Академический Документы
Профессиональный Документы
Культура Документы
CD30 Draft Collation
Загружено:
cgao30Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
CD30 Draft Collation
Загружено:
cgao30Авторское право:
Доступные форматы
CD30
What is CD30
The CD30 molecule and its ligand (CD30L) are newly
recognized members of the TNF receptor and TNF ligands
superfamilies
The biologic role of the CD30/
CD30L interaction is still largely unknown1
The soluble form of the CD30 antigen (sCD30) has
a smaller molecular weight (85 kD) than the membrane
bound form (120 kD), and is probably produced by proteolytic
cleavage of the membrane-bound CD302
CD30 shedding occurs as
an active process of viable CD30' cells and is not merely
caused by the release from dying or dead cells1
Ki-l (CD30) antigen is also expressed by highly activated
Band T cells and by certain large celllyrnphomas
(LCLs) of both T- and B-cell origin.3-8
Structure
The extracellular domain of CD30 has proved to be homologous
to that of the TNF receptor superfamily members6*
1's1(F2i g l), whose canonical motif is the presence of
several (usually three or four) cysteine-rich pseudorepeats,
each containing six cysteines and c40 amino acids in the
extracellular part of the molecule.9,10
CD30+ normal cells may represent activated proliferating lymphoid elements
of either B, T, or “null” type11
It has been shown that no other CD30-positive cells exist
in the human body1
Function
The CD30L induces pleiotropic biologic effects on human CD30+ cell lines; the specific
responses include differentiation, activation, proliferation,
and cell death, which depend on cell type, stage of differentiation,
transformation status, and the presence of other stimuli. 12
CD30 knock-out mice and
CD30L knock-out mice show no alteration of the immune
response whereas CD30L overexpression is a lethal mutation
(T. Mak and H.J. Gruss, personal communication, May
1 994).1
Detection of sCD30 could be used as a specific tool for CD30+ neoplasm 2
CD30 – clinical and therapeutic impact
The discovery that
the extracellular part of the membrane-bound CD30 antigen is
proteolpcally cleaved to produce a soluble form (sCD30) has
led to the development oef nzymelinked immunosorbent assays
(ELISAS) for the detction of sCD30 in the mum of patients
with CD30-expressing neoplasm2
the CD30 molecule appears to be an optimal target
for immune intervention with specific antibodies. 1
The Ber-H2 antibody, directed
against a fixative-resistant epitope of the CD30 molecule,
is particularly useful for the diagnosis of such entity.
CD30 role in disease
CD30 expression is also characteristic of ALCL, a recently
recognized type of lymphoma. The Ber-H2 antibody, directed
against a fixative-resistant epitope of the CD30 molecule,
is particularly useful for the diagnosis of such entity1
Mapping of the CD30 gene at lp36 excludes its
involvement in the (2;5) chromosomal translocation, which
has been found in a proportion of ALCL with T-cell phenotype.13
the CD30L has no effect on the HD-derived cell
lines with "B-cell-like'' phenotype (KMH2 and L-428) and
induces apoptotic cell death in the CD30+ ALCL cell lines12
previous studies have suggested
that patients with CD30-negative lymphomas have a
much worse prognosis than patients with CD30-positive
lymphoma14
However, the observation that four of five (80%)
patients with CD30-negative LCL with localized disease
at presentation died of progressive disease 4-46
months (median, 27 months) after diagnosis, compared
with 3 of 42 (7%) of the patients with CD30-positive
LCL, illustrates that CD30 expression is a much more
important prognostic parameter than is the extent of
skin disease at presentation.15
Unmodified anti-CD30 (Ber-H2 antibody)
Despite optimal in vivo targeting of tumor cells, none of our patients
with refractory HD showed a tumor regression in response
to the native Ber-H2 antibody
Little/no competition with tumour sites for
binding of Ber-H2
This implies that, for therapeutic
purposes, anti-CD30 antibodies should be conjugated to cytotoxic
agents (either isotopes or toxins)16-20
Anti-CD30 immunotoxins
Because of their different mechanism of action (eg, killing of tumor
cells by inhibition
of protein synthesis) and nonoverlapping toxicity with chem~
therapy17'~an ti-CD30 immunotoxins may be expected to
be effective against chemoresistant and/or resting residual
CD30' tumor cells in this setting.
