Animal Rights and Informed Consent This article does
not contain any studies with human or animal subjects performed by any
of the authors.
References
Papers of particular interest, published recently, have been
highlighted as:
Of importance
Of major importance
1.
2.
3.
4.
5.
6.
7.
Conclusions
In an increasingly sedentary culture, the need for increased
physical activity to improve the overall health of the global
population is more urgent than ever. Of all the potential health
benefits of exercise, improved insulin sensitivity is one that
appears to respond differently to different intensities of calorically equivalent exercise regimens: the balance of evidence
points toward improved insulin sensitivity with moderateintensity exercise compared with vigorous-intensity exercise.
The mechanisms responsible for this difference are undoubtedly complex. However, modern physiologic and molecular
investigative tools are beginning to shed light on the underlying biology. With these discoveries comes the potential to
improve our understanding of how and why exercise impacts
metabolic health.
8.
9.
10.
11.
12.
13.
14.
Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56:265567.
van der Ploeg HP, Chey T, Korda RJ, Banks E, Bauman A. Sitting
time and all-cause mortality risk in 222 497 Australian adults. Arch
Intern Med. 2012;172:494500.
Gennuso KP, Gangnon RE, Matthews CE, Thraen-Borowski KM,
Colbert LH. Sedentary behavior, physical activity, and markers of
health in older adults. Med Sci Sports Exerc. 2013;45:1493500.
Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS,
et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review
and meta-analysis. Ann Intern Med. 2015;162:12332.
Chau JY, Grunseit A, Midthjell K, Holmen J, Holmen TL, Bauman
AE, et al. Sedentary behaviour and risk of mortality from all-causes
and cardiometabolic diseases in adults: evidence from the HUNT3
population cohort. Br J Sports Med. 2015;49:73742.
Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle
glucose uptake. Physiol Rev. 2013;93:9931017. An excellent review of the molecular mechanisms underlying the effects of
exercise on skeletal muscle glucose homeostasis.
Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man.
J Physiol. 1985;366:23349.
Ploug T, Ralston E. Anatomy of glucose transporters in skeletal
muscle. Effects of insulin and contractions. Adv Exp Med Biol.
1998;441:1726.
Nesher R, Karl IE, Kipnis DM. Dissociation of effects of insulin
and contraction on glucose transport in rat epitrochlearis muscle.
Am J Physiol. 1985;249:C22632.
Ploug T, Galbo H, Vinten J, Jrgensen M, Richter EA. Kinetics of
glucose transport in rat muscle: effects of insulin and contractions.
Am J Physiol. 1987;253:E1220.
Constable SH, Favier RJ, Cartee GD, Young DA, Holloszy JO.
Muscle glucose transport: interactions of in vitro contractions, insulin, and exercise. J Appl Physiol. 1988;64:232932.
Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin
sensitivity. Annu Rev Med. 1998;49:23561.
US Department of Health and Human Services. Physical Activity
Guidelines Advisory Committee report, 2008. Nutr Rev. 2009;67:
11420.
Haskell WL, Lee I-M, Pate RR, Powell KE, Blair SN, Franklin BA,
et al. Physical activity and public health: updated recommendation
117
15.
16.
17.
18.
19.
20.
Curr Cardiol Rep (2016) 18:117
Page 6 of 6
for adults from the American College of Sports Medicine and the
American Heart Association. Circulation. 2007;116:108193.
Houmard JA, Tanner CJ, Slentz CA, Duscha BD, McCartney JS,
Kraus WE. Effect of the volume and intensity of exercise training
on insulin sensitivity. J Appl Physiol. 2004;96:1016. The
STRRIDE study examined the effect of moderate- and
vigorous-intensity aerobic exercise, controlled for energy expenditure, in sedentary overweight/obese individuals. After a 6month training program, insulin sensitivity improved more in
the moderate-intensity group.
Bajpeyi S, Tanner CJ, Slentz CA, Duscha BD, McCartney JS,
Hickner RC, et al. Effect of exercise intensity and volume on persistence of insulin sensitivity during training cessation. J Appl
Physiol. 2009;106:107985.
Slentz CA, Bateman LA, Willis LH, Granville EO, Piner LW,
Samsa GP, et al. Effects of exercise training alone vs a combined
exercise and nutritional lifestyle intervention on glucose homeostasis in prediabetic individuals: a randomised controlled trial.
Diabetologia. 2016. In press. The STRRIDE-PD study was similar to the STRRIDE study but enrolled individuals with impaired fasting glucose; it also included a group that underwent
a combined exercise and nutritional lifestyle intervention. As in
STRRIDE, the moderate-intensity group demonstrated a better
improvement in insulin sensitivity than the vigorous-intensity
group.
