Академический Документы
Профессиональный Документы
Культура Документы
2214
Загружено:
rjpatil19Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
2214
Загружено:
rjpatil19Авторское право:
Доступные форматы
16. - 18. 10.
2013, Brno, Czech Republic, EU
SYNTHESIS SUPPER HYDROPHOBIC NANO SILICA FOR APPLICATION ON COTTON
FABRIC
Laleh MALEKNIA, Ali BARZEGAR,Ramin KHAJAVI,Nahid ALIZADE GHAMSARI,Marzieh TAHERI
Islamic Azad University-South Tehran Branch, Tehran, Islamic Republic of Iran, Melika02@azad.ac.ir
Abstract
Cotton is extensively utilized in textile and clothing industry but since it is highly shrinkable because cellulose
chain as well as water absorption with hydroxyl group which exists in these chains, one can sometimes face
with hard ironing process and are not fixed at dimension; therefore, this paper deals with production, Nanosynthesis of the hydrophobic silica particles for making water proof and reduction of shrinkage of cotton
textiles. For this purpose, hydrophobic silica Nano-particles have simply and economically been produced
and then they have been dispersed in MT resin in different percents. Finally, the cotton goods turned to be
water proof through pad-dry-cure system. Scanning electron microscope was used to study the size of
synthesized Nano-particles and to study chemical structure of the Nano-particles FTIR test has been
employed. Image analyzer Software was utilized to measure the angle of a drop, also, to study shrinkage
rates, ASTM standard test has been employed. Micrographs resulted from SEM revealed that the average
size of the Nano-particles is 16nm.FTIR test also approved the existence of Methyl groups at hydrophobic
nanosilica structure. The results from experiments of contact angle of the drop and shrinkage rate revealed
that drop angle on cotton goods is 150 and the Nano-particles can extraordinarily make cotton goods water
proof and reduce the shrinkage rate remarkably.
Keywords: Silica nanoparticle aerogelcotton fabrichydrophobwrinkle
1.
INTRODUCTION
Silica aerogels are novel mesoporous materials with many intriguing properties such as low bulk density(~0.l
g/cm3), continuous porosities, high specific surface area (500-1000 m2/g) and extremely low thermal
conductivity (~0.02Wm1K1) [1-5]. These properties are derived from the nanoporous network of
interconnected primary particles. Because of their unique texture, silica aerogels are promising materials as
super-thermal insula tors, catalytic supports, adsorbents, host materials for drugdelivery systems [6-lo]. After
the pioneering contribution by Kistler in 1931 [11], it took nearly three decades for the aerogel to become
scientifically and industrially an interesting material. This is because of the tedious synthesis procedures
followed by Kistler which used to take several weeks to wash out the salts generated in hydrogel during the
process and supercritical drying of the wet-gel to get an aerogel. A decisive simplification and acceleration of
the aerogel synthesis was reported in 196os by Nicolaon and Teichner, who used expensive metal alkoxide
precursor such as TMOS and supercritical drying technique [12]. Further attempt to make the aerogels
commercially in intereting was made by brinker, who synthesized silica aerogel by drying a wet-gel at an
ambient pressure and thereby avoiding the risky supercritical drying method [13]. In principle, their process
involved a series of solvent exchanges and an organic modification of the inner surface of the wet-gel by
non-polar groups such as alkyl or aryl. The gel undergoes spring-back efficientduring the drying and thus
preserves the aerogel properties in the dried solid. However, the tedious solvent exchange and surface
modification steps prolong the processing with huge consumption of costly solvents [l4-l6]. Schwertfeger et
al. reported a process for the aerogel production using sodium silicate (SS), i.e. water-glass without solvent
exchange and supercritical drying [17]. In their process, they used silylating agents in bulk (e. g. for 100 g of
hydrogel, the quantities of HMDSO and TMCS used were minimum 50 g) which makes this process quite
expensive.Recently, we have focused our work on the development of silica aerogels using sodium silicate
precursor via ambient pressure drying (APD). Keeping in view the less processing time, low production cost
16. - 18. 10. 2013, Brno, Czech Republic, EU
and ease of production, we employed for the first time a co-precursor method for the organic modification of
hydrogels without solvent exchange as a pre-requisite [18,19]. In the present studies, our goal was to
develop a new approach which would allow for the cost-effective synthesis of silica aerogel powders. We
prepared hydrogels directly from sodium silicatewithout prior ion exchange (like Kistlers process) and
employed a co-precursor method (i.e. addition of the silylating agent directly to the sodium silicate) for the
organic modification of hydrogels in the aqueous phase using HMDS and nitric acid. The gels were then
immersed in n-hexane for one-step solvent exchange which essentially leads to the displacement of pore
water due to the organic modification in the aqueous phase and simultaneous intrusion of n-hexane into the
gel. During this process, the sodium ions present in the hydrogels were given out with the displaced pore
water. The novel aspect of this process is that all the three steps such as sodium ion removal, solvent
exchange and surface modification were accomplished simultaneously in one-step which makes this route a
very promising for the large scale production of powdered aerogels.
