Академический Документы
Профессиональный Документы
Культура Документы
Pip01 07
Загружено:
James BarlowОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Pip01 07
Загружено:
James BarlowАвторское право:
Доступные форматы
European Medicines Agency
Doc. Ref. EMEA/357907/2008
P/53/2008
EUROPEAN MEDICINES AGENCY DECISION
of 20 July 2008
on the application for agreement of a Paediatric Investigation Plan for Atorvastatin calcium
(Sortis and associated names) EMEA-000073-PIP01-07 in accordance with Regulation (EC) No
1901/2006 of the European Parliament and of the Council as amended
(ONLY THE ENGLISH TEXT IS AUTHENTIC)
7 Westferry Circus, Canary Wharf, London, E14 4HB, UK
Tel. (44-20) 74 18 84 00 Fax (44-20) 74 18 86 70
E-mail: mail@emea.europa.eu http://www.emea.europa.eu
EUROPEAN MEDICINES AGENCY DECISION
of 20 July 2008
on the application for agreement of a Paediatric Investigation Plan for Atorvastatin calcium,
(Sortis and associated names) EMEA-000073-PIP00-07 in accordance with Regulation (EC) No
1901/2006 of the European Parliament and of the Council as amended
THE EUROPEAN MEDICINES AGENCY,
Having regard to the Treaty establishing the European Community,
Having regard to Regulation (EC) No 1901/2006 of the European Parliament and of the Council of 12
December 2006 on medicinal products for paediatric use as amended and amending Regulation (EEC)
No. 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/20041,
Having regard to Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31
March 2004 laying down Community procedures for the authorisation and supervision of medicinal
products for human and veterinary use and establishing a European Medicines Agency2,
Having regard to the application submitted by Pfizer Limited on 19 October 2007 under Article 16(1)
also requesting a waiver under Article 13 of said Regulation,
Having regard to the opinion of the Paediatric Committee of the European Medicines Agency, issued
on 4 June 2008, in accordance with Article 18 of Regulation (EC) No 1901/2006 as amended, and
Article 13 of said Regulation,
Having regard to Article 25 of Regulation (EC) No 1901/2006 as amended,
WHEREAS:
(1) The Paediatric Committee of the European Medicines Agency has given a positive opinion,
(2) It is therefore appropriate to adopt a Decision following the Paediatric Committee’s opinion
on the Paediatric Investigation Plan.
(3) It is therefore appropriate to adopt a Decision granting a waiver.
1
OJ L 378, 27.12.2006, p.1
2
OJ L 136, 30.4.2004, p. 1
EMEA/357907/2008 Page 2/11
HAS ADOPTED THIS DECISION:
Article 1
A Paediatric Investigation Plan for Atorvastatin calcium, (Sortis and associated names), film-coated
tablet, age-appropriate oral formulation, oral use, the details of which are set out in the Opinion of the
Paediatric Committee of the European Medicines Agency annexed hereto, together with its
appendices, is hereby agreed.
Article 2
A waiver for Atorvastatin calcium, (Sortis and associated names), film-coated tablet, age-appropriate
oral formulation, oral use, the details of which are set out in the Opinion of the Paediatric Committee
the European Medicines Agency annexed hereto, together with its appendices, is hereby granted.
Article 3
This decision is addressed to Pfizer Limited, Ramsgate Road, Sandwich, Kent CT13 9NJ Sandwich .
Done at London, 20 July 2008
For the European Medicines Agency
Thomas Lönngren
Executive Director
(Signature on file)
EMEA/357907/2008 Page 3/11
European Medicines Agency
Pre-authorisation Evaluation of Medicines for Human Use
EMEA/PDCO/287022/2008
EMEA-000073-PIP01-07
POSITIVE OPINION OF THE PAEDIATRIC COMMITTEE ON
A REQUEST FOR AGREEMENT OF
A PAEDIATRIC INVESTIGATION PLAN FOR
Scope of the application
Active substance:
Atorvastatin calcium
Invented name:
Sortis and associated names
Condition(s):
Pure hypercholesterolaemia (heterozygous, homozygous, or otherwise primary
hypercholesterolaemia), combined (mixed) hyperlipidaemia; prevention of cardiovascular events
Pharmaceutical form(s):
Film-coated tablet
Age-appropriate oral formulation
Route(s) of administration:
Oral use
Name/corporate name of the PIP applicant:
Pfizer Limited
Information about the authorised medicinal product:
See Annex II.
