Академический Документы
Профессиональный Документы
Культура Документы
Suggested Answer - Tutorial 1 Chm510
Загружено:
Mark SullivanИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Suggested Answer - Tutorial 1 Chm510
Загружено:
Mark SullivanАвторское право:
Доступные форматы
SUGGESTED ANSWER FOR TUTORIAL 1
QUESTION 1
a) Very long columns are not often used in chromatographic separations in order to
achieve good separations because using infinitely long columns would allow more
separation process (plates) to occur, they would also lead to unacceptably long
separations. Longer separations are also more susceptible to broadening as a
result of longitudinal diffusion. As a result, overall separation quality is
diminished.
b) Two ways to increase efficiency in a chromatographic separation using packed column:
i) Use smaller packing material; This diminishes band broadening due to
multipath.
ii) Decrease the thickness of the stationary phase coating; This diminished
band broadening due to mass transfer terms.
(Q1 JAN 2012)
QUESTION 2
a) i) NA = 2568
NB = 1044
Higher N = more efficient
ii) RA = 1.65
RB = 1.37
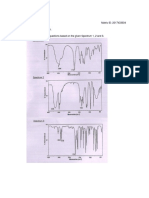
RA = 1.65 Baseline resolution between two peaks (as shown in the figure)
requires an Rs >1.5. Good separation for column A.
RB = 1.35. Resolution is more than 1.0, therefore the separation is considered
adequate for column B.
However, Column A better than column B
(Q1a JUN 2012)
b) i)
ii)
iii)
(Q1b JUN 2012)
QUESTION 3
Flow rate need to be optimized in order to improve the efficiency of a column:
For B/v, high flow rate will decrease the term and thus reduces band broadening
But for Cv, low flow rate is required to give the molecules enough time to reach
equilibrium.
Thus, an optimum flow rate is high enough to reduce diffusion and low enough to give
enough time for achieving equilibrium.
(Q1 JAN 2013)
QUESTION 4
(a)
Rs1 = 2.11 ; Rs2 = 0.78
Rs1 = 2.11. Peak X and Y were well separated as Rs > 1.5.
Rs2 = 0.78. Resolution is less than 1.0, therefore overlapping of peak Y and Z.
(b) i) 30 cms-1. Optimum flow rate is at minimum H.
ii) Increase in mass transfer. When the flow rate is high (above the optimum
value), the analyte has a strong affinity for the stationary phase and the
analyte in the mobile phase will move ahead of the analyte in the stationary
phase (without reaching equilibrium).
(Q1a & b JUN 2013)
QUESTION 5
In a chromatographic analysis, the effect of the following on the plate height of a column.
i) Increasing the flow rate
H increases. At high flow rate, term Cv increases. Analytes in mobile phase will
move faster than those in the stationary phase (without reaching equilibrium).
ii) Reducing the particle size of the packing
H decreases. Smaller particle size of packing will reduce differences in path of
molecules (reduce Eddy diffusion). Smaller particle size of packing will give
higher surface area, thus easier to reach equilibrium, reduce Cv.