DiPietro L, Dziura J, Yeckel CW, Neufer PD. Exercise and improved
insulin sensitivity in older women: evidence of the enduring benefits
of higher intensity training. J Appl Physiol. 2006;100:1429.
Coker RH, Hays NP, Williams RH, Brown AD, Freeling SA,
Kortebein PM, et al. Exercise-induced changes in insulin action
and glycogen metabolism in elderly adults. Med Sci Sports Exerc.
2006;38:4338.
Ross R, Hudson R, Stotz PJ, Lam M. Effects of exercise amount
and intensity on abdominal obesity and glucose tolerance in obese
21.
22.
23.
24.
25.
26.
27.
adults: a randomized trial. Ann Intern Med. 2015;162:32534. A
large randomized trial that enrolled abdominally obese individuals and examined the effects of exercise intensity on obesity
and glucose tolerance. In contrast to STRRIDE, only the
vigorous-intensity group had an improvement in insulin
sensitivity.
Kraus WE, Torgan CE, Duscha BD, Norris J, Brown SA, Cobb
FR, et al. Studies of a targeted risk reduction intervention through
defined exercise (STRRIDE). Med Sci Sports Exerc. 2001;33:
177484.
Slentz CA, Duscha BD, Johnson JL, Ketchum K, Aiken LB, Samsa
GP, et al. Effects of the amount of exercise on body weight, body
composition, and measures of central obesity: STRRIDEa randomized controlled study. Arch Intern Med. 2004;164:319.
Johnson JL, Slentz CA, Houmard JA, Samsa GP, Duscha BD,
Aiken LB, et al. Exercise training amount and intensity effects on
metabolic syndrome (from Studies of a Targeted Risk Reduction
Intervention through Defined Exercise). Am J Cardiol. 2007;100:
175966.
Duscha BD, Slentz CA, Johnson JL, Houmard JA, Bensimhon DR,
Knetzger KJ. Effects of exercise training amount and intensity on
peak oxygen consumption in middle-age men and women at risk for
cardiovascular disease. Chest. 2005;128:278893.
Slentz CA, Tanner CJ, Bateman LA, Durheim MT, Huffman KM,
Houmard JA, et al. Effects of exercise training intensity on pancreatic beta-cell function. Diabetes Care. 2009;32:180711.
Huffman KM, Koves TR, Hubal MJ, Abouassi H, Beri N, Bateman
LA, et al. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia. 2014;57:228295.
Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus
C, et al. Skeletal muscle triglyceride levels are inversely related to
insulin action. Diabetes. 1997;46:9838.
Вам также может понравиться
- DefaultДокумент43 страницыDefaultKassandra EggersОценок пока нет
- Nutrition Guidelines for Marathon, Triathlon, and Cycling AthletesДокумент9 страницNutrition Guidelines for Marathon, Triathlon, and Cycling AthletescakebakeshakeОценок пока нет
- Effects of Exercise On Insulin Sensitivity in Humans: John T. Devlin, MDДокумент4 страницыEffects of Exercise On Insulin Sensitivity in Humans: John T. Devlin, MDgod4alllОценок пока нет
- Exercise and Type 2 Diabetes: Molecular Mechanisms Regulating Glucose Uptake in Skeletal MuscleДокумент7 страницExercise and Type 2 Diabetes: Molecular Mechanisms Regulating Glucose Uptake in Skeletal MuscleArie AnggaОценок пока нет
- Carboidrato No Contexto Do Exercício Físico em Diabéticos Tipo 1Документ21 страницаCarboidrato No Contexto Do Exercício Físico em Diabéticos Tipo 1Duda FranchiОценок пока нет
- InglesДокумент6 страницInglesKuroKy KrausserОценок пока нет
- HHS Public Access: Exercise Training and Insulin Resistance: A Current ReviewДокумент16 страницHHS Public Access: Exercise Training and Insulin Resistance: A Current ReviewJerônimo CoelhoОценок пока нет
- Regulation of Skeletal Muscle Glucose Transport and Glucose Metabolism by Exercise TrainingДокумент24 страницыRegulation of Skeletal Muscle Glucose Transport and Glucose Metabolism by Exercise TrainingEnzoОценок пока нет
- Research Article: Acute Whole Body Vibration Decreases The Glucose Levels in Elderly Diabetic WomenДокумент8 страницResearch Article: Acute Whole Body Vibration Decreases The Glucose Levels in Elderly Diabetic WomenStefanny AmahorsejaОценок пока нет
