Академический Документы
Профессиональный Документы
Культура Документы
MM 311
Загружено:
Shavneel ChandОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
MM 311
Загружено:
Shavneel ChandАвторское право:
Доступные форматы
MM 311 Applied Thermo Fluid
The University of the South Pacific
School of Engineering and Physics
MM 311 Applied Thermo Fluids
Lab 1: Performance Study of a Vapor Compression Refrigerant System
Lab Session Time: Wednesday (2-5pm)
Student Name: Shavneel chand
Student ID#: S11108836
AIM
To calculate and compare the values of heat lost by air and heat gained by the refrigerant as by theory this
has to be equal.
EQUIPMENT
Vapor compression refrigerant system unit.
INTRODUCTION
In 1805, the American inventor Oliver Evans described a closed vapor-compression refrigeration cycle for
the production of ice by ether under vacuum. Heat would be removed from the environment by recycling
vaporized refrigerant, where it would move through a compressor and condenser and would eventually
revert to a liquid form in order to repeat the refrigeration process over again. However, no such refrigeration
unit was built by Evans [1]. In 1834, an American expatriate to Great Britain, Jacob Perkins, built the first
working vapor-compression refrigeration system in the world as it was a closed-cycle that could operate
continuously [2].
The vapor-compression uses a circulating liquid refrigerant as the medium which absorbs and removes heat
from the space to be cooled and subsequently rejects that heat elsewhere. Figure 1 depicts a typical, single-
stage vapor-compression system. All such systems have four components: a compressor, a condenser, a
thermal expansion valve also called a throttle valve, and an evaporator.
Figure 1. Typical single stage vapor compression refrigeration system [3]
Lab Report 1 Page 1
MM 311 Applied Thermo Fluid
THEORY
The Circulating refrigerant enters the compressor in the thermodynamic state known as a saturated vapor
and is compressed to a higher pressure, resulting in a higher temperature as well. The hot, compressed vapor
is then in the thermodynamic state known as a superheated vapor and it is at a temperature and pressure at
which it can be condensed with cooling air. That hot vapor is routed through a condenser where it is cooled
and condensed into a liquid by flowing through a coil or tubes with cool air flowing across the coil or tubes.
This is where the circulating refrigerant rejects heat from the system and the rejected heat is carried away by
the air. The condensed liquid refrigerant, in the thermodynamic state known as a saturated liquid, is next
routed through an expansion valve where it undergoes an abrupt reduction in pressure. That pressure
reduction results in the adiabatic flash evaporation of a part of the liquid refrigerant. The auto-refrigeration
effect of the adiabatic flash evaporation lowers the temperature of the liquid and vapor refrigerant mixture to
where it is colder than the temperature of the enclosed space to be refrigerated. The cold mixture is then
routed through the coil or tubes in the evaporator. A fan circulates the warm air in the enclosed space across
the coil or tubes carrying the cold refrigerant liquid and vapor mixture. That warm air evaporates the liquid
part of the cold refrigerant mixture. At the same time, the circulating air is cooled and thus lowers the
temperature of the enclosed space to the desired temperature. The evaporator is where the circulating
refrigerant absorbs and removes heat which is subsequently rejected in the condenser and transferred
elsewhere by the air used in the condenser.
To complete the refrigeration cycle, the refrigerant vapor from the evaporator is again a saturated vapor and
is routed back into the compressor [4].
Figure 2 schematic of a vapour compression refrigerant system with TS and PH graph. [5]
As shown in the figure 1 the standard single stage, vapour compression refrigeration system consists of the
following four processes:
Process 1-2: Isentropic compression of saturated vapour in compressor
Process 2-3: Isobaric heat rejection in condenser
Process 3-4: Isenthalpic expansion of saturated liquid in expansion device
Process 4-1: Isobaric heat extraction in the evaporator
Applying steady flow energy equation to each of the process the following equations were obtained:
Evaporator: Heat transfer rate at evaporator or refrigeration capacity is given by:
Lab Report 1 Page 2
MM 311 Applied Thermo Fluid
Compressor: Power input to the compressor is given by:
Condenser: Heat transfer rate at condenser is given by:
Expansion device: For the isenthalpic expansion across the expansion device could be considerable,
however, if we take the control volume, well downstream of the expansion device, then the kinetic energy
gets dissipated due to viscous effects is given by:
EXPERIMENTAL SETUP AND PROCEDURE
The vapour compression refrigerant system was set up in the lab which used Arcton 12 refrigerant gas in the
system. The input feed of H was set to 70mm of water which was then used to take the following readings
from the vapour compression refrigerant system:
Pressure 1 = 1.2 bar
Pressure 2 = 9 bar
Temperature 1 = 27.5c
Temperature in = 33c
Temperature out = 27c
r =46 g/s
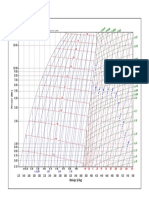
The following results were then taken and used in the Arcton 12 pressure Enthalpy diagram to locate point
3 as it lies on the saturated liquid line at pressure of 9 bars and point 4 was located using point 3 which was
dropped vertically down to meet with the pressure of 1.2 bars. The constant temperature line was then used
to locate point 1 which intersected with 1.2 bar pressure line and following the constant entropy lone which
intersected with 9 bar pressure point 2 was located. Moving on the enthalpy at each point was found where
h1 was found to be 270 kj/kg and similarly h2 was located to be 315 kj/kg and finally h3 and h4 was found to
be 138 kj/kg. The following enthalpy was then used to calculate for the following variables such as heat lost
by the refrigerant, work required by compressor, coefficient of performance.