2.
EXPERIMENTAL
2.1.
Synthesis of silica aerogel powders
The chemicals used for the synthesis of silica aerogel powders were sodium silicat ( 33%Merck KGaA),
nitric acid (65 %Merck KGaA), hexamethyldisilazaneand n-hexane) Merck KGaA(. The sodium silicate (SS)
solution was diluted first with the deionized water so as to obtain 4.35 wt.% of silica in the starting material.
The silylated hydrogels were then prepared by a co-precursor method wherein the nitric acid and
hexamethyldisilazane (HMDS) were added to the sodium silicate (4.35 wt.%) under constant stirring. The sol
undergoes gelation in 5 min at room temperature (27 C). The silylated hydrogels prepared by the coprecursor method were immersed in n-hexane for one-step solvent exchange and sodium ion removal which
could be accomplished in 3 hr. The displaced pore water was then removed from the beakers and the
silylatedorgano-gels were centrifugewith distilled water and then dried the hydrogel at ambient pressure in a
furnace in two steps: firstly at 170 0C for 20 min and secondly at 200 0C for 20 min to obtain the aerogel
powder.
2.2.
Impregnate cotton fabrics by resin and aerogel silica powder
4-8 ml of MT resin was taken in a beaker and diluted it by 100 ml distilled water and then added diffrent
percentage of Silica aerogel powder to beaker over a magnetic stirrer with constant stirring for 3 hr till the
powder disperss well in resin. amountof aerogel powder were 0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07 and
0.08gr. Fabric samples were prepared in 15*15cm and impregnated in different concentration of resin and
silica nano powders for 1hr and then dried and cured them at 1200C for 20 min.
2.3.
Methods and equipment of characterization
The microstructure of the aerogel powders was probed by a Field Emission Scanning Electron Microscope
(FE-SEM, JSM6700 F microscope, JEOL). The surface chemical modification of the aerogel powders was
confirmed by means of Fourier transform infrared spectroscopy (FT-IR, IFS 88, Bruker,Germany). The
contact angle ( ) measurements were done using a contact angle meter (Image analyzer ) to quantify the
degree of hydrophobicity. In order to Test the return of shrinkage in Cotton fabric impregnated by resin and
aerogel silica powder was used from the device wrinkle recovery tester AATCC 128(Shirley company
UK).Bending rigidity of fabrics measured by bending sensing device (SDL technology) M003B on the basis
of the standard ASTM D1388-96.
16. - 18. 10. 2013, Brno, Czech Republic, EU
3.
RESULTS AND DISCUSSION
3.1.
morphology
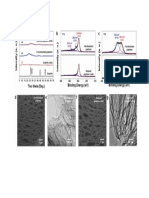
Microstructural studies were carried out for determine hydrophobic Silica nanoparticles by FESEM (FESEM, JSM6700 F microscope, JEOL). Fig.1 microscopic images show aerogel prepared with two different
magnifications. As the figures hows that nanoparticles have uniform distributionand the average particle size
is 31nm.
Fig 1 FESEM photogerafs of silica aerogel powder
3.2.