Basis for opinion
Pursuant to Article 16.1 of Regulation (EC) No 1901/2006 as amended, Pfizer Limited submitted for
agreement to the EMEA on 19 October 2007 a paediatric investigation plan for the above mentioned
medicinal product.
The procedure started on 22 November 2007.
Supplementary information was provided by the applicant on 10 April 2007.
A meeting with the Paediatric Committee took place on 2 June 2008.
7 Westferry Circus, Canary Wharf, London, E14 4HB, UK
Tel. (44-20) 74 18 84 00 Fax (44-20) 75 23 70 40
E-mail: mail@emea.europa.eu http://www.emea.europa.eu
Opinion
1. The Paediatric Committee, having assessed the proposed paediatric investigation plan in accordance
with Article 17 of Regulation (EC) No 1901/2006 as amended, recommends as set out in the appended
summary report:
• to agree the paediatric investigation plan in accordance with Article 18 of Regulation
(EC) No 1901/2006 as amended,
to grant a waiver for one or more subsets of the paediatric population in accordance
with Article 13 of Regulation (EC) No 1901/2006 as amended and concluded in
accordance with Article 11(1)(c) of Regulation (EC) No 1901/2006 as amended, on
the grounds that the specific medicinal product does not represent a significant
therapeutic benefit over existing treatments for paediatric patients.
The Icelandic and the Norwegian Paediatric Committee members agree with the above-mentioned
recommendation of the Paediatric Committee.
2. The measures and timelines of the agreed paediatric investigation plan and the subset(s) of the
paediatric population and condition(s) covered by the waiver are set out in the Annex I.
This opinion is forwarded to the applicant and the Executive Director of the Agency, together with its
annexes and appendices.
London, 4 June 2008
On behalf of the Paediatric Committee
Dr Daniel Brasseur, Chairman
(Signature on file)
EMEA/PDCO/287022/2008 Page 5/11
ANNEX I
THE MEASURES AND TIMELINES OF THE AGREED PAEDIATRIC INVESTIGATION
PLAN AND THE SUBSET(S) OF THE PAEDIATRIC POPULATION AND CONDITION(S)
COVERED BY THE WAIVER
EMEA/PDCO/287022/2008 Page 6/11
A. CONDITION(S) / DISEASE(S)
Pure hypercholesterolaemia (heterozygous, homozygous, or otherwise primary hypercholesterol-
aemia), combined (mixed) hyperlipidaemia; prevention of cardiovascular events
B. WAIVER
• Condition
Heterozygous hypercholesterolaemia
o Subset(s) of the paediatric population, pharmaceutical form(s) and route(s) of
administration covered
The waiver applies to children aged 0 to less than 6 years for the film-coated tablet and the
age-appropriate formulation for oral use.
• Condition
Homozygous familial hypercholesterolaemia, combined (mixed) hypercholesterolaemia, primary
hypercholesterolaemia; prevention of cardiovascular events
o Subset(s) of the paediatric population, pharmaceutical form(s) and route(s) of
administration covered
The waiver applies to children aged 0 to less than 18 years for the film-coated tablet and
the age-appropriate formulation for oral use.