RESULTS AND SAMPLE CALCULATION
H = 70 mm
Pressure 1 = 1.2 bar
Pressure 2 = 9 bar
Temperature 1 = 27.5c 300.5k
Temperature in = 33c 306k
Temperature out = 27c 300k
r =46 g/s 0.046kg/s
Lab Report 1 Page 3
MM 311 Applied Thermo Fluid
Values found from P-h graph:
h1= 270 KJ/Kg
h2= 315 KJ/Kg
h3= 138 KJ/Kg
h4= 138 KJ/Kg
COP of the VCR:
1 4 270138
= = 2.93
2 1 315270
Rate Of Work Done By The Compressor:
= (2 1 )
= (0.046kg/s) (315-270)
= 2.07 KJ/s
Rate Of Heat Gain By The Refrigerant:
= (1 4 )
= (0.046kg/s) (270-138)
= 6.072 KJ/s
Rate of Heat lost by the Air:
1. Find volume flow rate of air:
= 0.0247()
= 0.0247x (70)
=0.2066 mm3/s 2.07x10-10m3/s
2. Find the mass flow rate of air:
=
1
(101.3)(0.2066)
=
(287300.5)
= 0.243 Kg/s
3. Rate of Heat lost by the air:
= ( )
= 0.243 X 1005 X (306-300)
= 1.46 KJ/s
DISCUSSION
The results that was obtained from the experiment and which was used to do the analysis of the equations, it
was found that the heat lost by the air is not equal to the heat gained by the refrigerant, as stated by the
theory this values has to be equal but its not in practical. The aim of this experiment was to compare the
two values and there were slight difference between the heats lost by the air which came up to be 1.46 kj/kg
and the heat gained by the refrigerant came up to be 6.072kj/kg.
Lab Report 1 Page 4
MM 311 Applied Thermo Fluid
The reason for such difference in the results could be due to the body heat that can be absorbed by the
system as it was of metal while doing experiment. The difference in results also could be due to setup in the
lab exposed to atmospheric wind blowing hence change in the results of heat gained by refrigerant. The
difference would also arise if there were some of some misinterpretation of fine details in graph which might
have been approximated to the nearest value hence the values were so close to each other in the diagram this
could be reason to that calculating the results gave such difference in our experiments.
CONCLUSION
To conclude from this experiment, it can be said that the heat lost by air and heat gained by the refrigerant
should be equal but it is not due to the errors present as stated in the discussion. The major error that would
have caused such a difference could have been the interpretation of graph while taking the enthalpy reading
which may have differed from exact values.
REFERENCE
[1] Colin Hempstead and William E. Worthington (Editors) (2005). Encyclopedia of 20th-Century
Technology, Volume 2. Taylor& Francis. ISBN 1-57958-464-0.
[2] Robert T. Balmer (2011). Modern Engineering Thermodynamic. Academic Press. ISBN 978-0-12-
374996-3.
[3] Wikipedia. (2016). Vapor-compression refrigeration. [Online] Available at:
https://en.wikipedia.org/wiki/Vapor-compression_refrigeration#/media/File:Refrigeration.png [Accessed 15
Mar. 2016].
[4] Vapour compression refrigerant system. (2016). 1st ed. [eBook] Kharagpur: ME, IIT, pp.14-18.
Available at: http://nptel.ac.in/courses/112105129/pdf/RAC%20Lecture%2010.pdf [Accessed 15 Mar.
2016].
[5] Google.com. (2016). schematic of a vapour compression refrigeration system - Google Search. [Online]
Available at:
https://www.google.com/search?q=schematic+of+a+vapour+compression+refrigeration+system&biw=1366
&bih=657&source=lnms&tbm=isch&sa=X&ved=0ahUKEwj4opisxMLLAhUOx2MKHckKDokQ_AUIBig
B#imgrc=dN3tPTveUfryxM%3A [Accessed 15 Mar. 2016].