FTIR
FTIRwas used to study of surface modified aerogels produced using nonpolar solvents. The formulation of
hydrogel surface changes produced by themethyl groups of dimethylhexaSylazanis showns chematically in
following reactions:
Na2SiO3+ 2HNO3H2O [SiO2.xH2O]+ 2NaNO3
1
2)SiOH( + (CH3)3Si-NH-Si(CH3)3
HNO3 2(SiOSi(CH3)3) + NH3
Fig. 2 shows the FT-IR spectra of the aerogel powder synthesized with simultaneous surface modification,
solvent exchange and sodium ion removal. The peaks at 2963cm -1 correspond to the IR absorption with
exchanging OH in initial hydrogel by CH3 groups in Si bonds. [20,21] present in the aerogel powder which
confirms the surface modification and formation of hydrophobic Silica nanoparticles. During this process the
hydrogel was immersed in a water immiscible solvent such as n-hexane. As a consequence of water
displacement, n-hexane entered in the pore and eventually the hydrogel was transformed into
a silylatedorgano-gel and thus accomplished the solvent exchange.The solvent exchange in the present
studies is a one-step process and it is driven by the displacement of pore water from the hydrogel.
Fig. 2 FT-IR spectra of the aerogel powder
16. - 18. 10. 2013, Brno, Czech Republic, EU
3.3.
Super hydrophobicity of the aerogel powders
Fig. 3 show the micrographs of obtain result from water droplets placed in the surface of cotton fabric coated
with different concentrations hydrophobic silica aerogel powder. The drop maintained spheroidshape with
contact angle of 1400 on the fabric coated with 0.03gr aerogel powder. The result shows increasing the
contact angle by increasing amount of aerogel in a exact concentration of REZIN but surface gradually
becomes undesirable.
Table 1.duration of the shelf-life drops on the cotton fabric
Duration of
the shelflife drops
on the
goods
50min
70min
70min
110min
105min
85min
85min
65min
Mount of
aerogel
powders
0.01gr
0.015gr
0.02gr
0.03gr
0.04gr
0.05gr
0.06gr
0.07gr
Sample1
Sample2
Sample3
Sample4
Sample5
Sample6
Sample7
Sample8
Fig 3 duration of the shelf-life drops on the cotton fabric
Table 2 duration of the shelf-life drops on the cotton fabric after steam iron
duration of
the shelflife drops
on the
goods after
steam iron
0.5min
7min
10min
20min
12min
11min
5min
2min
mount of
aerogel
powders
0.01gr
0.015gr
0.02gr
0.03gr
0.04gr
0.05gr
0.06gr
0.07gr
Sample1
Sample2
Sample3
Sample4
Sample5
Sample6
Sample7
Sample8
Fig 4 duration of the shelf-life drops on the cotton fabric
after steam iron
Fig 3 micrographs of obtain result from water droplets placed in the surface of cottonfabric
16. - 18. 10. 2013, Brno, Czech Republic, EU
3.4.
wrinkle recovery test
result of fabrics wrinkle recovery test, determined that the silica aerogel powder have a salienteffect on the
decreasing of cotton fiber crimping.Cotton fabrics absorb moisture and it cause wrinkling the fabrics. the
water molecule cause the gliding cellulose chain on each other and then the side hydrogen bond of the chain
can make new graft in presence of water molecule in new places that be the reason of the
wrinkling.WrinkleRecovery Test shows that the superhydrophobic SiO2 nano particle on the cellulose chain
prevent moisture absorption so ase a result the chaingliding on each other and make new bonding and at
last it prevent to wrinkle the fabrics.
Table 3 angle of the wrinkles
mount
of
aerogel
powders
angle of
the
wrinkles
in the
direction
ofthe
weft end
0gr
85
angle of
the
wrinkles
in the
direction
of the
warp
yarn
90
0.01gr
0.02gr
0.03gr
0.04gr
0.05gr
0.06gr
0.07gr
99
109
118
96
108
119
97
105
123
132
118
115
119
115
Raw
sample
Sample1
Sample2
Sample3
Sample4
Sample5
Sample6
Sample7
3.5.
Fig 5 angle of the wrinkles in the directionof the warp yarn
and weft enddirection of the warp yarn and weft end
bending rigidity
Table 4 shows the results obtained for impregnated fabrics with difrrent percentages resin and Silica
aerogel.for investigation the bonding rigidity of coated cotton fabrics, SDLmtechnology M003B for measuring
bending rigidity was used. In this study different percentages of resin were analyzed on cotton fabrics.
