Академический Документы
Профессиональный Документы
Культура Документы
Calculation of Dew Point of Flue Gas 4x135 MW Jindal, Angul
Загружено:
Ashitava Sen0 оценок0% нашли этот документ полезным (0 голосов)
168 просмотров2 страницыThe document contains two tables that analyze the combustion products and flue gas composition from burning coal to generate electricity. Table 1 shows the molar amounts of combustion products produced per 100 kg of air-dried coal. Table 2 lists the molar percentages of components in the flue gas, including 12.02% water vapor. The dew point is calculated as 49.74°C, which is the temperature at which the water vapor partial pressure equals the saturation pressure based on the flue gas composition and pressure.
Исходное описание:
Acid Dew point
Оригинальное название
Acid Dewpoint Calculation
Авторское право
© © All Rights Reserved
Доступные форматы
XLS, PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документThe document contains two tables that analyze the combustion products and flue gas composition from burning coal to generate electricity. Table 1 shows the molar amounts of combustion products produced per 100 kg of air-dried coal. Table 2 lists the molar percentages of components in the flue gas, including 12.02% water vapor. The dew point is calculated as 49.74°C, which is the temperature at which the water vapor partial pressure equals the saturation pressure based on the flue gas composition and pressure.
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате XLS, PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
168 просмотров2 страницыCalculation of Dew Point of Flue Gas 4x135 MW Jindal, Angul
Загружено:
Ashitava SenThe document contains two tables that analyze the combustion products and flue gas composition from burning coal to generate electricity. Table 1 shows the molar amounts of combustion products produced per 100 kg of air-dried coal. Table 2 lists the molar percentages of components in the flue gas, including 12.02% water vapor. The dew point is calculated as 49.74°C, which is the temperature at which the water vapor partial pressure equals the saturation pressure based on the flue gas composition and pressure.
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате XLS, PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 2
Page 1 of 2
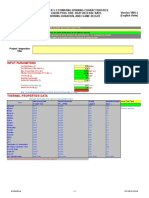
Calculation of Dew Point of flue gas
4x135 MW Jindal, Angul
Table 1: Combustion product analysis
kmol products/100 kg air-dried coal
Elements kg/100 Kg of Mol Wt kmol/100 kg kmol O2
air-dried coal of air-dried theoretically
coal required per 100
kg air-dried coal
CO2 H2O SO2 N2 O2 Total
C 24 12 2.00 2.00 2.00 - - - - 2.00
H 2.3 2 1.15 0.575 - 1.15 - - - 1.15
O 6.5 32 0.20 -0.20 - - - - - -
N 0.7 28 0.03 - - - - 0.03 - 0.03
S 0.5 32 0.02 0.02 - - 0.016 - - 0.02
Moisture 12 18 0.67 - - 0.67 - - - 0.67
Ash 54 - - - - - - - -
Total 100 2.39 2.000 1.817 0.016 0.025 0.000 3.857
Excess O2 at 20% of the theoretical (2.39 x 0.2 = 0.478) - - - - 0.478 0.478
N2 from total air supplied (Theoretical O2 x 3.76 x excess air factor = 2.39 x 3.76 x 1.2 = 10.784) - - - 10.784 - 10.784
2.000 1.817 0.016 10.809 0.478 15.119
Note : Coal analysis for Bakreshwar with maximum moisture content considered
Reference : Fuels and Combustion by Samir Sarkar
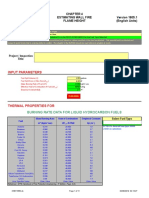
Page 2 of 2
Table 2 : Flue gas analysis
Components kmol Vol per
cent as
produced
CO2 2.000 13.23
SO2 0.016 0.10
O2 0.478 3.16
N2 10.809 71.49
H2O 1.817 12.02
Total 15.119 100.00
Dew Point
By definition, the dew point of a gaseous mixture is the temperature at which its water vapour
starts condensing. It is therefore the temperature at which the saturation pressure for water
equals the partial pressure of water vapour in the mixture.