- T1DM and ExerciseДокумент9 страницT1DM and ExerciserobertjoshuasantosОценок пока нет
- Diabetes GuidelineДокумент4 страницыDiabetes GuidelinenetoОценок пока нет
- Impacto Del Ejercicio FisicoДокумент27 страницImpacto Del Ejercicio FisicoOscarDavidGordilloGonzalezОценок пока нет
- Effect of Walking Exercise On Abdominal Fat, Insulin Resistance and Serum Cytokines in Obese WomenДокумент9 страницEffect of Walking Exercise On Abdominal Fat, Insulin Resistance and Serum Cytokines in Obese WomenankitsmkОценок пока нет
- Reuniao 25 04Документ12 страницReuniao 25 04Pia GrzegorczykОценок пока нет
- Resources To Guide Exercise Specialists Managing Adults With DiabetesДокумент11 страницResources To Guide Exercise Specialists Managing Adults With DiabetesArie AnggaОценок пока нет
- Resources To Guide Exercise Specialists Managing Adults With DiabetesДокумент13 страницResources To Guide Exercise Specialists Managing Adults With Diabeteslaura valentina ochoa ordoñezОценок пока нет
- The Essential Role of Exercise in The Management of Type 2 DiabetesДокумент11 страницThe Essential Role of Exercise in The Management of Type 2 DiabetesRadu MaricicaОценок пока нет
- Resistance Training Is An Effective Tool Against Metabolic and Frailty SyndromesДокумент7 страницResistance Training Is An Effective Tool Against Metabolic and Frailty SyndromesgiannidietОценок пока нет
- Impact of Endurance Exercise Training in the Fasted State on Muscle Metabolism and Insulin SensitivityДокумент14 страницImpact of Endurance Exercise Training in the Fasted State on Muscle Metabolism and Insulin SensitivityYo Vivo Fit Pablo y KarlaОценок пока нет
- Strength Training and Weight Loss: Treinamento de Força e EmagrecimentoДокумент4 страницыStrength Training and Weight Loss: Treinamento de Força e EmagrecimentoDevasyaОценок пока нет
- Clinical Aspects of Physical Exercise For Diabetes/metabolic SyndromeДокумент5 страницClinical Aspects of Physical Exercise For Diabetes/metabolic SyndromecokimasterОценок пока нет
- Benefits of Exercise in The Older PopДокумент11 страницBenefits of Exercise in The Older PopRafael PortoОценок пока нет
- Babu-Positive Effects of Exercise Intervention Without Weight Loss 2021Документ28 страницBabu-Positive Effects of Exercise Intervention Without Weight Loss 2021markwsheppardhotmail.comОценок пока нет
- 673 FullДокумент4 страницы673 FullIba SuprasabaОценок пока нет
- CipryanДокумент10 страницCipryan197Anzali CahyatiОценок пока нет
- Exercício Físico e Autofagia Seletiva Benefício e Risco Na Saúde CardiovascularДокумент18 страницExercício Físico e Autofagia Seletiva Benefício e Risco Na Saúde CardiovascularHenrique WilliamОценок пока нет
- Exercise Intervention Under Hypoxic Condition As A New TherapeuticДокумент14 страницExercise Intervention Under Hypoxic Condition As A New TherapeuticnorazmiОценок пока нет
- Prediabetes ExcsДокумент8 страницPrediabetes ExcsKamal JindalОценок пока нет
- Exercise and Adlib Eating in Obese Individuals Med Sci Sports Med Nov 2010Документ8 страницExercise and Adlib Eating in Obese Individuals Med Sci Sports Med Nov 2010Andrew OgundeleОценок пока нет
- Effects of exercise on lipids and blood clotting factorsДокумент11 страницEffects of exercise on lipids and blood clotting factorsJessica BarcelonaОценок пока нет
- Postprandial Light Physical Activity Blunts The Blood Glucose IncreaseДокумент3 страницыPostprandial Light Physical Activity Blunts The Blood Glucose IncreaseRemusLupinnОценок пока нет
- DANTAS 2020 Exercise-Induced Increases in Insulin Sensitivity After Bariatric Surgery Are Mediated by Muscle Extracellular Matrix RemodelingДокумент17 страницDANTAS 2020 Exercise-Induced Increases in Insulin Sensitivity After Bariatric Surgery Are Mediated by Muscle Extracellular Matrix RemodelingCris AquinoОценок пока нет