Systemic ALCL
Primary systemic ALCL, particularly of the common and
lymphohistiocytic types, frequently occurs in children and
young adults and is characterized in most cases by aggressive
clinical course, systemic symptoms, and multiple peripheral lymphadenopathy8,21
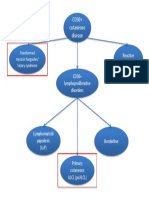
Cutaneous CD30+ disease
1. CD30+ lymphoproliferative disorder (pcALCL, LyP)
2. Cutaneous CD30+ LCLs e.g. MF, LyP
3. Skin involvement with a primary non-cutaneous CD30+ LCL or Hodgkin’s disease
The treatment of these patients is unsatisfactory.
Topical or systemic steroids or antibiotics are not
effective. Aggressive treatment modalities such as
systemic polychemotherapy or total-skin electron beam irradiation may produce complete remissions,
but these are generally short-lived.
It is more likely that these lesions represent
only variants of the same disease entity and that the clinical
differences reflect the variability of the biological behavior
of this tumor entity.22
It is important to note that cutaneous
Ki-1 + ALC lymphomas do not only occur primarily,
but also arise secondarily from other types of lymphomas
such as MF, pleomorphic T cell lymphoma, T cell lymphoma
of angioimmunoblastic type, and Lennert's lymphoma,
as well as Hodgkin's disease.23
Anaplastic large cell lymphoma (ALCL)
strongly suggest that ALCL is a tumor of highly
proliferating activated lymphoid cells
Characterized by subtotal effacement of the lymph
node architecture, paracortical growth pattern, and spread to
the sinuses, polymorphic appearance, and expression of the
CD30 antigen by virtually all neoplastic cells.3
morphologically
distinct type of lymphoid large cell neoplasms
that was designated as Ki-I/CD30+ ALCL.24
occurs predominantly in adults (median age, 60 years)
as a solitary, asymptomatic tumor that remains restricted to
the skin for a long time (evolution to a systemic disease
occurring in only 25% of cases), responds well to local treatment
(surgery or electron-beam therapy), and is associated
with good prognosis (median survival 42 months)22,25
Clinical characteristics of these lymphomas
include presentation with solitary or localized skin lesions,
frequent cutaneous relapses (often with the same
morphology and in the same area as the initial skin
lesion), a peculiar tendency to spontaneous regression,
and a favorable prognosis15
Because of the potential risk for the development of
a systemic lymphoma, long-term follow-up is required,
in particular in patients in group II, who may
have an increased risk to develop persistent tumors
requiring additional radiotherapy.26
The results of this study indicate that
primary cutaneous CD30-positive LCL, regardless of
their morphologic classification (anaplastic or nonanaplastic)
can be considered as a distinct type of cutaneous
T-cell lymphoma. Recognition of this type of cutaneous
lymphoma is important because it may prevent patients
from unnecessary aggressive treatment.15
Treatment
However, the results of the current study indicate that systemic polychemotherapy as initial treatment does not
result in higher cure rates, longer disease-free periods, or less recurrences compared with local radiation therapy or
simple excision.15
Because of their cytomorphology and large growth
fraction, primary cutaneous Ki-1 ALC lymphomas are
grouped among cutaneous lymphomas of high-grade
malignancy by the EORTC Cutaneous Lymphoma Study
Group.28 Such a grouping has prompted clinicians to treat
these lymphomas with aggressive therapeutic protocols.
Our observations, however, do not justify such measures
because some patients are certainly overtreated.22
Lymphomatoid papulosis
LyP is defined as a chronic, recurrent, self-healing
papulonecrotic or papulonodular skin disease
with histologic features suggestive of a malignant
lymphoma27
Type A – variable numbers of CD30+ large, atypical cells
Type B – CD30- atypical cerebriform mononuclear cells similar to MF24
Immunohistochemical findings were consistent with an activated T-cell phenotype for the atypical cells of
lymphomatoid papulosis, the Reed-Sternberg cells of Hodgkin's disease, and the malignant cells of the T-cell
lymphoma.
A t(8;9) genetic translocation may be involved in the pathogenesis of lymphomatoid papulosis or its progression to
malignant disease28
the risk for these patients to develop
systemic lymphoma is extremely low
Overlapping spectrum
The borderline cases already described
clearly illustrate that the differences between
primary cutaneous CD30+ LCL and LyP are gradual
and suggest that these conditions form a continuous
spectrum, both clinically a nd histologically24
Distinction must be made between cutaneous
CD30-positive lymphomas that develop de novo in the
skin and cutaneous CD30-positive lymphomas that develop in patients with MF or other types of CTCL. Patients
from this latter category, who were excluded in
the current study, generally have a poor progn~sis
1. Falini B, Pileri S, Pizzolo G, et al. CD30 (Ki-1) molecule: a new cytokine receptor of the tumor
necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood.
1995;85(1):1-14.
