Академический Документы
Профессиональный Документы
Культура Документы
Inflammation and Neuroprotection in Traumatic Brain Injury: Clinical Implications of Basic Neuroscience Research
Загружено:
Carlos GutierrezОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Inflammation and Neuroprotection in Traumatic Brain Injury: Clinical Implications of Basic Neuroscience Research
Загружено:
Carlos GutierrezАвторское право:
Доступные форматы
Clinical Review & Education
Clinical Implications of Basic Neuroscience Research
Inflammation and Neuroprotection in Traumatic Brain Injury
Kara N. Corps, DVM, DACVP; Theodore L. Roth, MS; Dorian B. McGavern, PhD
Video at jamaneurology.com
IMPORTANCE Traumatic brain injury (TBI) is a significant public health concern that affects
individuals in all demographics. With increasing interest in the medical and public
communities, understanding the inflammatory mechanisms that drive the pathologic and
consequent cognitive outcomes can inform future research and clinical decisions for patients
with TBI.
OBJECTIVES To review known inflammatory mechanisms in TBI and to highlight clinical trials
and neuroprotective therapeutic manipulations of pathologic and inflammatory mechanisms
of TBI.
EVIDENCE REVIEW We searched articles in PubMed published between 1960 and August 1,
2014, using the following keywords: traumatic brain injury, sterile injury, inflammation,
astrocytes, microglia, monocytes, macrophages, neutrophils, T cells, reactive oxygen species,
alarmins, danger-associated molecular patterns, purinergic receptors, neuroprotection, and
clinical trials. Previous clinical trials or therapeutic studies that involved manipulation of the
discussed mechanisms were considered for inclusion. The final list of selected studies was
assembled based on novelty and direct relevance to the primary focus of this review.
FINDINGS Traumatic brain injury is a diverse group of sterile injuries induced by primary and
secondary mechanisms that give rise to cell death, inflammation, and neurologic dysfunction
in patients of all demographics. Pathogenesis is driven by complex, interacting mechanisms
that include reactive oxygen species, ion channel and gap junction signaling, purinergic
receptor signaling, excitotoxic neurotransmitter signaling, perturbations in calcium
homeostasis, and damage-associated molecular pattern molecules, among others. Central
nervous system resident and peripherally derived inflammatory cells respond to TBI and can
provide neuroprotection or participate in maladaptive secondary injury reactions. The exact
contribution of inflammatory cells to a TBI lesion is dictated by their anatomical positioning as
well as the local cues to which they are exposed.
CONCLUSIONS AND RELEVANCE The mechanisms that drive TBI lesion development as well as
those that promote repair are exceedingly complex and often superimposed. Because
pathogenic mechanisms can diversify over time or even differ based on the injury type, it is
important that neuroprotective therapeutics be developed and administered with these
variables in mind. Due to its complexity, TBI has proven particularly challenging to treat;
however, a number of promising therapeutic approaches are now under pre-clinical
development, and recent clinical trials have even yielded a few successes. Given the
worldwide impact of TBI on the human population, it is imperative that research remains
active in this area and that we continue to develop therapeutics to improve outcome in
afflicted patients. Author Affiliations: Viral
Immunology and Intravital Imaging
Section, National Institutes of
Neurological Disorders and Stroke,
National Institutes of Health,
Bethesda, Maryland.
Corresponding Author: Dorian B.
McGavern, PhD, Viral Immunology
and Intravital Imaging Section,
National Institutes of Neurological
Disorders and Stroke, National
Institutes of Health, 10 Center Dr,
Bethesda, MD 20892 (mcgavernd
@mail.nih.gov).
JAMA Neurol. 2015;72(3):355-362. doi:10.1001/jamaneurol.2014.3558 Section Editor: Hassan M.
Published online January 19, 2015. Fathallah-Shaykh, MD, PhD.
(Reprinted) 355
Copyright 2015 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a NCSU Hunt Library User on 05/14/2015
Clinical Review & Education Clinical Implications of Basic Neuroscience Research Traumatic Brain Injury
T
raumatic brain injury (TBI) is a diverse group of brain inju- populations. Sterile immune reactions are at least initially designed
ries that vary in cause, severity, pathogenesis, and clinical to be beneficial but can become detrimental in certain situations.
outcome. As public awareness of TBI and its conse-
quences increases, there is a growing need to understand the un- Danger Signals
derlying mechanisms and develop therapeutic interventions. Within Pathogens can trigger innate immune activation via pathogen-
the United States alone, nearly 2 million people sustain a TBI annu- associated molecular pattern molecules, which are conserved struc-
ally, contributing to one-third of all injury-related deaths. Individu- tures within a class of microbes recognized by Toll-like receptors or
als from all nations and demographics are affected, including ath- pathogen-recognition receptors. These innate signaling pathways
letes, military troops, and individuals with unintentional injuries.1-3 allow plants and animals to respond quickly to invading microbes.
Traumatic brain injury is a significant cause of mortality in children However, it is now recognized that tissue damage in the absence of
and young adults, and the incidence in older individuals has in- microbial infection can trigger inflammasome and innate immune
creased with the average life span.4 Mild TBI (mTBI) is the most fre- activation through the release of damage-associated molecular pat-
quent type diagnosed, typically resulting in post-TBI survival. Trau- tern molecules (DAMPs), sometimes referred to as danger signals.12
matic brain injury is suspected to contribute to a variety of chronic Alarmins are endogenous DAMPs released by cells undergoing no-
degenerative processes, including chronic traumatic encephalopa- napoptotic death or by cells of the immune system.13 Some ex-
thy, Alzheimer disease, and Parkinson disease.5 Traumatic brain in- amples of alarmins include HMGB1, S-100 proteins, adenosine tri-
jury is initiated by the application of mechanical force to the head, phosphate (ATP), uric acid, DNA or RNA, and interleukin 1, among
which can occur with or without loss of consciousness. This then trig- others. After TBI, alarmins are undoubtedly released,14 and this trig-
gers a series of cerebral events that depend in part on the nature gers a sterile immune reaction designed to restore tissue homeo-
and location of the injury. A major challenge associated with treat- stasis. However, the severity and duration of injury can foster mal-
ing patients with TBI is the diverse pathologic and pathogenic mecha- adaptive immune reactions that become injurious. A previous study15
nisms that become operational after injuries. For example, TBI of- found that ATP release and detection via puringeric receptors elicit
ten promotes disruption of blood-brain barrier (BBB) integrity and an acutely neuroprotective inflammatory response after mild cor-
the neurovascular unit, which can result in vascular leakage, edema, tical injury, but sustained immune activation may not always be ben-
hemorrhage, and hypoxia. Other pathologic mechanisms include cell eficial. For example, therapeutic blockade of inflammasome activa-
death within the meninges and brain parenchyma, stretching and tion reduced innate immune activation and severe TBI lesion size.16
tearing of axonal fibers, and disruptions at the junctions between Thus, additional research is required to better understand the rules
white and gray matter, stemming from rotational forces that cause that govern pathogenic vs nonpathogenic innate immune reac-
shearing injuries.6 All these primary pathologic mechanisms are ac- tions after DAMP signaling in the injured brain.
