Академический Документы
Профессиональный Документы
Культура Документы
Flash (Equilibrium) Distillation
Загружено:
Timothy JonesОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Flash (Equilibrium) Distillation
Загружено:
Timothy JonesАвторское право:
Доступные форматы
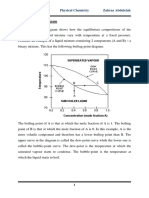
FLASH (EQUILIBRIUM) DISTILLATION
A single-stage continuous operation where a liquid mixture is partially vaporized: the vapour
produced and the residual liquid are in equilibrium, which are then separated and removed. See
the Figure below :
Set-up for Flash Distillation
We again consider a binary mixture of A (MVC) and B (LVC). The feed is preheated before
entering the separator. As such, part of the feed may be vaporized. The heated mixture then flows
through a pressure-reducing valve to the separator. In the separator, separation between the vapour
and liquid takes place. How much of A is produced in the vapour (and remained in the liquid)
depends on the condition of the feed, i.e. how much of the feed is entering as vapour state, which
in turn is controlled by the amount of heating. In other words, the degree of vapourization affects
the concentration (distribution) of A in vapour phase and liquid phase.
There is thus a certain relationship between the degree of heating (vapourization) and mole fraction
of A in vapour and liquid (y and x). This relationship is known as the Operating Line Equation.
Click here for the analysis and graphical solution.
http://www.separationprocesses.com/Distillation/DT_Chp03.htm
Вам также может понравиться
- Working Guide to Vapor-Liquid Phase Equilibria CalculationsОт EverandWorking Guide to Vapor-Liquid Phase Equilibria CalculationsРейтинг: 5 из 5 звезд5/5 (1)
- Vapour Pressure and Boiling: Distillation PrinciplesДокумент3 страницыVapour Pressure and Boiling: Distillation PrinciplesAldren Delina RiveraОценок пока нет
- Separation Process Chemical Engineering v2Документ7 страницSeparation Process Chemical Engineering v2Syed Zawar Shah KazmiОценок пока нет
- Continuous Distillation ColumnДокумент31 страницаContinuous Distillation ColumnRichard ObinnaОценок пока нет
- Mass Transfer: Distillation (Introduction and Phase Diagram)Документ8 страницMass Transfer: Distillation (Introduction and Phase Diagram)Gurunath EpiliОценок пока нет
- Chapter 1Документ111 страницChapter 1Radhi Abdullah100% (1)
- PMS Self StudyДокумент16 страницPMS Self StudyAbhishek KabburОценок пока нет
- Jawaharlal Nehru Technological University Kakinada: III Year B. Tech. Petrochemical Engineering II SemДокумент37 страницJawaharlal Nehru Technological University Kakinada: III Year B. Tech. Petrochemical Engineering II SemHashmi AshmalОценок пока нет
- MUCLecture 2021 12261237Документ6 страницMUCLecture 2021 12261237Shweta ChaudhariОценок пока нет
- Batch Distillation: D DT WX W DX DT X DW DTДокумент9 страницBatch Distillation: D DT WX W DX DT X DW DTZeny NaranjoОценок пока нет
- Unit-Iii 12Документ27 страницUnit-Iii 12Lakshya SaxenaОценок пока нет
- Report VLEДокумент10 страницReport VLEaizaqsyazwan0% (1)
- Separating Mixtures Volatility Unit Operation Chemical ReactionДокумент18 страницSeparating Mixtures Volatility Unit Operation Chemical ReactionmalavurОценок пока нет
- Distillation Lecture Note-2Документ20 страницDistillation Lecture Note-2BasseyОценок пока нет
- Distillation Typ 2Документ69 страницDistillation Typ 2Muhd HaniffОценок пока нет
- Distillation: Dr. Khalid Mahmood SHMTДокумент30 страницDistillation: Dr. Khalid Mahmood SHMTIqra MubeenОценок пока нет
- Mass Transfer - II 3350502: Parth Modi, LecturerДокумент39 страницMass Transfer - II 3350502: Parth Modi, LecturerSMIT CHRISTIANОценок пока нет