(Q1a DEC 2013)
QUESTION 6
a) Navg = (
Вам также может понравиться
- Vocology For The Singing Voice PDFДокумент120 страницVocology For The Singing Voice PDFNathalia Parra Garza100% (2)
- Suggested Answer - Tutorial 6 Chm510Документ7 страницSuggested Answer - Tutorial 6 Chm510Mark SullivanОценок пока нет
- Suggested Answer - Tutorial 4 Chm510Документ6 страницSuggested Answer - Tutorial 4 Chm510Mark SullivanОценок пока нет
- Suggested Answer - Tutorial 5 Chm510 EditДокумент8 страницSuggested Answer - Tutorial 5 Chm510 EditMark SullivanОценок пока нет
- Suggested Answer - Tutorial 5 Chm510 EditДокумент8 страницSuggested Answer - Tutorial 5 Chm510 EditMark SullivanОценок пока нет
- CH 9 Employee and Labour RelationsДокумент19 страницCH 9 Employee and Labour RelationsMark SullivanОценок пока нет
- CH 9 Employee and Labour RelationsДокумент19 страницCH 9 Employee and Labour RelationsMark SullivanОценок пока нет
- Digital Signal Processing Lab ManualДокумент61 страницаDigital Signal Processing Lab ManualOmer Sheikh100% (6)
- Centralized PurchasingДокумент2 страницыCentralized PurchasingbiyyamobulreddyОценок пока нет
- Lab Report Bio460: Name: Siti Nur Aqilah Binti Asrul MATRIX NO.: 2020963825 Class: AS2011AДокумент8 страницLab Report Bio460: Name: Siti Nur Aqilah Binti Asrul MATRIX NO.: 2020963825 Class: AS2011ASITI NUR AQILAH ASRULОценок пока нет
- Suggested Answer - Tutorial 3 Chm510 (Mac2017)Документ5 страницSuggested Answer - Tutorial 3 Chm510 (Mac2017)Mark Sullivan100% (2)
- Experiment 2Документ5 страницExperiment 2Alya HaifaОценок пока нет
- Physical Properties of Nylon 6-10Документ3 страницыPhysical Properties of Nylon 6-10sharmi2011Оценок пока нет
- Universiti Teknologi MARA Faculty of Applied Science B.SC (Hons) Environmental Technology (AS 229)Документ4 страницыUniversiti Teknologi MARA Faculty of Applied Science B.SC (Hons) Environmental Technology (AS 229)TarikTaliHidungОценок пока нет
- CHM260 Experiment 5Документ16 страницCHM260 Experiment 5Muhammad Azri HaziqОценок пока нет
- MEC681 Assignment 2Документ4 страницыMEC681 Assignment 2Kamariza SuhainiОценок пока нет
- CHM 524Документ10 страницCHM 524aisyah fauziОценок пока нет
- CHM510 - SpeДокумент7 страницCHM510 - SpeafifiОценок пока нет
- Lab Report Chm510 Final (All Reports)Документ54 страницыLab Report Chm510 Final (All Reports)Dang HumairahОценок пока нет
- CHM 3402 Experment 3Документ9 страницCHM 3402 Experment 3Luqman HakimОценок пока нет
- Direct Potentiometric Titration of Fluoride IonДокумент3 страницыDirect Potentiometric Titration of Fluoride IonDozdiОценок пока нет
- 10 May 2020 Before 5 PM at Author - Uthm.edu - My (I Will Create The Folder To Submit)Документ2 страницы10 May 2020 Before 5 PM at Author - Uthm.edu - My (I Will Create The Folder To Submit)Sky FireОценок пока нет
- (Final) ASSIGNMENT CMT405 - Leaching PDFДокумент11 страниц(Final) ASSIGNMENT CMT405 - Leaching PDFnur haslindaОценок пока нет
- Ecw568 Test Question 5 Feb 2021Документ4 страницыEcw568 Test Question 5 Feb 2021Syalmira LovetobelovedОценок пока нет
- Lab ReportДокумент8 страницLab ReportNurin BatrisyiaОценок пока нет
- Experiment 5 AASДокумент15 страницExperiment 5 AASnn bbОценок пока нет
- Lab Report CHM361Документ6 страницLab Report CHM361Nurin Izzati Zulkifli100% (1)