companied by cellular and molecular cascades leading to inflamma-
tion and additional cell death. This review focuses on our current un- Purinergic Receptor Signaling
derstanding of the sterile immune reaction to TBI and some clinical Purinergic receptors are an evolutionarily ancient family of trans-
successes in treating patients with TBI. membrane molecules that detect ATP, adenosine diphosphate (ADP),
We searched articles in PubMed published between 1960 or adenosine.10,11 The receptors are divided into 2 basic classes based
and August 1, 2014, using the following keywords: traumatic brain on whether they respond to adenosine (P1 receptors) or ATP or ADP
injury, sterile injury, inflammation, astrocytes, microglia, mono- (P2 receptors). Because ATP is a cellular source of energy, it is main-
cytes, macrophages, neutrophils, T cells, reactive oxygen species, tained at a high intracellular concentration during steady-state con-
alarmins, danger-associated molecular patterns, purinergic recep- ditions. After tissue injury, ATP is released from damaged cells, which
tors, neuroprotection, and clinical trials. Clinical trials or therapeu- triggers an immune reaction via purinergic receptor signaling. This
tic studies that involved manipulation of the discussed mecha- reaction can be amplified by pannexin and connexin hemichannels
nisms were considered for inclusion. The final reference list was that pump ATP from healthy cells into the extracellular space. Ster-
assembled based on novelty and direct relevance to the primary ile immune reactions generally subside as ATP is converted into aden-
focus of this review. osine through a 2-step reaction that involves ectonucleoside tri-
phosphate diphosphohydrolase 1 (CD39) and ecto-5-nucleotidase
(CD73). Astrocytes and microglia each express at least one these
ectoenzymes,17,18 allowing them to dampen ATP-mediated neuro-
Sterile Immune Reaction to TBI
inflammation.
Central nervous system (CNS) resident and peripherally derived in-
flammatory cells respond quickly to brain injuries and can even par- Microglia
ticipate in the repair process.7,8 These responses are commonly re- Microglia are highly dynamic CNS resident innate immune senti-
ferred to as sterile immune reactions. A previous study9 found that nels that originate from primitive myeloid progenitor cells during
the inflammatory gene expression profile is comparable between development.19,20 Microglia participate in a variety of homeostatic
mTBI and severe TBI, suggesting a common response to both forms CNS functions, including synaptic plasticity and learning,21 and are
of injury. The acute cellular reaction to TBI includes astrocytes, mi- often the first responders to any inflammatory event that occurs
croglia, monocytes or macrophages, neutrophils, and T cells, which within the parenchyma.20 Microglia mediate neuron removal dur-
are initially activated in part by purinergic receptor signaling.10,11 In ing development via release of reactive oxygen species (ROS) and
the following paragraphs, we describe the inflammatory response can acquire a phagocytic phenotype without an inflammatory
to TBI in more detail, focusing specifically on traditional immune cell response.20 Microglial expression of genes associated with neuro-
356 JAMA Neurology March 2015 Volume 72, Number 3 (Reprinted) jamaneurology.com
Copyright 2015 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a NCSU Hunt Library User on 05/14/2015
Traumatic Brain Injury Clinical Implications of Basic Neuroscience Research Clinical Review & Education
protection is upregulated with age.22 Microglia express a large ing from the blood do not reach peak numbers in the damaged brain
number of surface and cytoplasmic receptors, and cumulative sig- of animals and humans until 24 to 48 hours after injury.32,33 Mono-
naling through these receptors determines whether microglia cytes are capable of crossing the bloodcerebrospinal fluid barrier
remain in a ramified, sentinel state or take on various configura- with neutrophils into the injured brain as a result of CCL2 produc-
tions as a result of activation.23 In addition, microglia can sense a tion by choroid plexus epithelium.34 CCL2 is significantly increased
large repertoire of exogenous and endogenous signals, allowing in the cerebrospinal fluid of patients with TBI.33 Examination of
for dynamic responses to sterile injuries and infectious agents CCL2/ mice after TBI revealed slight alterations in cytokine expres-
that can be injurious or neuroprotective, depending on the sion but no changes in lesion size within the first week of injury.33
context.22 However, when followed for a longer timeframe of 2 to 4 weeks,
During CNS autoimmune disease, activated microglia phago- CCL2/ mice had improved functional recovery, suggesting a patho-
cytose debris and downregulate cellular metabolism in contrast to genic role for macrophages during the chronic phase of TBI. Similar
disease-initiating, peripherally derived macrophages, which ap- results were obtained in CCR2/ mice after TBI.35 CCR2 is the re-
pear to play a more destructive role by promoting demyelination.24 ceptor for CCL2, and deficiency significantly reduced the number
These data suggest that microglia are not inherently neurotoxic dur- of lesion macrophages and increased hippocampal neuronal den-
ing development of a CNS autoimmune disease. After acute focal sities, spatial learning, and locomotion when measured several
brain injury in rodents, microglia similarly appear to play a neuro- weeks after brain injury. Collectively, the data obtained in CCL2
protective role.15 Using 2-photon microscopy, we revealed that mi- and CCR2 knockout mice suggest that monocyte-derived macro-
croglia respond within minutes of brain injury by extending pro- phages play a pathogenic role in the chronic phase after TBI.
cesses to the glial limitans and circumscribing individual astrocytes, Additional studies are required to determine whether these cells
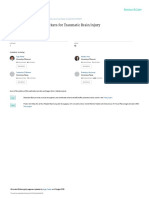
resembling a hexagonal honeycomb structure (Figure 1A-E, Figure 2E can participate in brain repair after TBI similar to what has been
and H, and Videos 1 and 2). This reaction was dependent on purin- described in models of spinal cord injury.36 Whether a macro-
ergic receptor signaling (P2X4 and P2Y12) and astrocytic ATP- phage is pathogenic or beneficial after tissue injury likely depends
dependent ATP release via connexin hemichannels. In response to on its state of differentiation.