According to the result increasing amount of rezin cause increasing the bending rigidity but as respect that
the bending rigidity affects on final operation and ZIR DAST the optimum mount af resin was 7 ml.
Table 4 bonding rigidity of coated cotton
bending
rigidity of
fabrics
Rawsample
Sample1
Sample2
0.01gf/cm2
0.017gf/cm2
0.019gf/cm2
the amount
of resin
impregnated
to goods
0ml
4ml
5ml
Sample3
0.025gf/cm2
6ml
Sample4
0.032gf/cm2
7ml
Sample5
0.035gf/cm2
8ml
Fig 6 bending rigidity of coated cotton fabrics
16. - 18. 10. 2013, Brno, Czech Republic, EU
4.
CONCLUSIONS
This method forsynthesising Silicaaergelpowder wasan easyand economicalwayby using cheap materials ,
such assodium silicate and reduce synthesis time.the total processing time of the aerogel powders could be
restricted to 4 hr. according to this parameters this method was more affordable than the previous synthesis
methods sodium silicate and HMDS and nitric acid were mixed for the organic modification of hydrogels in
the aqueous phase . The gels were then immersed in a nonpolar solvent such as, n-hexane for solvent
exchange . During this process, the sodium ions present in the hydrogels were given out with the displaced
pore water.Thepropertiesofthe aerogelpowder obtainedand usingitonthecotton textile for makeithydrophob
was an important step intheresearch processtobring other productshyrophob. The contact angle for the
sample which was impregnated by aerogel powder, was about 1400. According to wonderful proprties of this
nanoparticles, by using other resins on cottonsubstrate to reach contact angel up to 150 0. If the contact angle
being more than 1500, that substrate will be a super hydrophobic.Use of this nanoparticles on cotton fabric
perevent wrinkles on fabrics as well.
REFERENCES
[1]
L.W. Hrubesh, J. Non-Cryst. Solids 225 (1998) 335.
[2]
L. Kocon, F. Depetis, J. Non-Cryst. Solids 225 (1998) 96.
[3]
M.R. Ayers, A.J. Hunt, J. Non-Cryst. Solids 285 (2001) 123.
[4]
A.V. Rao, S.D. Bhagat, Solid State Sci. 6 (2004) 945.
[5]
A.V. Rao, M.M. Kulkarni, Mater. Chem. Phys. 77 (2002) 819.
[6]
P. Fabrizioli, T. Burgi, A. Baiker, J. Catal. 206 (2002) 143.
[7]
E.F. Murphy, A. Baiker, J. Mol. Catal. A: Chem. 179 (2002) 233.
[8]
C. Beck, T. Mallat, A. Baiker, J. Catal. 195 (2000) 79.
[9]
S. Yoda, S. Ohshima, F. Ikazaki, J. Non-Cryst. Solids 231 (1998) 41.
[10]
A.V. Rao, M.M. Kulkarni, S.D. Bhagat, J. Colloid Interf. Sci. 285(2005) 413.
[11]
S.S. Kistler, Nature 127 (1931) 741.
[12]
G.A. Nicolaon, S.J. Teichner, Bull. Soc. Chim. Fr. (1968) 1900.
[13]
C.J. Brinker, in: Mater. Res. Soc. Symp. Proc., 1992, p. 567.
[14]
C.J. Lee, G.S. Kim, S. Hyun, J. Mater. Sci. 37 (2002) 2237.
[15]
A.P. Rao, G.M. Pajonk, A.V. Rao, J. Mater. Sci. 40 (2005) 3481.
[16]
R. Deshpande, D.M. Smith, C.J. Brinker, J. Non-Cryst. Solids 144(1992) 32.
[17]
F. Schwertfeger, D. Frank, M. Schmidt, J. Non-Cryst. Solids 225(1998) 24.
[18]
S.D. Bhagat, Y.-H. Kim, Y.-S.Ahn, J.-G. Yeo, Micropor. Mesopor.Mater. 96 (2006) 237.
[19]
S.D. Bhagat, Y.-H. Kim, Y.-S.Ahn, J.-G. Yeo, Appl. Surf. Sci. 253(2006) 3231.