EMEA/PDCO/287022/2008 Page 7/11
C. PAEDIATRIC INVESTIGATION PLAN
Heterozygous hypercholesterolaemia
C.1. Condition to be investigated
Heterozygous hypercholesterolaemia
• Subset(s) covered
Children aged 6 to less than 18 years
• Formulation(s)
Film-coated tablet and age-appropriate oral formulation
• Studies / Measures
Study Area Subarea Description
Number
#1 Clinical Bioequivalence Bioequivalence study of the final age-appropriate oral
atorvastatin formulation to the existing atorvastatin
formulation in healthy adult volunteers
#2 Clinical Pharmacokinetic, Steady-state, eight week pharmacokinetic study of
safety atorvastatin in children and adolescents (aged 6 years to less
than 18 years) with heterozygous familial hypercholesterol-
aemia using sparse PK sampling methodology and including
flow-mediated artery dilatation assessments
#3 Clinical Safety A 3-year study of the safety and follow-up study of efficacy
of atorvastatin treatment of children and adolescents (aged 6
years to less than 18 years) with heterozygous familial
hypercholesterolaemia*
* Study to be initiated by the date of completion of the paediatric investigation plan
Date of completion of the paediatric investigation plan: 31 December 2009
Deferral compared to submission date: No
• Specific safety concern(s) / monitoring
Need for a EU-RMP: Yes
EMEA/PDCO/287022/2008 Page 8/11
ANNEX II
INFORMATION ABOUT THE AUTHORISED MEDICINAL PRODUCT
EMEA/PDCO/287022/2008 Page 9/11
Country Invented Strength Pharmaceutical Route of
Name form administration
AT Sortis 80 mg film-coated tablet oral
AT Sortis 10, 20, 40 mg film-coated tablet oral
BE Lipitor 10, 20, 40 mg film-coated tablet oral
BE Lipitor 80 mg film-coated tablet oral
BG Sortis 80 mg film-coated tablet oral
BG Sortis 10, 20, 40 mg film-coated tablet oral
CY Lipitor 10, 20, 40 mg film-coated tablet oral
CZ Sortis 10, 20, 40 mg film-coated tablet oral
CZ Sortis 80 mg film-coated tablet oral
DE Sortis 10, 20, 40 mg film-coated tablet oral
DE Sortis 80 mg film-coated tablet oral
DK Zarator 80 mg film-coated tablet oral
DK Zarator 10, 20, 40 mg film-coated tablet oral
EE Sortis 80 mg film-coated tablet oral
EE Sortis 10, 20, 40 mg film-coated tablet oral
ES Cardyl 10 mg film-coated tablet oral
ES Cardyl 20, 40 mg film-coated tablet oral
ES Zarator 10 mg film-coated tablet oral
ES Zarator 80 mg film-coated tablet oral
ES Cardyl 80 mg film-coated tablet oral
ES Zarator 10 mg film-coated tablet oral
ES Zarator 20, 40 mg film-coated tablet oral
FI Lipitor 10, 20, 40 mg film-coated tablet oral
FI Lipitor 80 mg film-coated tablet oral
FR Tahor 80 mg film-coated tablet oral
FR Tahor 10, 20, 40 mg film-coated tablet oral
GB Lipitor 80 mg film-coated tablet oral
GB Lipitor 10, 20, 40 mg film-coated tablet oral
GR Lipitor 10, 20, 40 mg film-coated tablet oral
GR Lipitor 80 mg film-coated tablet oral
GR Zarator 10, 20, 40 mg film-coated tablet oral
HU Sortis 80 mg film-coated tablet oral
HU Sortis 10, 20, 40 mg film-coated tablet oral
HU Liprimar 80 mg film-coated tablet oral
HU Liprimar 10, 20, 40 mg film-coated tablet oral
IE Lipitor 10, 20, 40 mg film-coated tablet oral
IE Lipitor 80 mg film-coated tablet oral
IS Zarator 10, 20, 40 mg film-coated tablet oral
IS Zarator 80 mg film-coated tablet oral
IT Totalip 80 mg film-coated tablet oral
IT Xarator 80 mg film-coated tablet oral