cell death (eg, astrocytic cell death in the glial limitans), microglia
transformed into phagocytic cells that resembled jellyfish Neutrophils
(Figure 1A-E, Figure 2F and I, and Videos 1 and 2). Jellyfish microg- Neutrophils are an abundant population of circulating leukocytes that
lia were highly mobile and often inserted themselves into the dam- are usually among the first responders to tissue injuries in the pe-
aged glial limitans in place of dead astrocytes, connecting together riphery and CNS.37 Neutrophils are often viewed as a proinflamma-
to form a phagocytic barrier. This reaction was also dependent on tory cell population but are known to play a vital role in wound heal-
purinergic receptor signaling (P2X4, P2Y6, P2Y12) and connexin hemi- ing through their involvement in phagocytosis, metalloproteinase
channels. When these microglia responses were inhibited locally release, and growth factor production. After tissue injury, neutro-
through blockade of purinergic receptor signaling or connexin hemi- phils can help prepare the damaged environment for repair. Neu-
channels, the pathologic mechanisms observed after brain injury trophils are rapidly recruited to the CNS after TBI and enter through
were more severe. One of the most notable changes was increased meningeal blood vessels and the choroid plexus.15,32,38,39 They can
leakage of materials through the glial limitans into the brain paren- also facilitate the recruitment of monocytes.37 A previous study40
chyma. These data suggest that microglia not only clean up debris focused on sterile injury of the liver found that ATP released from
from the injured brain but also help maintain glial limitans barrier in- the damaged tissue induced inflammasome activation in a P2X7R-
tegrity by sealing the gaps that result from dead or damaged astro- dependent manner. This activation in turn promoted rapid
cytes. Moreover, our data are consistent with previous studies25-28 recruitment of neutrophils through release of chemoattractants
that link microglia injury responses to ATP release and purinergic re- (CXCL1 and CXCL2) and formyl peptides that guided these cells to
ceptor signaling. Although it is conceivable that microglia re- the site of injury. After focal TBI, we observed that neutrophils are
sponses become maladaptive over time or after exposure to differ- similarly recruited in a P2X 7 R-dependent manner and arrive
ent combinations of stimuli,29 we propose that the acute role of within 1 hour of injury (Figure 2C). 15 Visualization of cellular
microglia in the focally injured brain is neuroprotective. dynamics and localization by 2-photon microscopy revealed that
neutrophils localized primarily to the damaged meninges (instead
Monocytes and Macrophages of the parenchyma), where they swarmed the area and interacted
Monocytes are a multipotent population of circulating bone marrow with dead cells. Antagonism of this response by blocking P2X7R
derived leukocytes capable of differentiating into macrophages or signaling increased the amount of cell death in the meninges, sug-
dendritic cells after invasion of an infected or injured tissue.30 They gesting a protective role for neutrophils in the meningeal space
are also known to participate in diverse functions, such as phago- after focal cortical injury.
cytosis, cytokine or chemokine release, antigen presentation, im- Neutrophils are not always neuroprotective and have the ca-
mune modulation, and tissue repair. In the naive brain, there are also pacity to break down the BBB by releasing metalloproteinases, pro-
populations of specialized macrophages that reside in the menin- teases, tumor necrosis factor , and ROS. Inflammatory mediators
ges, choroid plexus, and perivascular spaces.31 Their role in TBI patho- released after brain injury can facilitate this process by inducing a
genesis is unknown. Another study15 also found that meningeal mac- hyperactivated state that allows neutrophils to breach the BBB and
rophages are among the first cells to die after focal cortical injury and enter the CNS.41 On arrival, neutrophils have the potential to in-
may serve as an early source of alarmins and ROS (Figure 1A-C, duce neuronal cell death using the same soluble mediators that break
Figure 2A and B, and Video 1). Monocyte-derived macrophages com- down the BBB.42 A previous study43 revealed that neutrophils are
jamaneurology.com (Reprinted) JAMA Neurology March 2015 Volume 72, Number 3 357
Copyright 2015 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a NCSU Hunt Library User on 05/14/2015
Clinical Review & Education Clinical Implications of Basic Neuroscience Research Traumatic Brain Injury
Figure 1. Pathogenesis of Traumatic Brain Injury (TBI)
ATP UDP
A ROS Glutamate
Normal mTBI
Skull
A, Comparison of brain anatomy in
the meninges and superficial
neocortex before and after focal mild
Meningeal
macrophage TBI (mTBI). The dura mater contains
Dura mater numerous small vessels that are lined
Arachnoid mater
Blood vessels by thin, elongated meningeal
CSF macrophages. The subarachnoid
Subarachnoid Fluid leakage,
space space contains vessels, fibroblastlike
ROS meningeal
Pia mater cell death Neutrophils stromal cells, and cerebrospinal fluid
(CSF). The glial limitans, composed of
astrocytic foot processes, lies
beneath the pia mater and forms a
UDP barrier between the CSF and
Glial limitans Astrocyte ATP underlying parenchyma. Mild focal
Microglia Jellyfish microglia brain injury mechanically compresses
Microglial process
extension to the glial limitans the meningeal space, compromising
vascular integrity and inducing rapid
Glutamate
necrosis of meningeal macrophages
NMDA and structural cells. Leakage of fluid
Cortical from meningeal blood vessels results
neuron Ca2+ Necrotic
neuron in edema, and damaged cells within
the meninges release reactive oxygen
species (ROS) and adenosine
triphosphate (ATP), initiating a sterile
immune reaction. B and C, Maximum
projections (5-m wide) are shown in
the xz plane of 2-photon z-stacks
captured through the thinned skull of
CX3CR1GFP/+ mice (original
magnification 20).
B, A representative image of an
Meningeal macrophages uninjured mouse reveals the
B C presence of meningeal macrophages
Skull bone (green) in the dura and ramified
microglia (green) in the brain
Meninges parenchyma beneath the glial
limitans (white dotted line).
Microglia
C, Thirty minutes after focal mTBI,
Microglia
meningeal macrophages die and
20 m microglia relocate to the injured glial
Parenchyma
limitans (arrowheads). Skull bone is
shown in blue. D and E,
Parenchymal
Histopathologic analysis of the
D E cell death
superficial neocortex by confocal
microscopy 8 hours after mTBI
(original magnification 20). D, An
uninjured brain is shown for
comparison. Dead cells were labeled
transcranially with propidium iodide.
Cell nuclei are blue. E, A large lesion
250 m consisting of numerous dead cells
(red) (arrowhead). See Videos 1 and
2. UPD indicates uridine diphosphate.
the most abundant cell population in circulation after TBI and cause tical impact is highly disruptive to meningeal architecture and likely
increased expression of oxidative enzymes indicative of activation. favors neutrophil recruitment to the heavily damaged brain paren-
Depletion of neutrophils with antiGr-1 antibodies after controlled chyma. These findings contrast with mild closed-skull cortical in-
cortical impact in rodents reduced edema, microglia and macro- jury, which maintains meningeal architecture and fosters a more se-
phage activation, and TBI lesion size, but did not affect vascular leak- lective pattern of neutrophil recruitment.15 To definitively establish
age at 24 to 48 hours after injury.44 These data reveal that neutro- the contribution of neutrophils to TBI pathogenesis, these cells
phils can be pathogenic after open-skull cortical impact. However, should be evaluated in many different models of brain injury. It is con-
the contribution of neutrophils to a CNS lesion may depend on their ceivable that their contribution will differ based on the nature of the
precise localization and state of activation. Open-skull controlled cor- injury.
358 JAMA Neurology March 2015 Volume 72, Number 3 (Reprinted) jamaneurology.com
Copyright 2015 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a NCSU Hunt Library User on 05/14/2015
Traumatic Brain Injury Clinical Implications of Basic Neuroscience Research Clinical Review & Education
Figure 2. Inflammatory Reaction to Traumatic Brain Injury
Meningeal Macrophages
Normal mTBI Myelomonocytic Cells
A B C
A-I, The 25-m xy maximum
projections from CX3CR1GFP/+ (A, B,
and D-I) or LysMGFP/+ (C) mice were
captured by 2-photon microscopy
through a thinned skull. A, Meningeal
macrophages (green) are thin,
50 m 50 m elongated cells that reside along the
dural blood vessels in the uninjured
Normal Ramified Microglia Honeycomb Microglia Phagocytic Jellyfish Microglia
brain. B, After focal mild traumatic
D E F
brain injury (mTBI), meningeal
macrophages undergo necrosis
within 30 minutes and disappear
from the field of view.