2. Josimovic-Alasevic O, Dürkop H, Schwarting R, Backé E, Stein H, Diamantstein T. Ki-1 (CD30)
antigen is released by Ki-1-positive tumor cells in vitro and in vivo. I. Partial characterization
of soluble Ki-1 antigen and detection of the antigen in cell culture supernatants and in serum
by an enzyme-linked immunosorbent assay. European Journal of Immunology.
1989;19(1):157-162.
3. Stein H, Mason D, Gerdes J, et al. The expression of the Hodgkin's disease associated antigen
Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and
histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66(4):848-
858.
4. Suchi T, Lennert K, Tu LY, et al. Histopathology and immunohistochemistry of peripheral T
cell lymphomas: a proposal for their classification. Journal of Clinical Pathology.
1987;40(9):995-1015.
5. Agnarsson BA, Kadin ME. Ki-1 positive large cell lymphoma. A morphologic and immunologic
study of 19 cases. Am J Surg Pathol. 1988;12(4):264-274.
6. Chott A, Kaserer K, Augustin I, et al. Ki-1-positive large cell lymphoma. A clinicopathologic
study of 41 cases. Am J Surg Pathol. 1990;14(5):439-448.
7. Bitter MA, Franklin WA, Larson RA, et al. Morphology in Ki-1(CD30)-positive non-Hodgkin's
lymphoma is correlated with clinical features and the presence of a unique chromosomal
abnormality, t(2;5)(p23;q35). Am J Surg Pathol. 1990;14(4):305-316.
8. Greer JP, Kinney MC, Collins RD, et al. Clinical features of 31 patients with Ki-1 anaplastic
large-cell lymphoma. Journal of Clinical Oncology. 1991;9(4):539-547.
9. Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins:
Activation, costimulation, and death. Cell. 1994;76(6):959-962.
10. AN B, ML B, MH B, et al. The Leucocyte Antigen Facts Book. London, UK, Academic; 1993.
11. Falini B, Pileri S, Martelli MF, Taylor C. Histological and immunohistological analysis of
human lymphomas. Critical Reviews in Oncology/Hematology. 1989;9(4):351-419.
12. Gruss H, Dower S. Tumor necrosis factor ligand superfamily: involvement in the pathology of
malignant lymphomas. Blood. 1995;85(12):3378-3404.
13. Bastard C, Rimokh R, Dastugue N, et al. CD30-positive large cell lymphomas (‘Ki-1
lymphoma’) are associated with a chromosomal translocation involving 5q35. British Journal
of Haematology. 1990;74(2):161-168.
14. Beljaards RC, Meijer CJ, Scheffer E, et al. Prognostic significance of CD30 (Ki-1/Ber-H2)
expression in primary cutaneous large-cell lymphomas of T-cell origin. A clinicopathologic
and immunohistochemical study in 20 patients. The American Journal of Pathology.
1989;135(6):1169-1178.
15. Beljaards RC, Kaudewitz P, Berti E, et al. Primary cutaneous CD30‐positive large cell
lymphoma: Definition of a new type of cutaneous lymphoma with a favorable prognosis. A
European multicenter study of 47 patients. Cancer. 1993;71(6):2097-2104.
16. da Costa L, Carde P, Lumbroso JD, et al. Immunoscintigraphy in Hodgkin's disease and
anaplastic large cell lymphomas: results in 18 patients using the iodine radiolabeled
monoclonal antibody HRS-3. Annals of oncology : official journal of the European Society for
Medical Oncology. 1992;3 Suppl 4:53-57.
17. Grossbard M, Press O, Appelbaum F, Bernstein I, Nadler L. Monoclonal antibody-based
therapies of leukemia and lymphoma. Blood. 1992;80(4):863-878.
18. Engert A, Burrows F, Jung W, et al. Evaluation of ricin A chain-containing immunotoxins
directed against the CD30 antigen as potential reagents for the treatment of Hodgkin's
disease. Cancer research. 1990;50(1):84-88.
19. Tazzari PL, Bolognesi A, de Totero D, et al. Ber-H2 (anti-CD30)-saporin immunotoxin: a new
tool for the treatment of Hodgkin's disease and CD30+ lymphoma: in vitro evaluation. Br J
Haematol. 1992;81(2):203-211.
20. Engert A, Martin G, Pfreundschuh M, et al. Antitumor Effects of Ricin A Chain Immunotoxins
Prepared from Intact Antibodies and Fab′ Fragments on Solid Human Hodgkin's Disease
Tumors in Mice. Cancer research. 1990;50(10):2929-2935.
21. Pileri S, Falini B, Delsol G, et al. Lymphohistiocytic T-cell lymphoma (anaplastic large cell
lymphoma CD30+/Ki-1 + with a high content of reactive histiocytes). Histopathology.