IT Torvast 80 mg film-coated tablet oral
IT Xarator 10, 20, 40 mg film-coated tablet oral
IT Lipitor 10, 20, 40 mg film-coated tablet oral
IT Totalip 10, 20, 40 mg film-coated tablet oral
IT Torvast 10, 20, 40 mg film-coated tablet oral
IT Lipitor 80 mg film-coated tablet oral
LT Sortis 10, 20, 40 mg film-coated tablet oral
LT Sortis 80 mg film-coated tablet oral
LU Lipitor 80 mg film-coated tablet oral
LU Lipitor 10, 20, 40 mg film-coated tablet oral
LV Sortis 10, 20, 40 mg film-coated tablet oral
LV Sortis 80 mg film-coated tablet oral
MT Lipitor 10, 20, 40 mg film-coated tablet oral
EMEA/PDCO/287022/2008 Page 10/11
MT Lipitor 80 mg film-coated tablet oral
NL Lipitor 10, 20, 40 mg film-coated tablet oral
NL Lipitor 80 mg film-coated tablet oral
NO Lipitor 80 mg film-coated tablet oral
NO Lipitor 10, 20, 40 mg film-coated tablet oral
PL Sortis 10, 20, 40 mg film-coated tablet oral
PL Sortis 80 mg film-coated tablet oral
PT Sortis 10, 20, 40 mg film-coated tablet oral
PT Zarator 10, 20, 40 mg film-coated tablet oral
PT Zarator 80 mg film-coated tablet oral
RO Sortis 10, 20 mg film-coated tablet oral
RO Sortis 40, 80 mg film-coated tablet oral
SE Lipitor 10, 20, 40 mg film-coated tablet oral
SE Lipitor 80 mg film-coated tablet oral
SI Sortis 10, 20, 40 mg film-coated tablet oral
SK Sortis 80 mg film-coated tablet oral
SK Sortis 10, 20, 40 mg film-coated tablet oral
EMEA/PDCO/287022/2008 Page 11/11
Вам также может понравиться
- Childminding Ofsted Effective PracticeДокумент12 страницChildminding Ofsted Effective PracticeJames BarlowОценок пока нет
- Bristol City Council Transgender Guide v13Документ16 страницBristol City Council Transgender Guide v13James BarlowОценок пока нет
- UK Heatwave Plan (March 2010) Department of HealthДокумент47 страницUK Heatwave Plan (March 2010) Department of HealthJames BarlowОценок пока нет
- Newcastle University Alternate Student Prospectus 1986Документ34 страницыNewcastle University Alternate Student Prospectus 1986James BarlowОценок пока нет
- Charlottes Place Restaurants LimitedДокумент4 страницыCharlottes Place Restaurants LimitedJames BarlowОценок пока нет
- Ofsted Inspection of Children's Services - Grade Descriptors (PDF Format)Документ47 страницOfsted Inspection of Children's Services - Grade Descriptors (PDF Format)James BarlowОценок пока нет
- DSP App - Dspfinance - Com - A Document Received As Part of A Job Scam, Likely A Cheque Fraud NetworkДокумент3 страницыDSP App - Dspfinance - Com - A Document Received As Part of A Job Scam, Likely A Cheque Fraud NetworkJames BarlowОценок пока нет
- W102 Introduction To Law TMA 01 (2014)Документ9 страницW102 Introduction To Law TMA 01 (2014)James BarlowОценок пока нет
- DSP Info - Dspfinance - Com - A Document Received As Part of A Job Scam, Likely A Cheque Fraud NetworkДокумент4 страницыDSP Info - Dspfinance - Com - A Document Received As Part of A Job Scam, Likely A Cheque Fraud NetworkJames BarlowОценок пока нет
- Zombie Contingency PlanДокумент2 страницыZombie Contingency PlanJames BarlowОценок пока нет
- Article 50 Report 2010Документ34 страницыArticle 50 Report 2010James BarlowОценок пока нет
- Lidl Evoucher - 5 Off 12 Aug 10 To 15 Aug 10Документ1 страницаLidl Evoucher - 5 Off 12 Aug 10 To 15 Aug 10James BarlowОценок пока нет