- PST162 Chapter 4a Factors Affecting SMR PropertiesДокумент33 страницыPST162 Chapter 4a Factors Affecting SMR PropertiesMUADZ ARОценок пока нет
- Chapter 5 CHM510Документ90 страницChapter 5 CHM510syamimi zainalОценок пока нет
- Evt 531Документ8 страницEvt 531Yul100% (1)
- CHE515 Experiment 1Документ2 страницыCHE515 Experiment 1Amirul Assyraf NoorОценок пока нет
- CHM 510 Exp 1 GCДокумент8 страницCHM 510 Exp 1 GCNurul HaziqahОценок пока нет
- Lab Report: FRS 653 (Forensic Biology)Документ14 страницLab Report: FRS 653 (Forensic Biology)SHah LeLabahОценок пока нет
- Elc550 Test Brain Drain May 2021Документ5 страницElc550 Test Brain Drain May 2021Nurul IzzatiОценок пока нет
- Experiment of Gas ChromatographyДокумент10 страницExperiment of Gas Chromatographyadda93% (15)
- Tutorial 1 (CHAPTER 1: Introduction To Spectroscopic Method of Analysis)Документ1 страницаTutorial 1 (CHAPTER 1: Introduction To Spectroscopic Method of Analysis)Syaiful Ashraf Mohd Ashri100% (1)
- CHM 213 - Exp 5Документ9 страницCHM 213 - Exp 5hafiqahОценок пока нет
- Chm260 Tutorial 2Документ4 страницыChm260 Tutorial 2adlina asnawiОценок пока нет
- Full Lab 5 BiosepДокумент11 страницFull Lab 5 BiosepizuanieОценок пока нет
- Case Study FSGДокумент15 страницCase Study FSGmeklin0% (1)
- Phy400 Lab 3Документ7 страницPhy400 Lab 3Anonymous 5LtOddОценок пока нет
- CMT 581 Global Warning PresentationДокумент26 страницCMT 581 Global Warning PresentationAlia ShamsuddinОценок пока нет
- Exercise CHM420 3Документ2 страницыExercise CHM420 3syazaОценок пока нет
- CHM 477 Experiment 3 4 5 PDFДокумент10 страницCHM 477 Experiment 3 4 5 PDFAhmad ZakwanОценок пока нет
- Experiment 1Документ16 страницExperiment 1Izhharuddin100% (2)
- Chm260 Exp 1Документ6 страницChm260 Exp 1Ilya ZafirahОценок пока нет
- Bio320 Chap 1Документ43 страницыBio320 Chap 1qwefdfsОценок пока нет
- EXPERIMENT 1 chm260Документ10 страницEXPERIMENT 1 chm260Muhammad Azri HaziqОценок пока нет
- SWR Experiment 3 PDFДокумент9 страницSWR Experiment 3 PDFwnay100% (1)
- DR Tiang Report 1Документ7 страницDR Tiang Report 1KhAi EnОценок пока нет
- Exp 4 FlowabilityДокумент4 страницыExp 4 FlowabilityNur SyahirahОценок пока нет
- Lab Report Chm256 Exp 4Документ6 страницLab Report Chm256 Exp 4Miss KillerОценок пока нет
- Determination of The Percentage of Ligands in Coordination CompoundДокумент5 страницDetermination of The Percentage of Ligands in Coordination CompoundafifiОценок пока нет
- EVT577 Wastewater Exp1 FadzrilДокумент6 страницEVT577 Wastewater Exp1 FadzrilFadzrilОценок пока нет
- MIC481 Lab ReportДокумент6 страницMIC481 Lab ReportAbg Khairul Hannan Bin Abg AbdillahОценок пока нет
- Hardness of WaterДокумент6 страницHardness of WaterJamesShiq0% (1)
- SBL 1023 Exp 3Документ7 страницSBL 1023 Exp 3api-383623349Оценок пока нет
- AS120 - Diploma in ScienceДокумент2 страницыAS120 - Diploma in Scienceستي نوراسيقينОценок пока нет
- Chemistry Report 1Документ6 страницChemistry Report 1Athirah BidinОценок пока нет
- FST559 Exam 1 Question Feb Aug21Документ4 страницыFST559 Exam 1 Question Feb Aug21fatin umairahОценок пока нет
- Lab Report Exp 3 With Cover OCHEMДокумент3 страницыLab Report Exp 3 With Cover OCHEMFakhri Nazmi NorinОценок пока нет
- Case Study chm260 PDFДокумент11 страницCase Study chm260 PDFTaehyung KimОценок пока нет