1990;16(4):383-391.
22. Kaudewitz P, Stein H, Dallenbach F, et al. Primary and secondary cutaneous Ki-1+ (CD30+)
anaplastic large cell lymphomas. Morphologic, immunohistologic, and clinical-
characteristics. The American Journal of Pathology. 1989;135(2):359-367.
23. Stein H, Gerdes J. Phenotypical and genotypical marker in malignant lymphomas: Cellular
origin of Hodgkin and Stern-berg-Reed cells and implications on the classification of T-cell
and B-cell lymphomas. Verh Dtsch Ges Pathol. 1986;70:127-151.
24. Willemze R, Meyer CJ, Van Vloten WA, Scheffer E. The clinical and histological spectrum of
lymphomatoid papulosis. Br J Dermatol. 1982;107(2):131-144.
25. Kadin ME. The Spectrum of Ki-1+ Cutaneous Lymphomas. Curr Probl Dermatol. 1990;19:132-
143.
26. Willemze R, Beljaards RC. Spectrum of primary cutaneous CD30 (Ki-1)-positive
lymphoproliferative disorders. Journal of the American Academy of Dermatology.28(6):973-
980.
27. Macaulay WL. Lymphomatoid papulosis: A continuing self-healing eruption, clinically
benign—histologically malignant. Archives of Dermatology. 1968;97(1):23-30.
28. Davis TM. Hodgkin's disease, lymphomatoid papulosis and cutaneous T-cell lymphoma
derived from a common T-cell clone. N Engl J Med. 1992;326:1115.
Вам также может понравиться
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- Commonwealth Statutory Declaration Form (May 2011) PDFДокумент2 страницыCommonwealth Statutory Declaration Form (May 2011) PDFcgao30Оценок пока нет
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Theme IV Yr 5d Content Guide - MatrixДокумент9 страницTheme IV Yr 5d Content Guide - Matrixcgao30Оценок пока нет
- Pythagoras WorksheetДокумент2 страницыPythagoras Worksheetcgao30Оценок пока нет
- CD30 Cutaneous Disease FlowchartДокумент1 страницаCD30 Cutaneous Disease Flowchartcgao30Оценок пока нет
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- CPPREP4002 - Annotated Unit GuideДокумент8 страницCPPREP4002 - Annotated Unit Guidecgao30Оценок пока нет
- Paediatrics: Acyanotic Heart DiseaseДокумент5 страницPaediatrics: Acyanotic Heart Diseasecgao30Оценок пока нет
- CD Marker Panel Download 2Документ2 страницыCD Marker Panel Download 2cgao30Оценок пока нет
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- Matrix TopicsДокумент3 страницыMatrix Topicscgao30Оценок пока нет
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Fibroids: 1. Red DegenerationДокумент2 страницыFibroids: 1. Red Degenerationcgao30Оценок пока нет
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- Preterm LabourДокумент3 страницыPreterm Labourcgao30Оценок пока нет
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Pelvic Organ ProlapseДокумент5 страницPelvic Organ Prolapsecgao30Оценок пока нет
- Skin Terms: DescriptorsДокумент2 страницыSkin Terms: Descriptorscgao30Оценок пока нет
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Atomic Structure NotesДокумент9 страницAtomic Structure Notescgao30Оценок пока нет
- Equilibrium NotesДокумент5 страницEquilibrium Notescgao30Оценок пока нет
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- Pathology Lecture 7 - LiverДокумент11 страницPathology Lecture 7 - Livercgao30Оценок пока нет
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- Intro To Motor Systems: (Why Else Would You Go To The Gym, Right?)Документ13 страницIntro To Motor Systems: (Why Else Would You Go To The Gym, Right?)cgao30Оценок пока нет
- Acids and Bases NotesДокумент15 страницAcids and Bases Notescgao30Оценок пока нет
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- Kinetics Notes: 6.1 - Rates of ReactionДокумент15 страницKinetics Notes: 6.1 - Rates of Reactioncgao30Оценок пока нет
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Periodicity NotesДокумент5 страницPeriodicity Notescgao30Оценок пока нет
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- Atomic Structure NotesДокумент9 страницAtomic Structure Notescgao30Оценок пока нет
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Angelman Syndrome - Identification and ManagementДокумент10 страницAngelman Syndrome - Identification and ManagementFajar YuniftiadiОценок пока нет