C, Myelomonocytic cells (green)
invade the damaged meninges within
an hour of brain injury. D and G, In the
uninjured brain, microglia (green)
have small cell bodies and are highly
ramified. Focal brain injury induces
the rapid transformation of microglia
50 m into at least 2 distinct morphologic
patterns. E and H, Honeycomb
G H I microglia extend processes that
circumscribe the borders between
individual astrocytes in the glial
limitans. F and I, Phagocytic jellyfish
microglia are generated in response
to cell death and form a film across
the damaged glial limitans.
High-magnification views in panels G
through I are denoted with white
boxes in panels D through F (original
magnification 20). See Videos 1
20 m and 2.
T Cells The many reasons for these failures have been discussed in other
Although T cells play diverse roles in adaptive immune responses reviews.49,50 Rather than focus on the reasons for prior failures, we
and the regulation of inflammation, their role (if any) in TBI patho- instead briefly discuss some successes that pertain to mechanisms
genesis is not clear. It has been proposed that autoreactive T cells of pathogenesis and inflammation covered in this review.
against CNS antigens, such as myelin basic protein, can be neuro- The concept of free radicalmediated damage of CNS tissue af-
protective after spinal cord injury.45 After brain injury, activated T ter injury has existed for several decades.51,52 Administration of ef-
cells are recruited to sites of damage,46 and ROS release may facili- fective antioxidants has the potential to significantly limit the spread
tate this recruitment by activating endothelial barriers.47 To ad- of damage and inflammation if given soon after brain injury. In ani-
dress the role of T cells in TBI, a previous study48 examined the re- mal models, a number of previous studies53,54 have yielded prom-
sponse to closed-skull head injury in RAG1 knockout mice that lack ising results with antioxidants that neutralize ROS. For example, in-
mature T and B cells. No difference in any pathologic or neurologic travenous administration of the small-molecule free radical scavenger
parameters was observed between wild-type and RAG1-deficient edaravone at 2 and 12 hours after weight dropinduced TBI re-
mice for up to 1 week. These data suggest that T cells play no role in sulted in significantly reduced inflammation, edema, BBB break-
early TBI pathogenesis. Additional studies are required to deter- down, lesion size, and neurologic deficits.53 Inhibition of NADPH oxi-
mine whether T cells actively participate in chronic TBI lesions (be- dase complex assembly with apocynin also reduced ROS production,
yond 1 week) and/or the reparative process. BBB breakdown, and neuronal cell death after weight drop
induced TBI.54 The only caveat of this study was that the apocynin
was injected intraperitoneally 15 minutes before injury. Neverthe-
less, the favorable outcome implicates NADPH oxidase as a poten-
Therapeutic Modulation of TBI Pathogenesis
tial source of ROS after brain injury.
The pathogenesis of TBI is complex as reflected by the number of Using a new model of mild cortical injury, we found that trans-
clinical trials that have failed to improve outcomes in humans.49,50 cranial administration of the antioxidant glutathione at 15 minutes
jamaneurology.com (Reprinted) JAMA Neurology March 2015 Volume 72, Number 3 359
Copyright 2015 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a NCSU Hunt Library User on 05/14/2015
Clinical Review & Education Clinical Implications of Basic Neuroscience Research Traumatic Brain Injury
or 3 hours after injury significantly reduced inflammation, glial limi- creased meningeal cell death after mTBI, likely due to diminished
tans breakdown, and parenchymal (but not meningeal) cell death by neutrophil recruitment. Thus, purinergic receptor modulation can
up to approximately 70%.15 Pretreatment with glutathione reduced positively affect one CNS environment and negatively affect an-
meningeal cell death by approximately 50%. These data indicate that other. It will therefore be important in future studies to map out the
ROS are a primary inducer of cell death and inflammation after focal exact contributions of specific purinergic receptors to different TBI
brain injury and that an antioxidant can have a major effect on lesion lesion parameters before deciding which (if any) are best to target
expansion if given early. The advantage of passing a neuroprotective therapeutically in patients.
compounddirectlythroughtheskullbone(transcranialdelivery)isthat
a high local drug concentration can be achieved in the CNS with a lim-
ited off-target effect on the periphery.
Discussion
Previous studies55,56 have supported antioxidants as neuro-
protective agents in rats and humans, revealing that administra- The pathogenesis of TBI is initially induced by a mechanical injury
tion of N-acetylcysteine reduces brain damage and improves recov- that sets into motion a complex secondary reaction mediated by
ery after TBI. N-acetylcysteine is the cellular precursor to glutathione. ROS, purines, calcium ions, excitatory amino acids, and DAMPs,
A randomized, double-blind, placebo-controlled clinical trial55 was among others. This pathogenesis in turn triggers a robust sterile im-
performed to assess efficacy in members of the military who expe- mune reaction that consists of CNS resident and peripherally re-
rienced a mTBI that resulted from blast exposure. Patients who re- cruited inflammatory cells. The response is designed to be neuro-
ceived N-acetylcysteine within 24 hours had significantly im- protective and promote wound healing but can become maladaptive
proved recovery during a 7-day period when compared with a over time, especially if the lesion remains active for weeks. Among
placebo control group. These findings were corroborated in 2 dif- the earliest soluble mediators are ROS and purines. Both are re-
ferent rodent models of TBI (weight drop and fluid percussion), leased within minutes of brain injury and initiate an inflammatory
which revealed that N-acetylcysteine reversed the behavioral defi- cascade. Even after mild focal cortical injury, ROS can damage the
cits associated with mTBI and moderate TBI.56 Further studies are glial limitans that separate the meninges and parenchyma, which re-
needed to determine whether this promising neuroprotective in- sults in lesion expansion within brain tissue. Vascular damage and
tervention will be efficacious in patients with diverse types of brain leakage represent another early hallmark of TBI pathogenesis that
injury. can foster edema, hypoxia, and tissue destruction. After brain in-
Many clinical trials have been completed or are under way to jury, the innate immune system quickly mobilizes in response to pu-
assess the role of excitotoxic mechanisms in TBI pathogenesis.49,50 rines and alarmins, and astrocytes help orchestrate this response by
With the exception of amantadine, all drugs in this class tested to serving as inflammatory amplifiers. Within minutes, resident mi-
date have not been effective in promoting recovery in patients with croglia are among the first to react by fortifying CNS barriers and par-
TBI. Amantadine is thought to act as an N-methyl-D-aspartate re- ticipating in phagocytic cleanup. Neutrophils and monocytes ar-
ceptor antagonist and an indirect dopamine agonist. When pa- rive shortly thereafter and preferentially survey injured meningeal
tients with TBI were treated during a 4-week period beginning 4 to spaces if the CNS architecture remains intact. Focal brain injury elic-
16 weeks after injury, amantadine improved recovery relative to the its an anatomically partitioned immune reaction (at least acutely)
placebo control. The mechanism underlying this positive effect re- with myelomonocytic cells tending to the damaged meninges and
mains unclear. Prevention of N-methyl- D -aspartate receptor microglia responding within the parenchyma. Eventually, myelo-
mediated excitatory damage seems unlikely given that the drug was monocytic cells can enter the damaged brain, and studies40-42 have
administered a month or more after the initial injury.57 found that their presence there is sometimes neurotoxic. How-
Manipulation of purinergic receptor signaling is another thera- ever, sterile immune reactions are not inherently neurotoxic and are
peutic approach worth considering. Use of specific purinergic re- usually elicited to prepare a damaged tissue for wound healing. Thus,
ceptor agonists and antagonists should allow therapeutic amelio- the entire contribution of immune cell subsets to TBI lesions needs
ration of different TBI lesion parameters. A previous study15 found to be considered before targeted therapeutic interventions can
that microglia responses after mTBI were dependent on P2X4, P2Y6, be intelligently designed. Another important variable is time. The
and P2Y12 receptors, whereas P2X7R signaling was necessary for neu- exact contribution of immune cells to a TBI lesion may in fact shift
trophil recruitment. It might be possible to promote neuroprotec- over time. For example, an initially neuroprotective immune
tive inflammatory responses through therapeutic agonism of these response may become maladaptive as secondary inducers of tis-
pathways after brain injury. The challenge, however, with puriner- sue destruction diversify.