Вам также может понравиться
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (120)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Astm A266Документ4 страницыAstm A26609122912680Оценок пока нет
- HSS Cap Plate ConnectionДокумент7 страницHSS Cap Plate ConnectionlavignenoeОценок пока нет
- Drilling ProcessДокумент15 страницDrilling ProcessAl Fredo95% (21)
- Wind Load Calculation (9 MTR)Документ2 страницыWind Load Calculation (9 MTR)saravanakumarpalani100% (2)
- Superalloys - A Primer and HistoryДокумент4 страницыSuperalloys - A Primer and Historyhemakumars100% (1)
- How Power Factor Corection WorksДокумент16 страницHow Power Factor Corection Worksapi-3806201100% (6)
- Further Mechanics 1 Unit Test 5 Elastic Strings and Springs and Elastic Energy Mark SchemeДокумент7 страницFurther Mechanics 1 Unit Test 5 Elastic Strings and Springs and Elastic Energy Mark SchemeGavin Man100% (4)
- Intro To ShearographyДокумент23 страницыIntro To ShearographyizmitlimonОценок пока нет
- IR-12-structure DeterminationДокумент31 страницаIR-12-structure DeterminationSaji AlexОценок пока нет
- Battery Separators BATSOLДокумент2 страницыBattery Separators BATSOLrjpatil19Оценок пока нет
- 15mnt0016 Consolidated Samples & ResultsДокумент3 страницы15mnt0016 Consolidated Samples & Resultsrjpatil19Оценок пока нет
- How Does Nanosize Influence The Electron Band GapДокумент2 страницыHow Does Nanosize Influence The Electron Band Gaprjpatil19Оценок пока нет
- ALlGbatt ResultsДокумент1 страницаALlGbatt Resultsrjpatil19Оценок пока нет
- IC ch07 PDFДокумент45 страницIC ch07 PDFrjpatil19Оценок пока нет
- Applications Taylor SeriesДокумент5 страницApplications Taylor Seriesrjpatil19Оценок пока нет
- Limits at Infinity NotesДокумент10 страницLimits at Infinity Notesrjpatil19Оценок пока нет
- 15 MNT 0016Документ1 страница15 MNT 0016rjpatil19Оценок пока нет
- Convergence Power SeriesДокумент5 страницConvergence Power Seriesrjpatil19100% (1)
- Computing Taylor SeriesДокумент7 страницComputing Taylor Seriesrjpatil19Оценок пока нет
- Syllabus Diploma ElectricalДокумент136 страницSyllabus Diploma Electricalrjpatil19Оценок пока нет
- Probability Study GuideДокумент2 страницыProbability Study Guiderjpatil19Оценок пока нет
- Convergence Divergence NotesДокумент11 страницConvergence Divergence NotesRamaDinakaranОценок пока нет
- Development of Memory Chips, Memory Read Write OperationsДокумент6 страницDevelopment of Memory Chips, Memory Read Write Operationsrjpatil19Оценок пока нет
- Application Hrushi BagadeДокумент1 страницаApplication Hrushi Bagaderjpatil19Оценок пока нет
- Nikhilesh Pawar Resume - 21.10.13Документ4 страницыNikhilesh Pawar Resume - 21.10.13rjpatil19Оценок пока нет
- Linux Fasttrack 2006Документ177 страницLinux Fasttrack 2006mariusОценок пока нет
- Phys Sample GДокумент11 страницPhys Sample GAbhijit SanjeevОценок пока нет
- IITTM GwaliorДокумент10 страницIITTM Gwaliorrjpatil19Оценок пока нет
- BSNL Customer Care PDFДокумент4 страницыBSNL Customer Care PDFrjpatil19Оценок пока нет
- 8085 Microprocessor Pin ConfigurationДокумент7 страниц8085 Microprocessor Pin Configurationrjpatil19Оценок пока нет
- Basic Circuit Theory Problem SetДокумент9 страницBasic Circuit Theory Problem Setrjpatil19100% (1)
- MR AmbaniДокумент15 страницMR Ambanihemant100% (23)
- JEE ChemistryДокумент4 страницыJEE Chemistryrjpatil19Оценок пока нет