- RX Exercise For Weight Loss CCJM 2016Документ10 страницRX Exercise For Weight Loss CCJM 2016Sundar RamanathanОценок пока нет
- Hyperinsulinemia: Best Management Practice: ReviewДокумент11 страницHyperinsulinemia: Best Management Practice: Reviewprof_2011Оценок пока нет
- High-Intensity Interval Training: A Time-Efficient Strategy For Health Promotion?Документ3 страницыHigh-Intensity Interval Training: A Time-Efficient Strategy For Health Promotion?neilh39Оценок пока нет
- JSSM 11 495Документ7 страницJSSM 11 495Tahira RajputОценок пока нет
- Physiological Adaptations To Low-Volume, High-Intensity Interval Training in Health and DiseaseДокумент8 страницPhysiological Adaptations To Low-Volume, High-Intensity Interval Training in Health and DiseasetomОценок пока нет
- Abastecendo o Atleta Com Diabetes Tipo 1Документ7 страницAbastecendo o Atleta Com Diabetes Tipo 1josiОценок пока нет
- Low-Volume High-Intensity Interval Training Reduces Hyperglycemia and Increases Muscle Mitochondrial Capacity in Patients With Type 2 DiabetesДокумент7 страницLow-Volume High-Intensity Interval Training Reduces Hyperglycemia and Increases Muscle Mitochondrial Capacity in Patients With Type 2 DiabetesSafira Ridha UlyaОценок пока нет
- Billinger2012 2Документ12 страницBillinger2012 2GiorgianaОценок пока нет
- Exercise enhances insulin signaling and glycogen synthesis contributing to improved insulin actionДокумент15 страницExercise enhances insulin signaling and glycogen synthesis contributing to improved insulin actionkrishnans23Оценок пока нет
- Lakka 2007Документ13 страницLakka 2007Neha RauhilaОценок пока нет
- Management of Competitive Athletes With Diabetes: in BriefДокумент6 страницManagement of Competitive Athletes With Diabetes: in BriefDeritanti Fitria RiskianaОценок пока нет
- Dietary Protein and Occlusion Training for SarcopeniaДокумент6 страницDietary Protein and Occlusion Training for SarcopeniaNicolás HerreraОценок пока нет
- Impact of Resistance Circuit Training On Neuromuscular, Cardiorespiratory and Body Composition Adaptations in The ElderlyДокумент8 страницImpact of Resistance Circuit Training On Neuromuscular, Cardiorespiratory and Body Composition Adaptations in The ElderlyBryan HuaritaОценок пока нет
- Diet and TherapyДокумент3 страницыDiet and TherapyKatty AlevonОценок пока нет
- Clinical Aspects of Physical Exercise For Diabetes/metabolic SyndromeДокумент5 страницClinical Aspects of Physical Exercise For Diabetes/metabolic Syndromeprofesi nersОценок пока нет
- Diabetes, Sports and ExerciseДокумент8 страницDiabetes, Sports and ExerciseMateus AssisОценок пока нет
- Effectiveness and Safety of High-Intensity Interval Training in Patients With Type 2 DiabetesДокумент6 страницEffectiveness and Safety of High-Intensity Interval Training in Patients With Type 2 DiabetesMumtaz Maulana HidayatОценок пока нет
- Long QuizДокумент6 страницLong QuizMae EsmadeОценок пока нет
- As Stated Previously, in A Few Hours of Exercise or Work in The Heat, Water Loss orДокумент10 страницAs Stated Previously, in A Few Hours of Exercise or Work in The Heat, Water Loss orEric Mayo Dagradi Kabat100% (1)
- Exercise Guidelines in Pregnancy: Focus on Vigorous Intensity and Weekly Caloric ExpenditureДокумент16 страницExercise Guidelines in Pregnancy: Focus on Vigorous Intensity and Weekly Caloric ExpenditureCarlos VieiraОценок пока нет
- Menopause ExerciseДокумент8 страницMenopause ExerciseWittapong SinsoongsudОценок пока нет
- Sedentarismo e Inflexibilidade MetabolicaДокумент24 страницыSedentarismo e Inflexibilidade MetabolicaCristiano Kalata VarelaОценок пока нет
- Arad Et Al., 2015Документ11 страницArad Et Al., 2015Irene Chrysovalanto ThemistocleousОценок пока нет