gic receptor manipulation is that specific receptors are often ex- Although TBI has proven difficult to treat, promising interven-
pressed on multiple cell populations. A purinergic receptor agonist tions lie on the horizon. Given the importance of ROS in TBI patho-
or antagonist will likely affect multiple cell populations simultane- genesis and the success with N-acetylcysteine in patients with mTBI,
ously. As an example, a previous58 study found that P2X7R local- clinical pursuit of antioxidant therapy seems warranted. The likely
ized to astrocytic end feet and antagonism of this receptor re- key to success is early treatment with antioxidants so that TBI le-
duced astrocyte activation, cerebral edema, and neurobehavioral sion expansion and subsequent inflammation can be stopped as soon
abnormalities after controlled cortical impactinduced TBI. A simi- as they are initiated. Because TBI lesions begin to expand within
lar protective effect was obtained by blocking P2X7R after spinal cord hours of injury, development of strategies to rapidly preserve brain
injury, which was linked to receptor expression on spinal cord tissue is paramount. The kinetics of lesion expansion must be simi-
neurons.59 However, P2X7R is also expressed on inflammatory cells, larly considered when attempting to manipulate purinergic and ex-
and a previous study15 found that antagonism of this pathway in- citatory neurotransmitter pathways, which engage rapidly after in-
360 JAMA Neurology March 2015 Volume 72, Number 3 (Reprinted) jamaneurology.com
Copyright 2015 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a NCSU Hunt Library User on 05/14/2015
Traumatic Brain Injury Clinical Implications of Basic Neuroscience Research Clinical Review & Education
jury. Therapeutic targeting of these pathways has the greatest
likelihood of working if administered soon after injury. For the chronic Conclusions
phase of TBI pathogenesis, more research is required to under-
stand lesion dynamics. Over time, it may become necessary to Traumatic brain injury encompasses a complex spectrum of inju-
dampen maladaptive inflammatory responses and attempt to pro- ries that tax the neural-immune interface and can result in perma-
mote wound healing reactions, which would be challenging to nent neurologic dysfunction. Detailed knowledge of this interface
achieve without having a better understanding of chronic lesion during the acute and chronic phases of TBI will help us design the
dynamics. most efficacious interventions.
ARTICLE INFORMATION 7. Das M, Mohapatra S, Mohapatra SS. New 22. Hickman SE, Kingery ND, Ohsumi TK, et al. The
Accepted for Publication: October 2, 2014. perspectives on central and peripheral immune microglial sensome revealed by direct RNA
responses to acute traumatic brain injury. sequencing. Nat Neurosci. 2013;16(12):1896-1905.
Published Online: January 19, 2015. J Neuroinflammation. 2012;9:236.
doi:10.1001/jamaneurol.2014.3558. 23. Hu X, Liou AK, Leak RK, et al. Neurobiology of
8. Finnie JW. Neuroinflammation: beneficial and microglial action in CNS injuries: receptor-mediated
Author Contributions: Dr McGavern had full access detrimental effects after traumatic brain injury. signaling mechanisms and functional roles. Prog
to all the data in the study and takes responsibility Inflammopharmacology. 2013;21(4):309-320. Neurobiol. 2014;119-120(Jun):60-84.
for the integrity of the data and the accuracy of the
data analysis. 9. Lagraoui M, Latoche JR, Cartwright NG, 24. Yamasaki R, Lu H, Butovsky O, et al. Differential
Study concept and design: Corps, McGavern. Sukumar G, Dalgard CL, Schaefer BC. Controlled roles of microglia and monocytes in the inflamed
Acquisition, analysis, or interpretation of data: All cortical impact and craniotomy induce strikingly central nervous system. J Exp Med. 2014;211(8):
authors. similar profiles of inflammatory gene expression, 1533-1549.
Drafting of the manuscript: All authors. but with distinct kinetics. Front Neurol. 2012;3:155. 25. Nimmerjahn A, Kirchhoff F, Helmchen F.
Critical revision of the manuscript for important 10. Junger WG. Immune cell regulation by Resting microglial cells are highly dynamic
intellectual content: All authors. autocrine purinergic signalling. Nat Rev Immunol. surveillants of brain parenchyma in vivo. Science.
Obtained funding: McGavern. 2011;11(3):201-212. 2005;308(5726):1314-1318.
Administrative, technical, or material support: 11. Eltzschig HK, Sitkovsky MV, Robson SC. 26. Davalos D, Grutzendler J, Yang G, et al. ATP
McGavern. Purinergic signaling during inflammation. N Engl J mediates rapid microglial response to local brain
Study supervision: McGavern. Med. 2012;367(24):2322-2333. injury in vivo. Nat Neurosci. 2005;8(6):752-758.
Conflict of Interest Disclosures: None reported. 12. Matzinger P. Tolerance, danger, and the 27. Haynes SE, Hollopeter G, Yang G, et al. The
Funding/Support: This work was supported by the extended family. Annu Rev Immunol. 1994;12:991- P2Y12 receptor regulates microglial activation by
National Institutes of Health intramural program. 1045. extracellular nucleotides. Nat Neurosci. 2006;9(12):
Role of the Funder/Sponsor: The funding source 13. Bianchi ME. DAMPs, PAMPs and alarmins: all we 1512-1519.
had no role in the design and conduct of the study; need to know about danger. J Leukoc Biol. 2007;81 28. Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K,
collection, management, analysis, and (1):1-5. et al. UDP acting at P2Y6 receptors is a mediator of
interpretation of the data; preparation, review, or 14. Manson J, Thiemermann C, Brohi K. Trauma microglial phagocytosis. Nature. 2007;446(7139):
approval of the manuscript; and the decision to alarmins as activators of damage-induced 1091-1095.
submit the manuscript for publication. inflammation. Br J Surg. 2012;99(suppl 1):12-20. 29. Zhang J, Malik A, Choi HB, Ko RW,
Additional Contributions: Ethan Tyler, MA, and 15. Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Dissing-Olesen L, MacVicar BA. Microglial CR3
Alan Hoofring, MS, Medical Arts Design Section, Latour LL, McGavern DB. Transcranial amelioration activation triggers long-term synaptic depression in
National Institutes of Health, helped with the of inflammation and cell death after brain injury. the hippocampus via NADPH oxidase. Neuron.
illustration shown in Figure 1. Messrs Tyler and Nature. 2014;505(7482):223-228. 2014;82(1):195-207.