- Basic Instrumental Analysis Experiment 2: Uv-Visible Determination of An Unknown Concentration of Kmno4 SolutionДокумент7 страницBasic Instrumental Analysis Experiment 2: Uv-Visible Determination of An Unknown Concentration of Kmno4 SolutionSiti Maizatul AkmaОценок пока нет
- CHM 3402 Experiment 6Документ7 страницCHM 3402 Experiment 6Uma Villashini GunasekaranОценок пока нет
- Lecture 2, Fundamentals HPLCДокумент36 страницLecture 2, Fundamentals HPLCChevuru ManushaОценок пока нет
- Band Broadening and Column Efficiency 2020 PDFДокумент23 страницыBand Broadening and Column Efficiency 2020 PDFSalix MattОценок пока нет
- Van Deemter EquationДокумент12 страницVan Deemter EquationSANJEET YADAVОценок пока нет
- 1 LectureДокумент25 страниц1 LectureChemistry DepartmentОценок пока нет
- Basic ChromatographyДокумент41 страницаBasic ChromatographyLindelwa MthembuОценок пока нет
- Introduction Until Propagation Report AGR 302 GroundnutsДокумент7 страницIntroduction Until Propagation Report AGR 302 GroundnutsMark SullivanОценок пока нет
- Phy360 Solar EnergyДокумент9 страницPhy360 Solar EnergyMark SullivanОценок пока нет
- Lab Phy360 3 Velocity of SoundДокумент8 страницLab Phy360 3 Velocity of SoundMark SullivanОценок пока нет
- Phy360 Exp Heat PumpДокумент6 страницPhy360 Exp Heat PumpMark SullivanОценок пока нет
- CHM678Документ5 страницCHM678Mark SullivanОценок пока нет
- Assignmen T 2 GCДокумент9 страницAssignmen T 2 GCMark SullivanОценок пока нет
- CH 6 - Performance AppraisalДокумент50 страницCH 6 - Performance AppraisalMark SullivanОценок пока нет
- CH 9 Employee and Labour RelationsДокумент1 страницаCH 9 Employee and Labour RelationsMark SullivanОценок пока нет
- 2012566461Документ40 страниц2012566461Mark SullivanОценок пока нет
- CHM678Документ5 страницCHM678Mark SullivanОценок пока нет
- CH 8 Safety, Health & Security at WorkplaceДокумент18 страницCH 8 Safety, Health & Security at WorkplaceMark SullivanОценок пока нет
- CH 4a - RecruitmentДокумент35 страницCH 4a - RecruitmentMark SullivanОценок пока нет
- CH 2 - HR PlanningДокумент40 страницCH 2 - HR PlanningMark SullivanОценок пока нет
- INPUT NewДокумент1 страницаINPUT NewMark SullivanОценок пока нет
- Chapter 8 Safety, Health and Security at WorkplaceДокумент29 страницChapter 8 Safety, Health and Security at WorkplaceMark SullivanОценок пока нет
- CH 3 - Job Analysis and DesignДокумент26 страницCH 3 - Job Analysis and DesignMark SullivanОценок пока нет
- Suggested Answer - Tutorial 2 Chm510Документ6 страницSuggested Answer - Tutorial 2 Chm510Mark SullivanОценок пока нет
- ResultДокумент6 страницResultMark SullivanОценок пока нет
- Compact Bone and Spongy Bone: OsteocytesДокумент27 страницCompact Bone and Spongy Bone: OsteocytesMark SullivanОценок пока нет
- BIO2OO - Introduction Tissues, Classification of Living Things & Ecology 1.1.0 Animal TissueДокумент19 страницBIO2OO - Introduction Tissues, Classification of Living Things & Ecology 1.1.0 Animal TissueMark SullivanОценок пока нет
- Cable Systems For High and Extra-High Voltage: Development, Manufacture, Testing, Installation and Operation of Cables and Their AccessoriesДокумент1 страницаCable Systems For High and Extra-High Voltage: Development, Manufacture, Testing, Installation and Operation of Cables and Their AccessorieseddisonfhОценок пока нет