From the wet flue gas analysis, the content of water vapour is 12.02%.
The partial pressure of water vapour (assume gas pressure is 760 mm Hg) = 12.02/100 x 760
= 91.352 mm Hg
From steam table, the corresponding saturation temperature is 49.74C
This is the required dew point
Вам также может понравиться
- 2-CSWIP Practical Plate ExamplesДокумент31 страница2-CSWIP Practical Plate Examplesvibinkumars@gmail.com100% (42)
- HEI DeaeratorsДокумент24 страницыHEI Deaeratorsrajendrakagwade100% (3)
- Minimize Evaporation Losses by Calculating Boiloff Gas in LPG Storage TanksДокумент5 страницMinimize Evaporation Losses by Calculating Boiloff Gas in LPG Storage TanksgopalvivekОценок пока нет
- The Boudouard Reaction: C + CO2 2 CO: Thermodynamic Calculations Kj/mole T (C) T (K) LN (KR) KR KR KR Xco2 Xco GRДокумент6 страницThe Boudouard Reaction: C + CO2 2 CO: Thermodynamic Calculations Kj/mole T (C) T (K) LN (KR) KR KR KR Xco2 Xco GRmksayshiОценок пока нет
- Pipe Support Span CalculationДокумент1 страницаPipe Support Span CalculationAshitava SenОценок пока нет
- Closed Drain PaperДокумент4 страницыClosed Drain PaperBehnam Hosseinzadeh100% (1)
- A1.3 Rel UpgradingДокумент14 страницA1.3 Rel UpgradingGeppo RossiОценок пока нет
- 1055 Crude Stailization Systems-SperoidsДокумент0 страниц1055 Crude Stailization Systems-SperoidsgshdavidОценок пока нет
- Offer124033 Bom R0Документ2 страницыOffer124033 Bom R0t_syamprasad100% (1)
- MTG ProcessДокумент59 страницMTG ProcessShehzad Afzal MaharОценок пока нет
- Aln Group Clearance Form - v4 - Seadweller CorpДокумент4 страницыAln Group Clearance Form - v4 - Seadweller CorpJomar FrogosoОценок пока нет
- State-Of-The-Art Nitrogen Rejection TechnologyДокумент16 страницState-Of-The-Art Nitrogen Rejection Technologynabeel khanОценок пока нет
- Employee Final Settlement - Maria SabadoДокумент1 страницаEmployee Final Settlement - Maria SabadoZeeshan MirzaОценок пока нет
- Liquid Air Energy Storage: Pumped Hydro Capability No Geographical ConstraintsДокумент15 страницLiquid Air Energy Storage: Pumped Hydro Capability No Geographical ConstraintsAzrul Ikhwan AzharОценок пока нет
- Acid Dew PointДокумент3 страницыAcid Dew PointapminshullОценок пока нет
- HHV and LHVДокумент2 страницыHHV and LHVYuji OhkusuОценок пока нет
- Flare Gas Recovery Data Sheet PDFДокумент1 страницаFlare Gas Recovery Data Sheet PDFMohamed AdelОценок пока нет
- Cosa 9610 Wobbe Analyzer Users ManualДокумент44 страницыCosa 9610 Wobbe Analyzer Users ManualEnrique De Haro CortesОценок пока нет
- VBE - Aug2008 Pressure Loss CalculatorДокумент9 страницVBE - Aug2008 Pressure Loss Calculatorakhilendraa4074Оценок пока нет
- Applitek - Detector in Vinyl Process PDFДокумент24 страницыApplitek - Detector in Vinyl Process PDFwiboonwiОценок пока нет
- Slopo Compact and Safe Slop Oil Treatment Units WebДокумент6 страницSlopo Compact and Safe Slop Oil Treatment Units Webdomingos soaresОценок пока нет
- B 126063001 PaДокумент10 страницB 126063001 Pazizu1234Оценок пока нет