- Muscle Stem Cell and Physical Activity What Point Is The Debate atДокумент13 страницMuscle Stem Cell and Physical Activity What Point Is The Debate atZhao RuidiОценок пока нет
- Diabetology 03 00032Документ24 страницыDiabetology 03 00032Мөнх- АмарОценок пока нет
- Nutrition in The Perioperative Period: Metabolic Responses To Surgical StressДокумент10 страницNutrition in The Perioperative Period: Metabolic Responses To Surgical Stressanna_dianОценок пока нет
- Skeletal Muscle in The Fight Against Chronic Diseases: ExactaДокумент4 страницыSkeletal Muscle in The Fight Against Chronic Diseases: ExactaAgustin LopezОценок пока нет
- NEAT. TERMOGENESE. ADAPTAÇÃO METABÓLICA. ADAPTATIVE. Control of Energy Expenditure in Humans. Ejcn2016237a PDFДокумент5 страницNEAT. TERMOGENESE. ADAPTAÇÃO METABÓLICA. ADAPTATIVE. Control of Energy Expenditure in Humans. Ejcn2016237a PDFPaolo AltoéОценок пока нет
- Metabolic Effect. Cortisol. Hormones. HormôniosДокумент5 страницMetabolic Effect. Cortisol. Hormones. HormôniosPaolo AltoéОценок пока нет
- Neural Correlates of Frustration PDFДокумент4 страницыNeural Correlates of Frustration PDFPaolo AltoéОценок пока нет
- Listen To Your PhysiologistДокумент4 страницыListen To Your PhysiologistPaolo AltoéОценок пока нет
- PCT Protocol by CR Swale. TPC CR Swale. Michael ScallyДокумент3 страницыPCT Protocol by CR Swale. TPC CR Swale. Michael ScallyPaolo AltoéОценок пока нет
- EXERCÍCIO. Exercise Remodels Subcutaneous Fat Tissue. Metabolism. Wallberg-Henriksson2015Документ2 страницыEXERCÍCIO. Exercise Remodels Subcutaneous Fat Tissue. Metabolism. Wallberg-Henriksson2015Paolo AltoéОценок пока нет
- Sun C Et Al. (2016) - Naked Aggression - The Meaning and Practice of Ejaculation On A Woman's FaceДокумент20 страницSun C Et Al. (2016) - Naked Aggression - The Meaning and Practice of Ejaculation On A Woman's FacePaolo AltoéОценок пока нет
- HIPOTIROIDISMO FUNCIONAL. Subclinical Hypothyroidism in Children Can Normalize. FNS20120300018 - 72258988 PDFДокумент6 страницHIPOTIROIDISMO FUNCIONAL. Subclinical Hypothyroidism in Children Can Normalize. FNS20120300018 - 72258988 PDFPaolo AltoéОценок пока нет
- TIROIDE. LIVRO. Thyroid Disease Manager - Authoritative Source On The ThyroidThyroid Disease ManagerДокумент2 страницыTIROIDE. LIVRO. Thyroid Disease Manager - Authoritative Source On The ThyroidThyroid Disease ManagerPaolo AltoéОценок пока нет
- EXERCÍCIO. Exercise Remodels Subcutaneous Fat Tissue. Metabolism. Wallberg-Henriksson2015Документ2 страницыEXERCÍCIO. Exercise Remodels Subcutaneous Fat Tissue. Metabolism. Wallberg-Henriksson2015Paolo AltoéОценок пока нет
- Contrasts. Rapid Fat Loss. Neuro-Metabolic ContrastsДокумент22 страницыContrasts. Rapid Fat Loss. Neuro-Metabolic ContrastsPaolo Altoé0% (1)
- SuppVersity. HIIT. LISS. Estimativa Calórica Errada. 27300kcal. Estudo. ArtigoДокумент7 страницSuppVersity. HIIT. LISS. Estimativa Calórica Errada. 27300kcal. Estudo. ArtigoPaolo AltoéОценок пока нет
- Difference Between Cognitive Enhancers and Nootropics - PDFДокумент22 страницыDifference Between Cognitive Enhancers and Nootropics - PDFPaolo AltoéОценок пока нет
- Alkalinity Vs Acidity 2012, Ray PeatДокумент11 страницAlkalinity Vs Acidity 2012, Ray PeatPaolo AltoéОценок пока нет
- Shake. Paleo. Rob Wolf. Rob RegishДокумент1 страницаShake. Paleo. Rob Wolf. Rob RegishPaolo AltoéОценок пока нет
- Precision Nutrition. Nutrient TimingДокумент21 страницаPrecision Nutrition. Nutrient TimingPaolo AltoéОценок пока нет
- Heavy: Monday Reps Week 1 Week 2 Week 3 Week 4 SquatДокумент6 страницHeavy: Monday Reps Week 1 Week 2 Week 3 Week 4 SquatPaolo AltoéОценок пока нет
- The Energy Balance Equation ExplainedДокумент5 страницThe Energy Balance Equation ExplainedPaolo AltoéОценок пока нет