- CPE 601 Distillation - Topic 5 Design of Other EquipmentДокумент70 страницCPE 601 Distillation - Topic 5 Design of Other EquipmentRosa SinensisОценок пока нет
- Distillation & BPДокумент12 страницDistillation & BPAmirahKamaruddinОценок пока нет
- DistillationДокумент80 страницDistillationMatewos SadaОценок пока нет
- Chapter 1 DistillationДокумент110 страницChapter 1 DistillationSiti Nurshahira80% (5)
- Chapter 1 DistillationДокумент73 страницыChapter 1 DistillationNUR HIDAYAHОценок пока нет
- Lec 26Документ30 страницLec 26Abdul Hani MohammedОценок пока нет
- Pressure-Temperature-Concentration Phase DiagramДокумент8 страницPressure-Temperature-Concentration Phase DiagrammadhujayarajОценок пока нет
- CHT323A DistillationДокумент11 страницCHT323A DistillationVia Claire FloresОценок пока нет
- Distillation Background Summary and CalculationsRev2Документ5 страницDistillation Background Summary and CalculationsRev2Yonatan mebrateОценок пока нет
- Destilation: EVEN SEMESTER 2013/2014Документ24 страницыDestilation: EVEN SEMESTER 2013/2014انس خيرناОценок пока нет
- Chapter 4 Distillation Design: Subject: 1304 332 Unit Operation in Heat TransferДокумент71 страницаChapter 4 Distillation Design: Subject: 1304 332 Unit Operation in Heat TransferKirti DurhanОценок пока нет
- F 20130617 Chak Krit 46Документ71 страницаF 20130617 Chak Krit 46Nagwa MansyОценок пока нет
- DistillationДокумент124 страницыDistillationasharab70100% (1)
- Equilibrium Relationship Between P: A OA AДокумент19 страницEquilibrium Relationship Between P: A OA ATarek ChawafОценок пока нет
- Experiment 3Документ14 страницExperiment 3HafiniHambaliОценок пока нет
- Experiment D1 - Distillation ColumnДокумент16 страницExperiment D1 - Distillation ColumnchaitanyaОценок пока нет
- Che 411-Vapour Pressure and BoilingДокумент17 страницChe 411-Vapour Pressure and BoilingHannah CokerОценок пока нет
- Thermodynamics of Separation OperationsДокумент21 страницаThermodynamics of Separation OperationsgongweejieОценок пока нет
- 2018 Distillation NoteДокумент120 страниц2018 Distillation NoteemmanuelОценок пока нет
- Process and Significance of DistillationДокумент11 страницProcess and Significance of DistillationSiddiquer Rehman SidОценок пока нет
- Batch Distillation: Group 7 Errynne Yanza Hosleck Galasinao Aron BalinesДокумент18 страницBatch Distillation: Group 7 Errynne Yanza Hosleck Galasinao Aron BalinesAron BalinesОценок пока нет
- Equilibrium Separation ColumnsДокумент18 страницEquilibrium Separation ColumnsluckshimiОценок пока нет
- Continuous DistillationДокумент29 страницContinuous DistillationSAI P HARIHARAN 19BCM0030Оценок пока нет
- Distillation - Lectures 1 To 6 PDFДокумент45 страницDistillation - Lectures 1 To 6 PDFMayank PrasadОценок пока нет
- Notes On Mass TransferДокумент5 страницNotes On Mass Transferleonard katundaОценок пока нет
- Documentation of Distillation Column Design: Submitted To: Dr. Nadeem FerozeДокумент41 страницаDocumentation of Distillation Column Design: Submitted To: Dr. Nadeem Ferozerajtharun48Оценок пока нет
- Documentation of Distillation Column Design PDFДокумент41 страницаDocumentation of Distillation Column Design PDFFELIPE DURANОценок пока нет
- Chapter 1 - Distillation PDFДокумент107 страницChapter 1 - Distillation PDFFatin Natasha NazriОценок пока нет
- Distillation: Underlying Principles of Distillation Boiling A Binary MixtureДокумент19 страницDistillation: Underlying Principles of Distillation Boiling A Binary MixtureMukesh TiwariОценок пока нет
- Distillation PresentationДокумент61 страницаDistillation PresentationAli AmjadОценок пока нет
- DISTILLATION UNIT 1 28.2.22 - WatermarkДокумент26 страницDISTILLATION UNIT 1 28.2.22 - WatermarkHardik ChauhanОценок пока нет