- Assignment 2 Process and Dynamic System Modelling (PPSD)Документ15 страницAssignment 2 Process and Dynamic System Modelling (PPSD)Husaini ZaidanОценок пока нет
- Coke On Catalyst CalculationДокумент10 страницCoke On Catalyst CalculationSuryaprakash DigavalliОценок пока нет
- CH 10 Process IdentificationДокумент31 страницаCH 10 Process IdentificationMohammad YounesОценок пока нет
- Auto Thermal Reactor:: Secondary ReformerДокумент6 страницAuto Thermal Reactor:: Secondary ReformerAtif MehfoozОценок пока нет
- Drier Bed SizingДокумент14 страницDrier Bed SizingvkumaranОценок пока нет
- Typical Process / Facility Water Balance CalculationДокумент5 страницTypical Process / Facility Water Balance CalculationYesi CeballosОценок пока нет
- Hyoffwind Power To Gas End ReportДокумент68 страницHyoffwind Power To Gas End Reportapi-267204600Оценок пока нет
- PRPC - All CH - by Kenil JaganiДокумент102 страницыPRPC - All CH - by Kenil JaganiVarun pandeyОценок пока нет
- Sodium Bicarbonate For Flue Gas TreatmentДокумент12 страницSodium Bicarbonate For Flue Gas Treatmentblabla21Оценок пока нет
- HYSYS-Report Ammonia PlantДокумент21 страницаHYSYS-Report Ammonia PlantDouglas Ross HannyОценок пока нет
- LPG Energy IntegrationДокумент6 страницLPG Energy IntegrationBandaru KiranОценок пока нет
- Automated Operation of Topsoe Steam Reformers LTMДокумент8 страницAutomated Operation of Topsoe Steam Reformers LTMrajuОценок пока нет
- Distillation Column ReboilerДокумент13 страницDistillation Column ReboilerLouie GresulaОценок пока нет
- 03 HRR Flame Height Burning Duration Calculations Sup1Документ5 страниц03 HRR Flame Height Burning Duration Calculations Sup1Haris AbdulahОценок пока нет
- Biopproducts From Syngas: 1. Executive Summary/ConclusionsДокумент98 страницBiopproducts From Syngas: 1. Executive Summary/ConclusionsGonzalo TitoОценок пока нет
- PFDДокумент1 страницаPFDDenny FirmansyahОценок пока нет
- Fundamentals of Power PlantsДокумент48 страницFundamentals of Power Plantsknx175100% (1)
- Brochure Movialsa Gasification Plant EnglishДокумент6 страницBrochure Movialsa Gasification Plant EnglishSiwat Kiokaew100% (1)
- Ammonia Equvments DrawingДокумент43 страницыAmmonia Equvments DrawingSaad KhanОценок пока нет
- Ibp1502 12Документ9 страницIbp1502 12Marcelo Varejão CasarinОценок пока нет
- Random Packing Article PDFДокумент88 страницRandom Packing Article PDFAbizer Jamali100% (1)
- Coal Gasifier ProcessesДокумент28 страницCoal Gasifier ProcessesH Janardan PrabhuОценок пока нет
- PSV Releiving TempДокумент3 страницыPSV Releiving TempOthman Mat YamanОценок пока нет
- Dewatering Column AA Grade MethanolДокумент15 страницDewatering Column AA Grade MethanolIrma BrennanОценок пока нет
- Rigorous Method For Fire CaseДокумент4 страницыRigorous Method For Fire CaseFlorin Daniel AnghelОценок пока нет
- New Gas Fired Power Plant at Ressano Garcia - CER - Calculation - v5Документ11 страницNew Gas Fired Power Plant at Ressano Garcia - CER - Calculation - v5rym romdhanОценок пока нет