Lab Report 1 Page 5
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- Quiz 1Документ2 страницыQuiz 1jgd2080Оценок пока нет
- Dry-Wet Bulb TempДокумент4 страницыDry-Wet Bulb Tempmanpreetsodhi08Оценок пока нет
- Thermodynamics Type 1Документ24 страницыThermodynamics Type 1Balagovind BaluОценок пока нет
- ErP Information for AR12HSSFAWKN / AR12HSSFAWKX Air ConditionerДокумент1 страницаErP Information for AR12HSSFAWKN / AR12HSSFAWKX Air ConditionerpfsfosnecaОценок пока нет
- Equation Sheet: Boyang Qin Date Created: Jan 2013Документ3 страницыEquation Sheet: Boyang Qin Date Created: Jan 2013Boyang QinОценок пока нет
- Thermodynamic Properties Of: Dupont FluorochemicalsДокумент30 страницThermodynamic Properties Of: Dupont FluorochemicalstomkarazijaОценок пока нет
- Tutorial 3 ThermoДокумент27 страницTutorial 3 ThermoHaiqal AzizОценок пока нет
- Psychrometrics 20111212 PDFДокумент58 страницPsychrometrics 20111212 PDFjoeeoj189189Оценок пока нет
- ThermoДокумент3 страницыThermopranavОценок пока нет
- TE - Mech - RAC - Chapter 4 - VCC Cycles-1Документ98 страницTE - Mech - RAC - Chapter 4 - VCC Cycles-1Aniket MandalОценок пока нет
- HVAC Systems & Air Side EconimizersДокумент59 страницHVAC Systems & Air Side EconimizersDhirendra Singh RathoreОценок пока нет
- Determination of Changes in Internal Energy and Enthalpy:: Thermodynamics Session - 7Документ5 страницDetermination of Changes in Internal Energy and Enthalpy:: Thermodynamics Session - 7mukesh3021Оценок пока нет
- Refrigrant - R407AДокумент2 страницыRefrigrant - R407ASaor PakpahanОценок пока нет
- Psychrometrics - Theory and ExamplesДокумент45 страницPsychrometrics - Theory and ExamplesGatot NugrohoОценок пока нет
- Condensation CalculationДокумент40 страницCondensation Calculationbinhjuki100% (2)
- Dtu, Department of Energy Engineering S in (KJ/ (KG K) ) - V in (M 3/Kg) - T in (ºc) M.J. Skovrup & H.J.H Knudsen. 19-11-29 Ref:Peng-Robinson-Stryjek-Vera Equation and Dupont Suva Ac9000Документ1 страницаDtu, Department of Energy Engineering S in (KJ/ (KG K) ) - V in (M 3/Kg) - T in (ºc) M.J. Skovrup & H.J.H Knudsen. 19-11-29 Ref:Peng-Robinson-Stryjek-Vera Equation and Dupont Suva Ac9000raul correaОценок пока нет
- Isobaric VLE of Chloroform 2-Propanol With Margules EquationДокумент4 страницыIsobaric VLE of Chloroform 2-Propanol With Margules EquationMarcel ChevalierОценок пока нет
- Engineering Thermodynamics - Department of Mechanical EngineeringДокумент5 страницEngineering Thermodynamics - Department of Mechanical EngineeringKarthik P MuraliОценок пока нет
- 5refrigerant 134a Enters An Adiabatic Compressor As A Saturated Vapor at 120 KPa at A Rate of 0Документ3 страницы5refrigerant 134a Enters An Adiabatic Compressor As A Saturated Vapor at 120 KPa at A Rate of 0BryanChavezОценок пока нет
- R600a PXH ASHДокумент1 страницаR600a PXH ASHGiovani Renato ZontaОценок пока нет
- Thermodynamic Property RelationsДокумент26 страницThermodynamic Property RelationsSec CОценок пока нет
- Steam TablesДокумент12 страницSteam Tablesgrumpyfecker1988Оценок пока нет
- R-22 Enthalpy ChartДокумент16 страницR-22 Enthalpy ChartSuhail RanaОценок пока нет
- Addis Ababa UniversityДокумент2 страницыAddis Ababa Universitydeni ebit nugroho100% (1)
- Mollier Hs Diagram 500 A3 Free PDFДокумент1 страницаMollier Hs Diagram 500 A3 Free PDFWanko01 BaeОценок пока нет
- Basic LawsДокумент13 страницBasic LawsjubahewashereОценок пока нет
- Gas Laws and Thermodynamics ConceptsДокумент3 страницыGas Laws and Thermodynamics Conceptsmangiafzal100% (1)
- Thermowell Calculation GuideДокумент19 страницThermowell Calculation Guideprasanthk22Оценок пока нет
- Year-5-Place-Value End-Of-Block-AssessmentДокумент2 страницыYear-5-Place-Value End-Of-Block-Assessmentapi-368429342100% (1)
- 4-CE Thermodynamics Properties of FluidsДокумент70 страниц4-CE Thermodynamics Properties of FluidsApple EmiratessОценок пока нет