Hoofring are salaried employees of the National 30. Shi C, Pamer EG. Monocyte recruitment during
Institutes of Health and were not directly 16. de Rivero Vaccari JP, Lotocki G, Alonso OF,
Bramlett HM, Dietrich WD, Keane RW. Therapeutic infection and inflammation. Nat Rev Immunol.
compensated by our laboratory for their work. 2011;11(11):762-774.
neutralization of the NLRP1 inflammasome reduces
REFERENCES the innate immune response and improves 31. Nayak D, Zinselmeyer BH, Corps KN, McGavern
histopathology after traumatic brain injury. J Cereb DB. In vivo dynamics of innate immune sentinels in
1. Feigin VL, Theadom A, Barker-Collo S, et al; Blood Flow Metab. 2009;29(7):1251-1261. the CNS. Intravital. 2012;1(2):95-106.
BIONIC Study Group. Incidence of traumatic brain
injury in New Zealand: a population-based study. 17. Niemel J, Ifergan I, Yegutkin GG, Jalkanen S, 32. Soares HD, Hicks RR, Smith D, McIntosh TK.
Lancet Neurol. 2013;12(1):53-64. Prat A, Airas L. IFN-beta regulates CD73 and Inflammatory leukocytic recruitment and diffuse
adenosine expression at the blood-brain barrier. Eur neuronal degeneration are separate pathological
2. Jordan BD. The clinical spectrum of sport-related J Immunol. 2008;38(10):2718-2726. processes resulting from traumatic brain injury.
traumatic brain injury. Nat Rev Neurol. 2013;9(4): J Neurosci. 1995;15(12):8223-8233.
222-230. 18. Braun N, Svigny J, Robson SC, et al.
Assignment of ecto-nucleoside triphosphate 33. Semple BD, Bye N, Rancan M, Ziebell JM,
3. Logan BW, Goldman S, Zola M, Mackey A. diphosphohydrolase-1/cd39 expression to microglia Morganti-Kossmann MC. Role of CCL2 (MCP-1) in
Concussive brain injury in the military: September and vasculature of the brain. Eur J Neurosci. 2000; traumatic brain injury (TBI): evidence from severe
2001 to the present. Behav Sci Law. 2013;31(6): 12(12):4357-4366. TBI patients and CCL2-/- mice. J Cereb Blood Flow
803-813. Metab. 2010;30(4):769-782.
19. Ginhoux F, Greter M, Leboeuf M, et al. Fate
4. Roozenbeek B, Maas AI, Menon DK. Changing mapping analysis reveals that adult microglia derive 34. Szmydynger-Chodobska J, Strazielle N, Gandy
patterns in the epidemiology of traumatic brain from primitive macrophages. Science. 2010;330 JR, et al. Posttraumatic invasion of monocytes
injury. Nat Rev Neurol. 2013;9(4):231-236. (6005):841-845. across the blood-cerebrospinal fluid barrier. J Cereb
5. Smith DH, Johnson VE, Stewart W. Chronic 20. Nayak D, Roth TL, McGavern DB. Microglia Blood Flow Metab. 2012;32(1):93-104.
neuropathologies of single and repetitive TBI: development and function. Annu Rev Immunol. 35. Hsieh CL, Niemi EC, Wang SH, et al. CCR2
substrates of dementia? Nat Rev Neurol. 2013;9(4): 2014;32:367-402. deficiency impairs macrophage infiltration and
211-221. improves cognitive function after traumatic brain
21. Parkhurst CN, Yang G, Ninan I, et al. Microglia
6. Blennow K, Hardy J, Zetterberg H. The promote learning-dependent synapse formation injury. J Neurotrauma. 2014;31(20):1677-1688.
neuropathology and neurobiology of traumatic through brain-derived neurotrophic factor. Cell. 36. Shechter R, London A, Varol C, et al. Infiltrating
brain injury. Neuron. 2012;76(5):886-899. 2013;155(7):1596-1609. blood-derived macrophages are vital cells playing
jamaneurology.com (Reprinted) JAMA Neurology March 2015 Volume 72, Number 3 361
Copyright 2015 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a NCSU Hunt Library User on 05/14/2015
Clinical Review & Education Clinical Implications of Basic Neuroscience Research Traumatic Brain Injury
an anti-inflammatory role in recovery from spinal 44. Kenne E, Erlandsson A, Lindbom L, Hillered L, 53. Wang GH, Jiang ZL, Li YC, et al. Free-radical
cord injury in mice. PLoS Med. 2009;6(7):e1000113. Clausen F. Neutrophil depletion reduces edema scavenger edaravone treatment confers
37. Kolaczkowska E, Kubes P. Neutrophil formation and tissue loss following traumatic brain neuroprotection against traumatic brain injury in
recruitment and function in health and injury in mice. J Neuroinflammation. 2012;9:17. rats. J Neurotrauma. 2011;28(10):2123-2134.
inflammation. Nat Rev Immunol. 2013;13(3):159-175. 45. Cohen IR, Schwartz M. Autoimmune 54. Choi BY, Jang BG, Kim JH, et al. Prevention of
38. Carlos TM, Clark RS, Franicola-Higgins D, maintenance and neuroprotection of the central traumatic brain injury-induced neuronal death by
Schiding JK, Kochanek PM. Expression of nervous system. J Neuroimmunol. 1999;100(1-2): inhibition of NADPH oxidase activation. Brain Res.
endothelial adhesion molecules and recruitment of 111-114. 2012;1481:49-58.
neutrophils after traumatic brain injury in rats. 46. Ling C, Sandor M, Suresh M, Fabry Z. Traumatic 55. Hoffer ME, Balaban C, Slade MD, Tsao JW,
J Leukoc Biol. 1997;61(3):279-285. injury and the presence of antigen differentially Hoffer B. Amelioration of acute sequelae of blast
39. Szmydynger-Chodobska J, Strazielle N, Zink BJ, contribute to T-cell recruitment in the CNS. J Neurosci. induced mild traumatic brain injury by N-acetyl
Ghersi-Egea JF, Chodobski A. The role of the 2006;26(3):731-741. cysteine: a double-blind, placebo controlled study.
choroid plexus in neutrophil invasion after 47. Clausen F, Lorant T, Lewn A, Hillered L. T PLoS One. 2013;8(1):e54163.
traumatic brain injury. J Cereb Blood Flow Metab. lymphocyte trafficking: a novel target for 56. Eakin K, Baratz-Goldstein R, Pick CG, et al.