- Gas (Amine) Sweetening Process - Jonell Filtration Applications GasSweet 1015Документ2 страницыGas (Amine) Sweetening Process - Jonell Filtration Applications GasSweet 1015Bob PeppingОценок пока нет
- Revised Process Datasheet For Deaerator Ma-1018 - Rev 001 - SignedДокумент10 страницRevised Process Datasheet For Deaerator Ma-1018 - Rev 001 - SignedAnonymous bHh1L1Оценок пока нет
- Comtaminents in Amine Gas Treating UnitДокумент13 страницComtaminents in Amine Gas Treating Unitarvindgupta_2005Оценок пока нет
- NIR Implementataion For Fuels Blending DEPДокумент34 страницыNIR Implementataion For Fuels Blending DEPKumarОценок пока нет
- MetoxidoДокумент8 страницMetoxidocessavelinoОценок пока нет
- Mass Balance 3Документ22 страницыMass Balance 3barbadosiyОценок пока нет
- Basics of Steam System DesignДокумент5 страницBasics of Steam System Designarun89000100% (1)
- Wake Freequency CalculationДокумент5 страницWake Freequency CalculationManoranjan Kumar ChoudharyОценок пока нет
- 04 Flame Height Calculations Sup1Документ11 страниц04 Flame Height Calculations Sup1Adam MaulanaОценок пока нет
- Ammonia Synthesis Material Balence CalulДокумент1 страницаAmmonia Synthesis Material Balence CalulDhruv RanaОценок пока нет
- Naimat Phase-4 Glycol Dehydration Unit VesselsДокумент1 страницаNaimat Phase-4 Glycol Dehydration Unit VesselsTahir Amin (Descon)Оценок пока нет
- Combustion of Fossil FuelsДокумент5 страницCombustion of Fossil FuelsRahul ChandrawarОценок пока нет
- Fuel Analysis CalculationДокумент2 страницыFuel Analysis Calculationmohamed ElsayedОценок пока нет
- Garbage IncineratorДокумент59 страницGarbage IncineratorgsdaundhОценок пока нет
- 20V12 Wissbaum Getting The Most Out of Your SRU Performance Test PDFДокумент25 страниц20V12 Wissbaum Getting The Most Out of Your SRU Performance Test PDFRiyesh KpОценок пока нет
- Metro ViewerДокумент5 страницMetro ViewerSteve WanОценок пока нет
- EC - YadadriДокумент10 страницEC - YadadriAshitava SenОценок пока нет
- CW Pipe Thickness Calculation - 80% Vacuum - With RCCДокумент39 страницCW Pipe Thickness Calculation - 80% Vacuum - With RCCAshitava Sen0% (1)
- Fsa PSJ 701 06 PDFДокумент9 страницFsa PSJ 701 06 PDFAshitava SenОценок пока нет
- Expansion Joint - Engineering GuideДокумент50 страницExpansion Joint - Engineering GuideAshitava SenОценок пока нет
- List of Material Used For FlangesДокумент1 страницаList of Material Used For FlangesAshitava SenОценок пока нет
- Iso 12944Документ15 страницIso 12944Ashitava Sen90% (10)
- Power Test Code-Centrifugals PTC10Документ3 страницыPower Test Code-Centrifugals PTC10Ashitava SenОценок пока нет
- HEI Heater Part01 Closed FWHДокумент36 страницHEI Heater Part01 Closed FWHAshitava Sen100% (2)
- HEI CondenserДокумент53 страницыHEI CondenserAshitava Sen100% (1)
- Maaping With 3D DataДокумент5 страницMaaping With 3D Datanasir.hdip8468Оценок пока нет
- MANDA-TOWNHOUSE - Technical SpecificationsДокумент10 страницMANDA-TOWNHOUSE - Technical SpecificationsMark Nathan Sta. MonicaОценок пока нет