- Cheap Meeting and Conference Venues in BristolДокумент23 страницыCheap Meeting and Conference Venues in BristolJames BarlowОценок пока нет
- British Computer Society - Extraordinary General Meeting 10 Explanatory NoteДокумент8 страницBritish Computer Society - Extraordinary General Meeting 10 Explanatory NoteJames BarlowОценок пока нет
- UK Parliamentary General Election Nominations Factsheet 2010Документ5 страницUK Parliamentary General Election Nominations Factsheet 2010James BarlowОценок пока нет
- Corey Helford BristolДокумент4 страницыCorey Helford BristolJames BarlowОценок пока нет
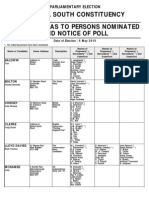
- Statement of Persons Nominated - Bristol South Parliamentary Constituency 2010Документ2 страницыStatement of Persons Nominated - Bristol South Parliamentary Constituency 2010James BarlowОценок пока нет
- Cheap Meeting and Conference Venues in BristolДокумент23 страницыCheap Meeting and Conference Venues in BristolJames BarlowОценок пока нет
- Statement of Persons Nominated - Bristol East Parliamentary Constituency 2010Документ2 страницыStatement of Persons Nominated - Bristol East Parliamentary Constituency 2010James BarlowОценок пока нет
- Statement of Persons Nominated - Bristol West Parliamentary Constituency 2010Документ2 страницыStatement of Persons Nominated - Bristol West Parliamentary Constituency 2010James BarlowОценок пока нет
- Local Statement of Persons Nominated (All Wards)Документ3 страницыLocal Statement of Persons Nominated (All Wards)James BarlowОценок пока нет
- Statement of Persons Nominated - Bristol North West Parliamentary Constituency 2010Документ2 страницыStatement of Persons Nominated - Bristol North West Parliamentary Constituency 2010James BarlowОценок пока нет
- Notice of Election (Bristol Council - by Thirds) - May 6 2010Документ1 страницаNotice of Election (Bristol Council - by Thirds) - May 6 2010James BarlowОценок пока нет
- Association of Chief Police Officers New Psychoactive Substances Guidance WebsiteДокумент15 страницAssociation of Chief Police Officers New Psychoactive Substances Guidance WebsiteJames BarlowОценок пока нет
- Bristol City Council Polling Stations (2010)Документ13 страницBristol City Council Polling Stations (2010)James BarlowОценок пока нет
- Analytics WWW - Bristol.gov - Uk Public 20060501-20100401 (Death of Dial-Up)Документ1 страницаAnalytics WWW - Bristol.gov - Uk Public 20060501-20100401 (Death of Dial-Up)James BarlowОценок пока нет
- Khat Use Among Somalis in Four English Cities - UK Home Office ReportДокумент65 страницKhat Use Among Somalis in Four English Cities - UK Home Office ReportJames BarlowОценок пока нет
- Bristol City Council - Election GuidelinesДокумент8 страницBristol City Council - Election GuidelinesJames BarlowОценок пока нет
- Bristol City Council - Electoral Arrangement 2010 - 2013Документ1 страницаBristol City Council - Electoral Arrangement 2010 - 2013James BarlowОценок пока нет
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (894)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- General Characters of MicroorganismsДокумент3 страницыGeneral Characters of MicroorganismssadafОценок пока нет
- TS051 Project Structural Analysis ResultsДокумент6 страницTS051 Project Structural Analysis ResultsFabiano PimentelОценок пока нет
- Aoac 972.44 PDFДокумент2 страницыAoac 972.44 PDFPablo Emilio Martinez GarciaОценок пока нет