- Engineering Mathematics SyllabusДокумент2 страницыEngineering Mathematics Syllabusrjpatil19Оценок пока нет
- Cooper Bussman NSC CRДокумент21 страницаCooper Bussman NSC CRrjpatil19Оценок пока нет
- Yg Pocket 2014 En18572 PDFДокумент437 страницYg Pocket 2014 En18572 PDFliceuОценок пока нет
- A One-Equationturbulencetransport: Model For High Reynolds Number Wall-Bounded FlowsДокумент26 страницA One-Equationturbulencetransport: Model For High Reynolds Number Wall-Bounded FlowsAlirezaKhoshkoneshОценок пока нет
- Photonic Crystal - Wikipedia, The Free EncyclopediaДокумент8 страницPhotonic Crystal - Wikipedia, The Free EncyclopediaWasyhun AsefaОценок пока нет
- Thermal Spray Coating For Steel ProcessingДокумент5 страницThermal Spray Coating For Steel ProcessingRamkiyengarОценок пока нет
- Spring Supplement Designers GДокумент6 страницSpring Supplement Designers Gbell_15477Оценок пока нет
- Eglin SteelДокумент3 страницыEglin SteelShaun LeeОценок пока нет
- r050210104 Fluid MechanicsДокумент10 страницr050210104 Fluid MechanicsSrinivasa Rao G100% (1)
- Beam Deflection Tables - MechaniCalcqweqdsdДокумент9 страницBeam Deflection Tables - MechaniCalcqweqdsdKenneth LuceroОценок пока нет
- Gate Steel DesignДокумент21 страницаGate Steel DesignTejas PatilОценок пока нет
- Generalized Water Hammer Algorithm For Piping Systems With Unsteady Friction PDFДокумент115 страницGeneralized Water Hammer Algorithm For Piping Systems With Unsteady Friction PDFHugo RuizОценок пока нет
- A.E.E'S (Assistant Executive Engineers) : (Civil Engineering - 1984)Документ12 страницA.E.E'S (Assistant Executive Engineers) : (Civil Engineering - 1984)SHIRISHA YADAVОценок пока нет
- Gonzalez 21399Документ11 страницGonzalez 21399Yogesh S Yogi SОценок пока нет
- Presentation PosterДокумент1 страницаPresentation PosterMihir PatelОценок пока нет
- Reservoir Fluid PropertiesДокумент9 страницReservoir Fluid PropertiesAnonymous LLLK3pqОценок пока нет
- Microstructural, Mechanical and Strain Hardening Behaviour of NiTi Alloy Subjected To Constrained Groove Pressing and Ageing TreatmentДокумент14 страницMicrostructural, Mechanical and Strain Hardening Behaviour of NiTi Alloy Subjected To Constrained Groove Pressing and Ageing TreatmentMoin AОценок пока нет
- Material PropertiesДокумент25 страницMaterial PropertiesAbhijit SanjeevОценок пока нет
- Effects of On-Line Melt Blending of Polypropylene With Polyamide 6 On The Bulk and Strength of The Resulting BCF YarnДокумент10 страницEffects of On-Line Melt Blending of Polypropylene With Polyamide 6 On The Bulk and Strength of The Resulting BCF YarnianОценок пока нет
- RT - Casting DefectsДокумент5 страницRT - Casting DefectsShanawas Abdul RazakОценок пока нет
- AMPT2018 - Programme Booklet PDFДокумент44 страницыAMPT2018 - Programme Booklet PDFMazurchevici Andrei DănuţОценок пока нет
- M.SC PhysicsДокумент73 страницыM.SC Physicsnitu kumariОценок пока нет
- Sol Xe Doc s88 Exs020 High 1Документ2 страницыSol Xe Doc s88 Exs020 High 1Hafid Papeda SaguОценок пока нет
- Multiple Choice Questions (MCQ) : Assignment 2 - Cutting ToolsДокумент2 страницыMultiple Choice Questions (MCQ) : Assignment 2 - Cutting ToolsAbdullah ALdajaniОценок пока нет
- Handout 6Документ21 страницаHandout 6coppernitrateОценок пока нет
- 4 FOUNDATION IN ROCK (Compatibility Mode) PDFДокумент28 страниц4 FOUNDATION IN ROCK (Compatibility Mode) PDFxdhrts54 yОценок пока нет