- Sequencias Numéricas e Seus SignificadosДокумент7 страницSequencias Numéricas e Seus SignificadosPaolo AltoéОценок пока нет
- Glycogen Storage Capacity and de Novo Lipogenesis. ESTUDO .ARTIGO. QUANTO de CARBO. VIRA GORDURA. Am J Clin Nutr-1988-Acheson-240-7Документ8 страницGlycogen Storage Capacity and de Novo Lipogenesis. ESTUDO .ARTIGO. QUANTO de CARBO. VIRA GORDURA. Am J Clin Nutr-1988-Acheson-240-7Paolo AltoéОценок пока нет
- High Fat Diet. Leptin ResistanceДокумент2 страницыHigh Fat Diet. Leptin ResistancePaolo AltoéОценок пока нет
- Chris Thibaudeau. 9 Ways To Keep Getting BetterДокумент2 страницыChris Thibaudeau. 9 Ways To Keep Getting BetterPaolo AltoéОценок пока нет
- CaloriesДокумент5 страницCaloriesallhailjimbowalesОценок пока нет
- Payam Mohamad PanahiДокумент6 страницPayam Mohamad PanahiPaolo AltoéОценок пока нет
- Breaking The Vicious Cycle. ReviewДокумент3 страницыBreaking The Vicious Cycle. ReviewPaolo AltoéОценок пока нет
- Antioxidants Block Exercise Recovery. AOX e EXERCÍCIO. TOPДокумент9 страницAntioxidants Block Exercise Recovery. AOX e EXERCÍCIO. TOPPaolo AltoéОценок пока нет
- 10 Secrets To Insane GainsДокумент8 страниц10 Secrets To Insane GainsPaolo Altoé100% (4)
- AST Sports Science 12 Week Max-OT Online Training CourseДокумент165 страницAST Sports Science 12 Week Max-OT Online Training CourseNel JerezОценок пока нет
- Mango Modulates Blood GlucoseДокумент12 страницMango Modulates Blood GlucosePaolo AltoéОценок пока нет
- PCOS Evidence-Based Guideline For Assessment and Management PcosДокумент167 страницPCOS Evidence-Based Guideline For Assessment and Management PcosJalajarani AridassОценок пока нет
- Heart Failure in Patients With Metabolic Syndrome XДокумент12 страницHeart Failure in Patients With Metabolic Syndrome XErwin SiahaanОценок пока нет
- More On Mediterranean Diets (Artemis P. Simopoulos, Francesco Visioli) (Z-Library)Документ255 страницMore On Mediterranean Diets (Artemis P. Simopoulos, Francesco Visioli) (Z-Library)qrscentralОценок пока нет
- Atherosclerosis, Dyslipidaemia and Diabetes SlidesДокумент83 страницыAtherosclerosis, Dyslipidaemia and Diabetes SlidesWee DaliОценок пока нет
- Journal of Clinical Gerontology & Geriatrics: Original ArticleДокумент6 страницJournal of Clinical Gerontology & Geriatrics: Original ArticleDeedee RenovaldiОценок пока нет
- Ovario Poliquístico/Polycystic Ovary SyndromeДокумент14 страницOvario Poliquístico/Polycystic Ovary SyndromeJosé María Lauricella100% (1)
- The Clinical Biochemistry of ObesityДокумент28 страницThe Clinical Biochemistry of ObesityAnonymous vxLhRlVgjHОценок пока нет
- The 8 Second Secret The Scientifically Proven Method For Lasting WeightlossДокумент204 страницыThe 8 Second Secret The Scientifically Proven Method For Lasting WeightlossGlen HutchinsonОценок пока нет
- Pathophysiology of Type 2 DiabetesДокумент5 страницPathophysiology of Type 2 DiabetesLouella Mae CoraldeОценок пока нет
- Polycystic Ovary Syndrome, Fertility, Diet, and Lifestyle ModificationsДокумент17 страницPolycystic Ovary Syndrome, Fertility, Diet, and Lifestyle ModificationsMauricio EsparzaОценок пока нет
- Sindroma MetabolikДокумент24 страницыSindroma MetabolikAnissa Aulia AdjaniОценок пока нет
- NTRN 516 Case Study 2 - DM 10 12 15 UpdateДокумент18 страницNTRN 516 Case Study 2 - DM 10 12 15 Updateapi-315324296Оценок пока нет
- Final ProgrammeДокумент204 страницыFinal ProgrammeimagigatoОценок пока нет
- Book Diacylglycerol OilsДокумент268 страницBook Diacylglycerol OilsTeera YimyooОценок пока нет
- Mediterranean Diet May Reduce Stroke RiskДокумент12 страницMediterranean Diet May Reduce Stroke RiskDijana Dencic NalovskaОценок пока нет
- Diabetes NotesДокумент2 страницыDiabetes NotesUmer MasoodОценок пока нет
- Art 1Документ12 страницArt 1Irene Ramirez ChavesОценок пока нет