- BERKLEY - Catalogo Caà As 2011Документ8 страницBERKLEY - Catalogo Caà As 2011dondepescasОценок пока нет
- Advantages of ForgingДокумент20 страницAdvantages of ForgingPramod DhaigudeОценок пока нет
- CH 3 Chemical Reaction WorksheetДокумент19 страницCH 3 Chemical Reaction WorksheetStephanus AbednegoОценок пока нет
- Paints and Varnishes - General Tests Methods - Vol 1.1 - IndexДокумент5 страницPaints and Varnishes - General Tests Methods - Vol 1.1 - IndexGilberto ManhattanОценок пока нет
- Chapter 3Документ25 страницChapter 3Abel JacobОценок пока нет
- Actvated Sludge Process SSWMДокумент28 страницActvated Sludge Process SSWMGREENLAB ECL TECHОценок пока нет
- Failure Analysis Question BankДокумент29 страницFailure Analysis Question BankAbd-Elaleem Abdallah SosaОценок пока нет
- Scraper Wiper LimpiadorДокумент100 страницScraper Wiper LimpiadorRPINILLA (EICO S.A.)Оценок пока нет
- Cross Ref 1Документ7 страницCross Ref 1Devendra KhadeОценок пока нет
- Bumper Systems - An IntroductionДокумент25 страницBumper Systems - An IntroductionMichaelОценок пока нет
- Chemicals Used & Modes of Actions of DisinfectantsДокумент25 страницChemicals Used & Modes of Actions of DisinfectantsjayОценок пока нет
- Uhde Dual-Pressure Process For Large-Scale Ammonia Plants: - Saskferco Ammonia-Urea Complex, CanadaДокумент6 страницUhde Dual-Pressure Process For Large-Scale Ammonia Plants: - Saskferco Ammonia-Urea Complex, CanadaMUHAMMAD USMAN0% (1)
- Exam Steel DesignДокумент2 страницыExam Steel DesignAndrew PortugalОценок пока нет
- Buckling of Spherical Shells Subjected To External PressureДокумент7 страницBuckling of Spherical Shells Subjected To External PressureSUBHASH100% (1)
- Spent Bleaching Earth Sbe The Hidden Treasure From Waste of The Palm Oil Refinery PlantДокумент6 страницSpent Bleaching Earth Sbe The Hidden Treasure From Waste of The Palm Oil Refinery PlantAgustina TriyaniОценок пока нет
- Anna Sheryl F. Dimacali-Le-Science-5-Q1-Wk-3-5Документ6 страницAnna Sheryl F. Dimacali-Le-Science-5-Q1-Wk-3-5Anna Sheryl DimacaliОценок пока нет
- Alcohols, Phenols and Ethers - MCQs Test - 3Документ3 страницыAlcohols, Phenols and Ethers - MCQs Test - 3Prasant KumarОценок пока нет
- Unit Test-II (SOM) 1Документ2 страницыUnit Test-II (SOM) 1hasanОценок пока нет
- Rubio Monocoat Furniture DataДокумент1 страницаRubio Monocoat Furniture DataJosh PiersmaОценок пока нет
- Guia de Aditivos para Resolucao de ProblemasДокумент20 страницGuia de Aditivos para Resolucao de ProblemasFabiano DesangiacomoОценок пока нет
- Cascade Data Sheet PDFДокумент28 страницCascade Data Sheet PDFvisutsiОценок пока нет
- LAKBAY A Proposed PNR StationДокумент5 страницLAKBAY A Proposed PNR StationJohn Paul RamosОценок пока нет
- Typical Roadway SectionДокумент1 страницаTypical Roadway SectionCarmela Ayessa PiguerraОценок пока нет
- Coating Thickness GaugesДокумент5 страницCoating Thickness GaugesSheikh Muhammad AsifОценок пока нет
- Sulfate AttackДокумент1 страницаSulfate AttackbozarromegustaОценок пока нет