- Century Vemap PDFДокумент5 страницCentury Vemap PDFMaster MirrorОценок пока нет
- SOL-Logarithm, Surds and IndicesДокумент12 страницSOL-Logarithm, Surds and Indicesdevli falduОценок пока нет
- Electromechanical Instruments: Permanent-Magnet Moving-Coil InstrumentsДокумент13 страницElectromechanical Instruments: Permanent-Magnet Moving-Coil InstrumentsTaimur ShahzadОценок пока нет
- Canadian-Solar Datasheet Inverter 3ph 75-100KДокумент2 страницыCanadian-Solar Datasheet Inverter 3ph 75-100KItaloОценок пока нет
- DCS Ground Charts v350Документ34 страницыDCS Ground Charts v350lkjsdflkjОценок пока нет
- Barriers of CommunicationДокумент5 страницBarriers of CommunicationIVY YBAÑEZОценок пока нет
- Silenat Berhanu SimaДокумент6 страницSilenat Berhanu SimaSilenat BerhanuОценок пока нет
- UntitledДокумент37 страницUntitledUnknown UserОценок пока нет
- Systematic Literature Review SvenskaДокумент6 страницSystematic Literature Review Svenskafihum1hadej2100% (1)
- 9A02505 Electrical Machines-IIIДокумент4 страницы9A02505 Electrical Machines-IIIsivabharathamurthyОценок пока нет
- Python - Data EngineeringДокумент34 страницыPython - Data EngineeringChetan PatilОценок пока нет
- 2021 3 AbstractsДокумент168 страниц2021 3 AbstractsLong An ĐỗОценок пока нет
- 1:100 Scale: SPACE-X "Crew Dragon 2" Demo Mission-1 CapsuleДокумент9 страниц1:100 Scale: SPACE-X "Crew Dragon 2" Demo Mission-1 CapsuleBearium YTОценок пока нет
- Case Study: Medisys Corp The Intense Care Product Development TeamДокумент10 страницCase Study: Medisys Corp The Intense Care Product Development TeamBig BОценок пока нет
- One Wavelength To Loop SkywireДокумент2 страницыOne Wavelength To Loop SkywireRobert TurnerОценок пока нет
- Sap Retail Two Step PricingДокумент4 страницыSap Retail Two Step PricingShams TabrezОценок пока нет
- Ficha Tecnica Reflector 2000W Led Lluminacion de Campos de Futbol Estadios Goled Philips Osram Opalux LedДокумент5 страницFicha Tecnica Reflector 2000W Led Lluminacion de Campos de Futbol Estadios Goled Philips Osram Opalux Ledluis perdigonОценок пока нет
- Researchpaper Should Removable Media Be Encrypted - PDF - ReportДокумент15 страницResearchpaper Should Removable Media Be Encrypted - PDF - ReportSakshi Dhananjay KambleОценок пока нет
- Advanced Laser Al170: Instruction ManualДокумент35 страницAdvanced Laser Al170: Instruction ManualJuan Camilo100% (1)
- Interjections NotesДокумент2 страницыInterjections NotesKanna ImuiОценок пока нет
- Results 2020: Climate Change Performance IndexДокумент32 страницыResults 2020: Climate Change Performance IndexTonyОценок пока нет
- Lithospheric Evolution of The Pre-And Early Andean Convergent Margin, ChileДокумент29 страницLithospheric Evolution of The Pre-And Early Andean Convergent Margin, ChileAbdiel MuñozОценок пока нет
- ENGLISH TOEFL Structure (3rd Exercise)Документ5 страницENGLISH TOEFL Structure (3rd Exercise)susannnnnnОценок пока нет
- UnitPlan (P.E) Grade 6Документ13 страницUnitPlan (P.E) Grade 6Lou At CamellaОценок пока нет
- MiningДокумент3 страницыMiningDherick RaleighОценок пока нет
- Himachal Pradesh Staff Selection Commission Hamirpur - 177001Документ2 страницыHimachal Pradesh Staff Selection Commission Hamirpur - 177001Verma JagdeepОценок пока нет