- Nature Wants Us To Be FatДокумент12 страницNature Wants Us To Be FathaydendevОценок пока нет
- Hari Sharma ChapterДокумент14 страницHari Sharma Chapterdynamic2004Оценок пока нет
- Hypothyroid Newsletter 01.20.09Документ24 страницыHypothyroid Newsletter 01.20.09Powerbug777100% (8)
- Lipids and MembranesДокумент9 страницLipids and MembranesPatricia Bianca BunagОценок пока нет
- Tipe Tubuh (Somatotype) Dengan Sindrom Metabolik Pada Wanita Dewasa Non-Obesitas Usia 25-40 TahunДокумент9 страницTipe Tubuh (Somatotype) Dengan Sindrom Metabolik Pada Wanita Dewasa Non-Obesitas Usia 25-40 TahunJessica Ester ExaudiaОценок пока нет
- 2023 2024 - Nutrition Care For ObesityДокумент73 страницы2023 2024 - Nutrition Care For ObesityDinanti OktianaОценок пока нет
- Efficacy of Itrifal Saghir', A Combination of Three Medicinal Plants in The Treatment of Obesity A Randomized Controlled TrialДокумент8 страницEfficacy of Itrifal Saghir', A Combination of Three Medicinal Plants in The Treatment of Obesity A Randomized Controlled TrialAhmad Yar SukheraОценок пока нет
- Does Family History of Obesity, Cardiovascular, and Metabolic Diseases Influence Onset and Severity of Childhood ObesityДокумент6 страницDoes Family History of Obesity, Cardiovascular, and Metabolic Diseases Influence Onset and Severity of Childhood ObesityTony Miguel Saba SabaОценок пока нет
- Fact Sheeton Canola OilДокумент15 страницFact Sheeton Canola OilMonika ThadeaОценок пока нет
- Abdominal Obesity, Adipokines and Non-Communicable Diseases 1-S2.0-S0960076020302624-MainДокумент6 страницAbdominal Obesity, Adipokines and Non-Communicable Diseases 1-S2.0-S0960076020302624-MainDeepika DhawanОценок пока нет
- Ok1 Hiperferritin Indonesia (Prof Ugro)Документ20 страницOk1 Hiperferritin Indonesia (Prof Ugro)Wijayadi SuyonoОценок пока нет
- Obesity: Dr. Princess Aliza LorezoДокумент13 страницObesity: Dr. Princess Aliza LorezorosamundraeОценок пока нет
- Forbidden Secrets From Nature's Pharmacy To Reverse Diabetes and Blood Sugar ProblemsДокумент54 страницыForbidden Secrets From Nature's Pharmacy To Reverse Diabetes and Blood Sugar Problemsa1khanОценок пока нет
- The Fast800 Diet: Discover the Ideal Fasting Formula to Shed Pounds, Fight Disease, and Boost Your Overall HealthОт EverandThe Fast800 Diet: Discover the Ideal Fasting Formula to Shed Pounds, Fight Disease, and Boost Your Overall HealthРейтинг: 5 из 5 звезд5/5 (37)
- The Raw Food Detox Diet: The Five-Step Plan for Vibrant Health and Maximum Weight LossОт EverandThe Raw Food Detox Diet: The Five-Step Plan for Vibrant Health and Maximum Weight LossРейтинг: 4 из 5 звезд4/5 (22)
- The Diabetes Code: Prevent and Reverse Type 2 Diabetes NaturallyОт EverandThe Diabetes Code: Prevent and Reverse Type 2 Diabetes NaturallyРейтинг: 5 из 5 звезд5/5 (1)
- Summary: Fast Like a Girl: A Woman’s Guide to Using the Healing Power of Fasting to Burn Fat, Boost Energy, and Balance Hormones: Key Takeaways, Summary and AnalysisОт EverandSummary: Fast Like a Girl: A Woman’s Guide to Using the Healing Power of Fasting to Burn Fat, Boost Energy, and Balance Hormones: Key Takeaways, Summary and AnalysisРейтинг: 3 из 5 звезд3/5 (2)
- Power Souping: 3-Day Detox, 3-Week Weight-Loss PlanОт EverandPower Souping: 3-Day Detox, 3-Week Weight-Loss PlanРейтинг: 3.5 из 5 звезд3.5/5 (3)
- Summary of Mary Claire Haver's The Galveston DietОт EverandSummary of Mary Claire Haver's The Galveston DietРейтинг: 5 из 5 звезд5/5 (1)
- Glucose Goddess Method: A 4-Week Guide to Cutting Cravings, Getting Your Energy Back, and Feeling AmazingОт EverandGlucose Goddess Method: A 4-Week Guide to Cutting Cravings, Getting Your Energy Back, and Feeling AmazingРейтинг: 5 из 5 звезд5/5 (59)
- Eat to Lose, Eat to Win: Your Grab-n-Go Action Plan for a Slimmer, Healthier YouОт EverandEat to Lose, Eat to Win: Your Grab-n-Go Action Plan for a Slimmer, Healthier YouОценок пока нет