- Explaination:: Flash DistillationДокумент9 страницExplaination:: Flash DistillationKaleemОценок пока нет
- Liquid Liquid ExtractionДокумент40 страницLiquid Liquid ExtractionApurba Sarker Apu93% (29)
- Buckley and Leverett (1942) : A.Fractional Flow EquationДокумент6 страницBuckley and Leverett (1942) : A.Fractional Flow Equationحسين رامي كريم A 12Оценок пока нет
- Distillation Column DesignДокумент5 страницDistillation Column DesignChemsys SunnyОценок пока нет
- OnLine Lecture 12Документ8 страницOnLine Lecture 12shamsul aminОценок пока нет
- The Mccabe-Thiele Method: Stream Total Molar Flowrate Composition (Mole Fraction MVC) V YДокумент10 страницThe Mccabe-Thiele Method: Stream Total Molar Flowrate Composition (Mole Fraction MVC) V YRose Dane Escobedo DiestaОценок пока нет
- CLP302 - MT (SG 6) - Merged (1) 2Документ62 страницыCLP302 - MT (SG 6) - Merged (1) 2hedavo3338Оценок пока нет
- Equilibrio AbsorciónДокумент47 страницEquilibrio AbsorciónFernando Quispe AliagaОценок пока нет
- Working Guide to Reservoir Rock Properties and Fluid FlowОт EverandWorking Guide to Reservoir Rock Properties and Fluid FlowРейтинг: 3 из 5 звезд3/5 (1)
- Fanning EquationДокумент2 страницыFanning EquationTimothy JonesОценок пока нет
- Air DryingДокумент1 страницаAir DryingTimothy JonesОценок пока нет
- Chemical Reaction Engineering DefinitionДокумент1 страницаChemical Reaction Engineering DefinitionTimothy JonesОценок пока нет
- Enzyme Kinetics DefinitionДокумент2 страницыEnzyme Kinetics DefinitionTimothy JonesОценок пока нет
- Reynolds NumberДокумент1 страницаReynolds NumberTimothy JonesОценок пока нет
- Understanding Mass TransferДокумент1 страницаUnderstanding Mass TransferTimothy JonesОценок пока нет
- Week 6Документ1 страницаWeek 6Timothy JonesОценок пока нет
- Gas Absorption & DesorptionДокумент1 страницаGas Absorption & DesorptionTimothy JonesОценок пока нет
- Newtonian FluidsДокумент3 страницыNewtonian FluidsTimothy JonesОценок пока нет
- LeachingДокумент1 страницаLeachingTimothy JonesОценок пока нет
- Evaporation - Inorganic Chemistry For IndustriesДокумент2 страницыEvaporation - Inorganic Chemistry For IndustriesTimothy JonesОценок пока нет
- Chocolate Chip-Coffee MuffinsДокумент1 страницаChocolate Chip-Coffee MuffinsTimothy JonesОценок пока нет
- Fluid DynamicsДокумент1 страницаFluid DynamicsTimothy JonesОценок пока нет
- Understanding Water Quality, Wastewater Treatment and Complying With DAO 34-35 and 2016-08 and PN SDWДокумент2 страницыUnderstanding Water Quality, Wastewater Treatment and Complying With DAO 34-35 and 2016-08 and PN SDWTimothy JonesОценок пока нет
- Gas Absorption ReportДокумент6 страницGas Absorption ReportTimothy JonesОценок пока нет
- Republic Act No. 8293Документ47 страницRepublic Act No. 8293Timothy JonesОценок пока нет
- Week 5Документ1 страницаWeek 5Timothy JonesОценок пока нет
- Week 1Документ1 страницаWeek 1Timothy JonesОценок пока нет
- Week 2Документ1 страницаWeek 2Timothy JonesОценок пока нет
- Week 3Документ1 страницаWeek 3Timothy JonesОценок пока нет
- Acetyl Co-A RRLДокумент8 страницAcetyl Co-A RRLTimothy JonesОценок пока нет
- Acetyl Co-AДокумент8 страницAcetyl Co-ATimothy JonesОценок пока нет
- Table of ContentsДокумент1 страницаTable of ContentsTimothy JonesОценок пока нет
- Week 4Документ1 страницаWeek 4Timothy JonesОценок пока нет
- Corn Muffin RecipeДокумент5 страницCorn Muffin RecipeTimothy JonesОценок пока нет
- Bulacan - Style Leche Flan RecipeДокумент1 страницаBulacan - Style Leche Flan RecipeTimothy JonesОценок пока нет
- DATA - Drying Part 2Документ1 страницаDATA - Drying Part 2Timothy JonesОценок пока нет
