Академический Документы
Профессиональный Документы
Культура Документы
Phenyl Eph Rine
Загружено:
Sam SonИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Phenyl Eph Rine
Загружено:
Sam SonАвторское право:
Доступные форматы
1568 Cough Suppressants Expectorants Mucolytics and Nasal Decongestants
Preparations Pharmacopoeias. In Chin., Eur. (see p.vii), Jpn, and US. cases. Dermatoconjunctivitis2 has also been reported after use of
Proprietary Preparations (details are given in Part 3) Ph. Eur. 6.2 (Phenylephrine Hydrochloride). A white or almost phenylephrine eye drops.
Austral.: Nyal Dry Cough†; Austria: Atenos; Sedotussin; Belg.: Balsoclase white, crystalline powder. Freely soluble in water and in alcohol. 1. Soparkar CN, et al. Acute and chronic conjunctivitis due to over-
Antitussivum; Tuclase; Cz.: Sedotussin†; Denm.: Toclase†; Fin.: Toclase; USP 31 (Phenylephrine Hydrochloride). White or practically the-counter ophthalmic decongestants. Arch Ophthalmol 1997;
Fr.: Pectosan Toux Seche; Toclase Toux Seche; Vicks Pectoral; Ger.: Pertix- white, odourless, crystals. Freely soluble in water and in alcohol. 115: 34–8.
Solo-N, Pertix-T, Pertix-Z, and Pertix-L†; Sedotussin; Gr.: Tuclase; Hong 2. Moreno-Ancillo A, et al. Allergic contact reactions due to phe-
Store in airtight containers at a temperature of 25°, excursions nylephrine hydrochloride in eyedrops. Ann Allergy Asthma Im-
Kong: Toclase; Hung.: Sedotussin; Ital.: Tuclase; Neth.: Balsoclase; Tucla-
se; Norw.: Toclase; Philipp.: Toclase; Swed.: Toclase; Thai.: Toclase; permitted between 15° and 30°. Protect from light. munol 1997; 78: 569–72.
Turk.: Toclase; USA: Solotuss; Venez.: Carbin†. Incompatibility. Phenylephrine is stated to be incompatible Effects on mental function. Hallucinations and paranoid de-
Multi-ingredient: Arg.: Bio Grip Plus; Rynatus†; Wilpan Antigripal; with the local anaesthetic butacaine. lusions have been reported1 in a patient after excessive use of a
Wilpan C†; Austral.: Vicks Cough Syrup; Austria: Tussoretardin; Belg.: nasal spray containing phenylephrine 0.5%. Mania has also fol-
Balsoclase Expectorans; Braz.: Alergo Glucalbet†; Coldrin; Gegrip†; Re-
sprin; Fin.: Toclase Expectorant; Ger.: Sedotussin plus†; Hong Kong: Coci- Adverse Effects and Precautions lowed the use of large oral doses.2
Fedra; Marflu-X; Vida Cough; Neth.: Balsoclase Compositum; Balsoclase-E; As for Sympathomimetics, p.1407; phenylephrine has 1. Snow SS, et al. Nasal spray ‘addiction’ and psychosis: a case
S.Afr.: Vicks Acta Plus; Switz.: Sedotussin†; Turk.: Gayaben; USA: AMBI report. Br J Psychiatry 1980; 136: 297–9.
1000/5; Aridex; BetaVent; C-Tanna 12D; Carb Pseudo-Tan; Carbatab; mainly alpha-agonist effects. It has a longer duration of 2. Waters BGH, Lapierre YD. Secondary mania associated with
Diphen Tann/ PE Tann/ CT Tann; Duratuss CS; Dynex VR; Dytan-AT; action than noradrenaline and an excessive vasopressor sympathomimetic drug use. Am J Psychiatry 1981; 138: 837–40.
Dytan-CD; Dytan-CS; Exratuss; Extendryl GCP; Levall; Levall 12; Oratuss;
Pyrlex CB; Re-Tann; Rentamine Pediatric; Respi-Tann G; Ry-Tuss†; Rynatuss; response may cause a prolonged rise in blood pressure. Hypersensitivity. Cross-sensitivity to phenylephrine has been
Tannic-12; Tri-Tannate Plus Pediatric; Tuss-Tan; Tussi-12; Tussi-12 D; Tussi- It induces tachycardia or reflex bradycardia and should reported in a patient hypersensitive to pseudoephedrine.1 See
12D S; Tussizone; Vazotan; XiraTuss; Xpect-AT; Venez.: Resprin; Tolmex; also Effects on the Eyes, above.