- Happy Gut: The Cleansing Program to Help You Lose Weight, Gain Energy, and Eliminate PainОт EverandHappy Gut: The Cleansing Program to Help You Lose Weight, Gain Energy, and Eliminate PainРейтинг: 3.5 из 5 звезд3.5/5 (6)
- Keto Friendly Recipes: Easy Keto For Busy PeopleОт EverandKeto Friendly Recipes: Easy Keto For Busy PeopleРейтинг: 2 из 5 звезд2/5 (1)
- Allen Carr's Easy Way for Women to Lose Weight: The original Easyway methodОт EverandAllen Carr's Easy Way for Women to Lose Weight: The original Easyway methodРейтинг: 4.5 из 5 звезд4.5/5 (18)
- The Arm: Inside the Billion-Dollar Mystery of the Most Valuable Commodity in SportsОт EverandThe Arm: Inside the Billion-Dollar Mystery of the Most Valuable Commodity in SportsРейтинг: 4 из 5 звезд4/5 (49)
- Metabolism Revolution: Lose 14 Pounds in 14 Days and Keep It Off for LifeОт EverandMetabolism Revolution: Lose 14 Pounds in 14 Days and Keep It Off for LifeОценок пока нет
- Proteinaholic: How Our Obsession with Meat Is Killing Us and What We Can Do About ItОт EverandProteinaholic: How Our Obsession with Meat Is Killing Us and What We Can Do About ItРейтинг: 4.5 из 5 звезд4.5/5 (19)
- Grit & Grace: Train the Mind, Train the Body, Own Your LifeОт EverandGrit & Grace: Train the Mind, Train the Body, Own Your LifeРейтинг: 4 из 5 звезд4/5 (3)
- Lose Weight by Eating: 130 Amazing Clean-Eating Makeovers for Guilt-Free Comfort FoodОт EverandLose Weight by Eating: 130 Amazing Clean-Eating Makeovers for Guilt-Free Comfort FoodРейтинг: 2 из 5 звезд2/5 (1)
- Rapid Weight Loss Hypnosis: How to Lose Weight with Self-Hypnosis, Positive Affirmations, Guided Meditations, and Hypnotherapy to Stop Emotional Eating, Food Addiction, Binge Eating and MoreОт EverandRapid Weight Loss Hypnosis: How to Lose Weight with Self-Hypnosis, Positive Affirmations, Guided Meditations, and Hypnotherapy to Stop Emotional Eating, Food Addiction, Binge Eating and MoreРейтинг: 5 из 5 звезд5/5 (17)
- The Candida Cure: The 90-Day Program to Balance Your Gut, Beat Candida, and Restore Vibrant HealthОт EverandThe Candida Cure: The 90-Day Program to Balance Your Gut, Beat Candida, and Restore Vibrant HealthОценок пока нет
- How to Be Well: The 6 Keys to a Happy and Healthy LifeОт EverandHow to Be Well: The 6 Keys to a Happy and Healthy LifeРейтинг: 5 из 5 звезд5/5 (1)
- The Longevity Plan: Seven Life-Transforming Lessons from Ancient ChinaОт EverandThe Longevity Plan: Seven Life-Transforming Lessons from Ancient ChinaОценок пока нет
- The Ultimate Volumetrics Diet: Smart, Simple, Science-Based Strategies for Losing Weight and Keeping It OffОт EverandThe Ultimate Volumetrics Diet: Smart, Simple, Science-Based Strategies for Losing Weight and Keeping It OffОценок пока нет
- Ultrametabolism: The Simple Plan for Automatic Weight LossОт EverandUltrametabolism: The Simple Plan for Automatic Weight LossРейтинг: 4.5 из 5 звезд4.5/5 (28)
- The Toxin Solution: How Hidden Poisons in the Air, Water, Food, and Products We Use Are Destroying Our Health—AND WHAT WE CAN DO TO FIX ITОт EverandThe Toxin Solution: How Hidden Poisons in the Air, Water, Food, and Products We Use Are Destroying Our Health—AND WHAT WE CAN DO TO FIX ITРейтинг: 5 из 5 звезд5/5 (1)
- Brain Body Diet: 40 Days to a Lean, Calm, Energized, and Happy SelfОт EverandBrain Body Diet: 40 Days to a Lean, Calm, Energized, and Happy SelfРейтинг: 5 из 5 звезд5/5 (2)
- Think Yourself Thin: A 30-Day Guide to Permanent Weight LossОт EverandThink Yourself Thin: A 30-Day Guide to Permanent Weight LossРейтинг: 4.5 из 5 звезд4.5/5 (22)
- Kintsugi Wellness: The Japanese Art of Nourishing Mind, Body, and SpiritОт EverandKintsugi Wellness: The Japanese Art of Nourishing Mind, Body, and SpiritРейтинг: 4.5 из 5 звезд4.5/5 (3)