- Acetic Acid Bacteria Prospective Applications in Food BiotechnologyДокумент16 страницAcetic Acid Bacteria Prospective Applications in Food BiotechnologyMarcelina Mendoza SalazarОценок пока нет
- ZFN, Talens, Crispr PDFДокумент9 страницZFN, Talens, Crispr PDFjezelle lividОценок пока нет
- The Human Genome ProjectДокумент22 страницыThe Human Genome ProjectAashianaThiyam100% (1)
- 3rd GenerationДокумент3 страницы3rd GenerationlorrainebarandonОценок пока нет
- Levine H-Challenges in The CGMP Manufacturing of Hescs-Lessons Learned From Monoclonal Antibodies-RprintДокумент24 страницыLevine H-Challenges in The CGMP Manufacturing of Hescs-Lessons Learned From Monoclonal Antibodies-RprintChilliG35Оценок пока нет
- Bigdye Terminator Protocol v1.1Документ74 страницыBigdye Terminator Protocol v1.1Citrawati Dyah Kencono WunguОценок пока нет
- CourseInfo Booklet PDFДокумент130 страницCourseInfo Booklet PDFtanmay mandalОценок пока нет
- EU Pharma Draft2Документ1 страницаEU Pharma Draft2মোঃ এমদাদুল হকОценок пока нет
- Revised Vacant Seats For Ph.D. Admission 2017-18-27!7!17Документ48 страницRevised Vacant Seats For Ph.D. Admission 2017-18-27!7!17vitthalmech8687Оценок пока нет
- Transcription in ProkaryotesДокумент16 страницTranscription in ProkaryotesAditya Kanwal100% (1)
- Golden Rice Genetic PresentationДокумент23 страницыGolden Rice Genetic PresentationPeter FadoulОценок пока нет
- Healthcare DatabaseДокумент1 400 страницHealthcare DatabaseAatish Mohite33% (3)
- Upda Revis: Ted EdДокумент34 страницыUpda Revis: Ted EdmrthumbОценок пока нет
- Essentials of Genetics 8th Edition by Klug Cummings Spencer and Palladino ISBN Test BankДокумент14 страницEssentials of Genetics 8th Edition by Klug Cummings Spencer and Palladino ISBN Test Banklester100% (23)
- Innovation Is Great WorksheetsДокумент2 страницыInnovation Is Great WorksheetsAngel Angeleri-priftis.Оценок пока нет
- Bio in For MaticsДокумент17 страницBio in For MaticsSayan MandalОценок пока нет
- Euthenics 2 Quiz 5Документ14 страницEuthenics 2 Quiz 5Yoo Jung100% (1)
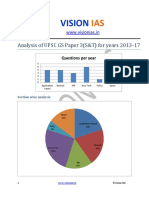
- Analysis of Upsc Gs Paper 3 Science and Tech Without ApproachДокумент2 страницыAnalysis of Upsc Gs Paper 3 Science and Tech Without ApproachJames KnotОценок пока нет
- Yoshida 2016 Science Ideonella Sakaiensis Grows On PETДокумент5 страницYoshida 2016 Science Ideonella Sakaiensis Grows On PETAnonymous 0zrCNQОценок пока нет
- Langton Et Al-2010-International Journal of Cosmetic ScienceДокумент10 страницLangton Et Al-2010-International Journal of Cosmetic SciencegenzizuОценок пока нет
- MAN0010408 QuantStudioDesign Analysis Desktop Software UGДокумент106 страницMAN0010408 QuantStudioDesign Analysis Desktop Software UGSairam06Оценок пока нет
- Antibiotic Sensitivity Test GuideДокумент3 страницыAntibiotic Sensitivity Test GuideRajeshОценок пока нет
- Chapter 6.3 TranslationДокумент9 страницChapter 6.3 TranslationMatthew ShieldsОценок пока нет
- A. Identitas DiriДокумент5 страницA. Identitas DirinellieauthorОценок пока нет
- Punnet QuizДокумент2 страницыPunnet Quizapi-351157612Оценок пока нет
- Resumes For Industry Scientist Job ApplicationsДокумент19 страницResumes For Industry Scientist Job ApplicationsJeannette CraigОценок пока нет
- Complete Listing (Sorted by Title)Документ86 страницComplete Listing (Sorted by Title)mildeithbidaОценок пока нет