- Determination of Dissolved OxygenДокумент1 страницаDetermination of Dissolved OxygenTimothy JonesОценок пока нет
- Republic Act No. 8293Документ26 страницRepublic Act No. 8293Timothy JonesОценок пока нет
- Antalia HeaterДокумент1 страницаAntalia HeaterDaniel MocanuОценок пока нет
- Diagnostic Trouble Code (DTC) Charts and Descriptions: Procedure Revision Date: 03/29/2006Документ147 страницDiagnostic Trouble Code (DTC) Charts and Descriptions: Procedure Revision Date: 03/29/2006efasaravananОценок пока нет
- SaeДокумент8 страницSaeahmadОценок пока нет
- Control Valve Calibration Procedure (Fisher HC6010)Документ14 страницControl Valve Calibration Procedure (Fisher HC6010)Karen Cain93% (15)
- Eileen IntroductionДокумент43 страницыEileen IntroductionYork ZengОценок пока нет
- Free Body Diagram Work Book StudentДокумент15 страницFree Body Diagram Work Book StudentwshhytОценок пока нет
- Dynamic Depressurisation Calculations LNG Regasification UnitДокумент15 страницDynamic Depressurisation Calculations LNG Regasification Unitilmu2Оценок пока нет
- RR320306 HeattransferДокумент8 страницRR320306 HeattransferAnil Frivolous AbstemiousОценок пока нет
- Vacuum Bag MoldingДокумент36 страницVacuum Bag MoldingKarthick PrasadОценок пока нет
- GEA32129A Heavy Duty Gas Turbine Monitoring Protection R1Документ48 страницGEA32129A Heavy Duty Gas Turbine Monitoring Protection R1Manuel L LombarderoОценок пока нет
- Thermodynamics (5 Hours) : PhysicsДокумент56 страницThermodynamics (5 Hours) : PhysicsSaiful Nizam0% (1)
- 2927-Connecting Rod AlignmentДокумент1 страница2927-Connecting Rod AlignmentCr250r100% (1)
- EzoBord - Ceiling Baffle - Installation InstructionsДокумент3 страницыEzoBord - Ceiling Baffle - Installation InstructionsMEPОценок пока нет
- 4JH3 HteДокумент2 страницы4JH3 HteIvana Muratti JerkovicОценок пока нет
- CT - 100 - SPC - Rev01 Mar 2015Документ67 страницCT - 100 - SPC - Rev01 Mar 2015Ittoop and sons AutomobileОценок пока нет
- Self-Opening Die Head Instruction Sheet: WarningДокумент4 страницыSelf-Opening Die Head Instruction Sheet: WarningCesar Carito ValenciaОценок пока нет
- Problem Set 4Документ5 страницProblem Set 4Jobelle SDОценок пока нет
- Amal Float 691 622069 PDFДокумент2 страницыAmal Float 691 622069 PDFjvdkjdlkkОценок пока нет
- 1400SRM0046 (06 2017) Uk en PDFДокумент46 страниц1400SRM0046 (06 2017) Uk en PDFCosmin GîrleanuОценок пока нет
- User List PDFДокумент2 страницыUser List PDFroooyyОценок пока нет
- D3967-08. Splitting Tensile Strength of Intact Rock Core SpecimensДокумент4 страницыD3967-08. Splitting Tensile Strength of Intact Rock Core SpecimensIgnacio Padilla100% (2)
- MechanicalДокумент2 страницыMechanicalHalar MaymonОценок пока нет
- Factors Affecting MachinabilityДокумент1 страницаFactors Affecting MachinabilityPrasad WasteОценок пока нет
- Rothenberger R600 ManualДокумент10 страницRothenberger R600 Manualdorin stoicuОценок пока нет
- Raw Mill Patroller Checklist Route 1Документ5 страницRaw Mill Patroller Checklist Route 1AbasiemekaОценок пока нет
- Lemken Spare Parts Catalogue enДокумент116 страницLemken Spare Parts Catalogue enVlad Ptashnichenko100% (2)
- TKC40升降机维修说明书TKC Man Lift Maintenance ManualДокумент36 страницTKC40升降机维修说明书TKC Man Lift Maintenance Manualkiên phạm trungОценок пока нет
- Instruction Manual Unique Sanitary Mixproof Valve Including Us Version Ese00923enДокумент120 страницInstruction Manual Unique Sanitary Mixproof Valve Including Us Version Ese00923enAnonymous KdnOsd9Оценок пока нет
- TRD Supercharger InstallationДокумент42 страницыTRD Supercharger InstallationErnesto Lopez100% (2)
- Solis - 60N 75N 90N PDFДокумент255 страницSolis - 60N 75N 90N PDFkvsj2001Оценок пока нет