2009;29(9):1503-1516. neuroprotection in traumatic brain injury. Efficacy of N-acetyl cysteine in traumatic brain
40. McDonald B, Pittman K, Menezes GB, et al. J Neurotrauma. 2007;24(8):1295-1307. injury. PLoS One. 2014;9(4):e90617.
Intravascular danger signals guide neutrophils to 48. Weckbach S, Neher M, Losacco JT, et al. 57. Giacino JT, Whyte J, Bagiella E, et al.
sites of sterile inflammation. Science. 2010;330 Challenging the role of adaptive immunity in Placebo-controlled trial of amantadine for severe
(6002):362-366. neurotrauma: Rag1(-/-) mice lacking mature B and T traumatic brain injury. N Engl J Med. 2012;366(9):
41. Scholz M, Cinatl J, Schdel-Hpfner M, Windolf cells do not show neuroprotection after closed 819-826.
J. Neutrophils and the blood-brain barrier head injury. J Neurotrauma. 2012;29(6):1233-1242. 58. Kimbler DE, Shields J, Yanasak N, Vender JR,
dysfunction after trauma. Med Res Rev. 2007;27(3): 49. Janowitz T, Menon DK. Exploring new routes Dhandapani KM. Activation of P2X7 promotes
401-416. for neuroprotective drug development in traumatic cerebral edema and neurological injury after
42. Nguyen HX, OBarr TJ, Anderson AJ. brain injury. Sci Transl Med. 2010;2(27):27rv1. traumatic brain injury in mice. PLoS One. 2012;7(7):
Polymorphonuclear leukocytes promote 50. McConeghy KW, Hatton J, Hughes L, Cook AM. e41229.
neurotoxicity through release of matrix A review of neuroprotection pharmacology and 59. Wang X, Arcuino G, Takano T, et al. P2X7
metalloproteinases, reactive oxygen species, and therapies in patients with acute traumatic brain receptor inhibition improves recovery after spinal
TNF-alpha. J Neurochem. 2007;102(3):900-912. injury. CNS Drugs. 2012;26(7):613-636. cord injury. Nat Med. 2004;10(8):821-827.
43. Liao Y, Liu P, Guo F, Zhang ZY, Zhang Z. 51. Kontos HA. Oxygen radicals in CNS damage.
Oxidative burst of circulating neutrophils following Chem Biol Interact. 1989;72(3):229-255.
traumatic brain injury in human. PLoS One. 2013;8 52. Bains M, Hall ED. Antioxidant therapies in
(7):e68963. traumatic brain and spinal cord injury. Biochim
Biophys Acta. 2012;1822(5):675-684.
362 JAMA Neurology March 2015 Volume 72, Number 3 (Reprinted) jamaneurology.com
Copyright 2015 American Medical Association. All rights reserved.
Downloaded From: http://archneur.jamanetwork.com/ by a NCSU Hunt Library User on 05/14/2015
Вам также может понравиться
- Ni Hms 809839Документ15 страницNi Hms 809839SDОценок пока нет
- Position Statement: Definition of Traumatic Brain Injury: Special CommunicationДокумент4 страницыPosition Statement: Definition of Traumatic Brain Injury: Special CommunicationLee ValievaОценок пока нет
- Brain, Behavior, and Immunity: Alok Kumar, David J. LoaneДокумент11 страницBrain, Behavior, and Immunity: Alok Kumar, David J. LoaneRinaldy TejaОценок пока нет
- Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic TargetsДокумент23 страницыTraumatic Brain Injuries: Pathophysiology and Potential Therapeutic TargetsTrajceОценок пока нет
- Mixture model framework for TBI prognosis using heterogeneous dataДокумент13 страницMixture model framework for TBI prognosis using heterogeneous dataCaroline FelicianoОценок пока нет
- How Do We Indentify The Crasinh Traumatic Brain Injury PatientДокумент8 страницHow Do We Indentify The Crasinh Traumatic Brain Injury PatientRodrigo PintoОценок пока нет
- KumarR ILSДокумент13 страницKumarR ILSHrishit ShahОценок пока нет
- Trauma CraneoencefalicoДокумент18 страницTrauma CraneoencefalicoUCIADULTO UPEDОценок пока нет
- The Spectrum of Mild Traumatic Brain Injury: A ReviewДокумент11 страницThe Spectrum of Mild Traumatic Brain Injury: A ReviewKomangОценок пока нет
- Assessing The Severity of Traumatic Brain Injury-Time For A Change?Документ12 страницAssessing The Severity of Traumatic Brain Injury-Time For A Change?Anonymous R6ex8BM0Оценок пока нет
- Savitz Et Al-2016-Stem CellsДокумент6 страницSavitz Et Al-2016-Stem CellsMaha Satya Dwi PalgunaОценок пока нет
- jamaneurology_fischer_2024_rv_230004_1711137409.29747Документ11 страницjamaneurology_fischer_2024_rv_230004_1711137409.29747samuel anchondoОценок пока нет
- Fnmol 12 00057Документ9 страницFnmol 12 00057Uziko 10Оценок пока нет
- Effect of Photobiomodulation in MultiplesclerosisДокумент4 страницыEffect of Photobiomodulation in MultiplesclerosisMuhammad Imam NoorОценок пока нет
- NIH Public Access: Severe Traumatic Head Injury Affects Systemic Cytokine ExpressionДокумент15 страницNIH Public Access: Severe Traumatic Head Injury Affects Systemic Cytokine ExpressionDeVisShoppОценок пока нет
- Treat Concussion, TBI, and PTSD with Vitamins and AntioxidantsОт EverandTreat Concussion, TBI, and PTSD with Vitamins and AntioxidantsОценок пока нет
- 2021_Brain Death_Death by Neurologic Criteria DeterminationДокумент25 страниц2021_Brain Death_Death by Neurologic Criteria DeterminationOlga Manco GuzmánОценок пока нет
- MinervaAnestesiol-12294 ProofinPDF V3 2018-03-16Документ10 страницMinervaAnestesiol-12294 ProofinPDF V3 2018-03-16Marcela Sanchez ParraОценок пока нет
- Prediction of Minimally Conscious State With Brain Stem Reflexes in Unconscious Patients After Traumatic Brain InjuryДокумент5 страницPrediction of Minimally Conscious State With Brain Stem Reflexes in Unconscious Patients After Traumatic Brain InjuryMARIANA LINETH OROZCO RICARDOОценок пока нет
- PRP2-9-e00795Документ13 страницPRP2-9-e00795diego.battiatoОценок пока нет
- Severe Traumatic Brain Injury Targeted Management in The Intensive Care UnitДокумент24 страницыSevere Traumatic Brain Injury Targeted Management in The Intensive Care UnitJuan Sebastian Rodriguez GarciaОценок пока нет
- Traumatic Brain Injury 1: SeriesДокумент13 страницTraumatic Brain Injury 1: SeriesIntan HaddadОценок пока нет
- Current Concepts: Diffuse Axonal Injury-Associated Traumatic Brain InjuryДокумент11 страницCurrent Concepts: Diffuse Axonal Injury-Associated Traumatic Brain InjuryAryantoОценок пока нет
- Fnins 13 01178Документ12 страницFnins 13 01178Hrishit ShahОценок пока нет