- NCP For PostpartumДокумент1 страницаNCP For PostpartumMary Hope BacutaОценок пока нет
- Using MBCT in A Chronic Pain Setting: A Qualitative Analysis of Participants' ExperiencesДокумент11 страницUsing MBCT in A Chronic Pain Setting: A Qualitative Analysis of Participants' ExperiencesJay JalaliОценок пока нет
- Forensic Aspects of Dissociative Identity DisorderДокумент240 страницForensic Aspects of Dissociative Identity DisorderDeath Tiscat100% (1)
- Assisting Delivery Name: - Grade: - Year and Section: - DateДокумент5 страницAssisting Delivery Name: - Grade: - Year and Section: - DateCrisia Jane LotaОценок пока нет
- TNF-dental PulpДокумент3 страницыTNF-dental PulpNicolas PintoОценок пока нет
- MFDS FAQsДокумент2 страницыMFDS FAQsSuhesh HydrosОценок пока нет
- Pulmon Ficha Técnica PDFДокумент2 страницыPulmon Ficha Técnica PDFderlingОценок пока нет
- Block-D FinalДокумент47 страницBlock-D FinalAnonymous 7IKdlmОценок пока нет
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- VedicReport10 29 202211 43 48AMДокумент1 страницаVedicReport10 29 202211 43 48AMAvish DussoyeОценок пока нет
- Sustainable Rural Water, Sanitation and Hygiene Project PROPOSALДокумент13 страницSustainable Rural Water, Sanitation and Hygiene Project PROPOSALLawrence WatssonОценок пока нет
- Hafiz Fizalia - Acupuncture Hack - The Easiest Way To Learn Classical Theories of Acupuncture (2018) PDFДокумент66 страницHafiz Fizalia - Acupuncture Hack - The Easiest Way To Learn Classical Theories of Acupuncture (2018) PDFkhalid Sijilmassi100% (3)
- African National Congress: Department of Information and PublicityДокумент9 страницAfrican National Congress: Department of Information and PublicityeNCA.com100% (2)
- Global Source Healthcare Case StudyДокумент6 страницGlobal Source Healthcare Case StudyEszterОценок пока нет
- Cancer Treatment - Capsaicin - Oil RecipeДокумент4 страницыCancer Treatment - Capsaicin - Oil RecipeJESUS IS RETURNING DURING OUR GENERATIONОценок пока нет
- BURNS SoftДокумент3 страницыBURNS SoftErlo John Asentista0% (1)
- Rdramirez Aota 2018 Poster For PortfolioДокумент1 страницаRdramirez Aota 2018 Poster For Portfolioapi-437843157Оценок пока нет
- Chapter 3 Definition - DisabilityДокумент12 страницChapter 3 Definition - DisabilityAnimesh KumarОценок пока нет
- UrethralstricturesДокумент37 страницUrethralstricturesNinaОценок пока нет
- Concurrent Visit Report 7-12Документ14 страницConcurrent Visit Report 7-12Tizita ChakaОценок пока нет
- Craniofacial SyndromesДокумент101 страницаCraniofacial SyndromesSaranya MohanОценок пока нет
- Hematinics: Dr. Monalisa Mondal Demonstrator Department of PharmacologyДокумент28 страницHematinics: Dr. Monalisa Mondal Demonstrator Department of PharmacologyShirsh JauriharОценок пока нет
- Engineering Practice - Workplace Safety and HealthДокумент68 страницEngineering Practice - Workplace Safety and HealthignatiusОценок пока нет
- Abdominal PainДокумент39 страницAbdominal PainIsma Resti PratiwiОценок пока нет
- Syba Economics Syll 20-21Документ13 страницSyba Economics Syll 20-21Saniya ShaikhОценок пока нет
- Labor and Delivery OB Concept MapДокумент2 страницыLabor and Delivery OB Concept MapMissy Johnson75% (4)
- Eeh455 Assignment2b MaharpДокумент22 страницыEeh455 Assignment2b Maharpapi-267478292Оценок пока нет
- CircCircuit Protection in Health Care Facilitiesuit Protection in Health Care FacilitiesДокумент43 страницыCircCircuit Protection in Health Care Facilitiesuit Protection in Health Care FacilitiesMenaОценок пока нет
- Fractures NoteДокумент31 страницаFractures NoteNoor AlblushiОценок пока нет
- Professional Review Industry Route Guidance NotesДокумент10 страницProfessional Review Industry Route Guidance NotesAnonymous TlYmhkОценок пока нет
- Love Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)От EverandLove Life: How to Raise Your Standards, Find Your Person, and Live Happily (No Matter What)Рейтинг: 3 из 5 звезд3/5 (1)
- The Age of Magical Overthinking: Notes on Modern IrrationalityОт EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityРейтинг: 4 из 5 звезд4/5 (30)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsОт EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsОценок пока нет