- A Blood-Based Biomarker Panel To Risk-Stratify Mild Traumatic Brain InjuryДокумент18 страницA Blood-Based Biomarker Panel To Risk-Stratify Mild Traumatic Brain InjuryNurul FajriОценок пока нет
- Pharmaceutics 14 00152 v2Документ26 страницPharmaceutics 14 00152 v2Carlos CalderonОценок пока нет
- Biomarkers in Traumatic Brain Injury (Tbi) : A Review: Neuropsychiatric Disease and Treatment DoveДокумент12 страницBiomarkers in Traumatic Brain Injury (Tbi) : A Review: Neuropsychiatric Disease and Treatment DoveShafira WidiaОценок пока нет
- TraumaДокумент16 страницTraumaJuan Pablo PérezОценок пока нет
- Dissertation Brain InjuryДокумент7 страницDissertation Brain InjuryBuyCustomPapersOnlineChicago100% (1)
- Imaging Chronic Traumatic Brain Injury As A Risk Factor For Neurodegeneration Alzheimers DementiaДокумент8 страницImaging Chronic Traumatic Brain Injury As A Risk Factor For Neurodegeneration Alzheimers Dementiaapi-308065432Оценок пока нет
- Post Op Cognitive DysfunctionДокумент23 страницыPost Op Cognitive Dysfunctionlakshminivas PingaliОценок пока нет
- Optimistic Stratagems of Machine Based Deep LearningДокумент8 страницOptimistic Stratagems of Machine Based Deep LearningHerald Scholarly Open AccessОценок пока нет
- Neuroprotección en TEC, Revisión ActualizadaДокумент11 страницNeuroprotección en TEC, Revisión ActualizadaGustavo Delgado ReyesОценок пока нет
- Bacterial Brain Abscess: An Outline For Diagnosis and ManagementДокумент10 страницBacterial Brain Abscess: An Outline For Diagnosis and ManagementWIWI HRОценок пока нет
- Clinical Patterns and Biological Correlates of Cognitive Dysfunction Associated To Cancer TreatmentДокумент12 страницClinical Patterns and Biological Correlates of Cognitive Dysfunction Associated To Cancer TreatmentRodolfo BenavidesОценок пока нет
- Infeksi PanggulДокумент13 страницInfeksi PanggulAndalisОценок пока нет
- Jurnal 6Документ11 страницJurnal 6ichamarichaОценок пока нет
- Diagnosis, Prognosis and Clinical Management of Mild TbiДокумент12 страницDiagnosis, Prognosis and Clinical Management of Mild TbiMacros FitОценок пока нет
- Efeitos Dos Alopáticos Na DepressãoДокумент11 страницEfeitos Dos Alopáticos Na DepressãoTatiane PaulinoОценок пока нет
- Pone 0228947Документ12 страницPone 0228947M Ali AdrianОценок пока нет
- Genetic Stroke Syndromes.16Документ13 страницGenetic Stroke Syndromes.16mhd.mamdohОценок пока нет
- Current Trends in Biomarkers For Traumatic Brain Injury: ArticleДокумент15 страницCurrent Trends in Biomarkers For Traumatic Brain Injury: ArticleVandana OzaОценок пока нет
- Brambilla Et Al. 2013 - The Effect of Stroke On Immune FunctionДокумент24 страницыBrambilla Et Al. 2013 - The Effect of Stroke On Immune FunctionNiluh ItaОценок пока нет
- Kra Nick 2012Документ19 страницKra Nick 2012Yanuar Ahsan OfficialОценок пока нет
- Treatment of Radiation-Induced Cognitive DeclineДокумент17 страницTreatment of Radiation-Induced Cognitive Declinemadalena limaОценок пока нет
- Research Paper On TbiДокумент6 страницResearch Paper On Tbifvf3f9h1100% (1)
- Car Gade Delirium e Nuc IДокумент20 страницCar Gade Delirium e Nuc INataly Audrey Romero DiazОценок пока нет
- Tugas Jurnal 1Документ13 страницTugas Jurnal 1Aulia Putri AОценок пока нет
- Management of Concussion and Mild Traumatic Brain Injury: A Synthesis of Practice GuidelinesДокумент12 страницManagement of Concussion and Mild Traumatic Brain Injury: A Synthesis of Practice GuidelinesKomangОценок пока нет
- Assess the traumatic brain injury outcomes in pakistani emergency departmentДокумент27 страницAssess the traumatic brain injury outcomes in pakistani emergency departmentsanamaqsoodmalikОценок пока нет
- Akmal Hidayat FixДокумент17 страницAkmal Hidayat FixAndryana AgrevitaОценок пока нет
- Neuroinflammation and Neuroprogression in DepressionДокумент14 страницNeuroinflammation and Neuroprogression in DepressionAldehydeОценок пока нет
- Early Measurement of Interleukin-10 Predicts The Absence of CT Scan Lesions in Mild Traumatic Brain InjuryДокумент12 страницEarly Measurement of Interleukin-10 Predicts The Absence of CT Scan Lesions in Mild Traumatic Brain InjuryDesyОценок пока нет
- Metotreksat I FajzerДокумент11 страницMetotreksat I FajzergregorimОценок пока нет
- Neurofeedback and Traumatic Brain Injury A Literature ReviewДокумент6 страницNeurofeedback and Traumatic Brain Injury A Literature ReviewafmzmrigwaeeraОценок пока нет
- Ivig Supplement Septoct10Документ12 страницIvig Supplement Septoct10MuhammadRizalNОценок пока нет
- Jurnal 1Документ10 страницJurnal 1Hafizh Rafi RabbaniОценок пока нет
- 1-s2.0-S096808962300216X-mainДокумент23 страницы1-s2.0-S096808962300216X-mainPT Sedoyo Sami SehatОценок пока нет
- UntitledДокумент38 страницUntitledjoyce kabasogaОценок пока нет
- Journal 2 Gaming AdictionДокумент8 страницJournal 2 Gaming AdictionIlham PrayogoОценок пока нет
- Disruption of The Na+K+-ATPase-purinergic P2X7 Receptor Complex in Microglia Promotes Stress-Induced Anxiety NДокумент30 страницDisruption of The Na+K+-ATPase-purinergic P2X7 Receptor Complex in Microglia Promotes Stress-Induced Anxiety NSara Tenjo NaviaОценок пока нет
- Publication RC - PosterModena - ITALYДокумент1 страницаPublication RC - PosterModena - ITALYDr RC MishraОценок пока нет
- Fizyoloji Kongresi FEPS 2011 Abstract Book PDFДокумент522 страницыFizyoloji Kongresi FEPS 2011 Abstract Book PDFH. Fehmi OZELОценок пока нет
- Austin AndrologyДокумент4 страницыAustin AndrologyAustin Publishing GroupОценок пока нет
- Reichert 2021Документ18 страницReichert 2021Yash PatilОценок пока нет
- Physiology of The Carotid Body - From Molecules To DiseaseДокумент23 страницыPhysiology of The Carotid Body - From Molecules To DiseaseBladimir CentenoОценок пока нет
- Borea, P. A., Varani, K., Gessi, S., Merighi, S., & Vincenzi, F. (Eds.) - (2018) - The Adenosine Receptors PDFДокумент603 страницыBorea, P. A., Varani, K., Gessi, S., Merighi, S., & Vincenzi, F. (Eds.) - (2018) - The Adenosine Receptors PDFBreiner PeñarandaОценок пока нет
- Adenosine ReceptorДокумент53 страницыAdenosine Receptormirza_baig_46100% (1)