Yerba Santa. therefore be avoided in severe hyperthyroidism and
1. Buzo-Sanchez G, et al. Stereoisomeric cutaneous hypersensitiv-
used with caution in severe ischaemic heart disease. ity. Ann Pharmacother 1997; 31: 1091.
Patients with diabetes mellitus or prostatic hyperplasia
should also avoid phenylephrine. Interactions
Phenylephrine (BAN, rINN) Since phenylephrine is absorbed through the mucosa As for Sympathomimetics, p.1407. Phenylephrine has
Fenilefrin; Fenilefrina; Fenilefrinas; Fenylefrin; Fenyyliefriini; Phé- systemic effects may follow application to the eyes or mainly direct alpha-agonist properties and is less liable
nyléphrine; Phenylephrinum; m-Synephrine. (1R)-1-(3-Hydroxy- the nasal mucosa. In particular, phenylephrine 10% eye than adrenaline or noradrenaline to induce ventricular
phenyl)-2-methylaminoethanol. drops can have powerful systemic effects. They should fibrillation if used as a pressor agent during anaesthesia
Фенилэфрин be avoided or only used with extreme caution in in- with inhalational anaesthetics such as cyclopropane
C 9 H 13 NO 2 = 167.2. fants, the elderly, and in patients with cardiac disease, and halothane; nevertheless, caution is necessary.
C AS — 59-42-7. significant hypertension, or advanced arteriosclerosis. Since phenylephrine is absorbed through the mucosa,
ATC — C01C A06; R01AA04; R01AB01; R01BA03; Fatalities have been reported in patients with pre-exist- interactions may also follow topical application, partic-
S01FB01; S01GA05. ing cardiovascular disease. ularly in patients receiving an MAOI (including a RI-
ATC Vet — QC01C A06; QR01AA04; QR01AB01;
Use of phenylephrine in the eye may liberate pigment MA). See also under Phenelzine (p.418) and Mo-
QR01BA03; QS01FB01; QS01GA05. clobemide (p.411).
granules from the iris, especially when given in high
doses to elderly patients. Ophthalmic solutions of phe- Cardiovascular drugs. Hypertensive reactions have been re-
nylephrine are contra-indicated in patients with angle- ported in a patient stabilised on debrisoquine when given phe-
H OH nylephrine orally,1 in patients receiving reserpine or guanethi-
H
HO N closure glaucoma. Corneal clouding may occur if cor- dine when given phenylephrine eye drops,2 and a fatal reaction
CH3 neal epithelium has been denuded or damaged. occurred in a patient receiving propranolol and hydrochlorothi-
Excessive or prolonged use of phenylephrine nasal azide also after the instillation of phenylephrine eye drops.3
1. Aminu J, et al. Interaction between debrisoquine and phenyle-
drops can lead to rebound congestion. phrine. Lancet 1970; ii: 935–6.
Phenylephrine hydrochloride is irritant and may cause 2. Kim JM, et al. Hypertensive reactions to phenylephrine eyedrops
NOTE. Synephrine has been used as a synonym for oxedrine local discomfort at the site of application; extravasa- in patients with sympathetic denervation. Am J Ophthalmol
(p.1364). Care should be taken to avoid confusion with phenyle- 1978; 85: 862–8.
phrine (m-synephrine). tion of the injection may even cause local tissue necro- 3. Cass E, et al. Hazards of phenylephrine topical medication in
sis. persons taking propranolol. Can Med Assoc J 1979; 120:
Pharmacopoeias. In Eur. (see p.vii). 1261–2.
Ph. Eur. 6.2 (Phenylephrine). A white or almost white crystal- Effects on the cardiovascular system. Systemic adverse ef-
line powder. Slightly soluble in water and in alcohol; sparingly fects have occurred after the use of phenylephrine as eye drops Pharmacokinetics
soluble in methyl alcohol. It dissolves in dilute mineral acids and (particularly at a strength of 10%), or nasal drops. Phenylephrine has low oral bioavailability owing to ir-
in solutions of alkali hydroxides. Store in airtight containers. Pro- Hypertension1 and hypertension with pulmonary oedema2 have
tect from light. regular absorption and first-pass metabolism by
been described in infants and children after the use of phenyl-
ephrine 10% eye drops. Hypertension with arrhythmias has also monoamine oxidase in the gut and liver. When injected
Phenylephrine Acid Tartrate been reported in an 8-year-old child3 and in an adult4 after phe- subcutaneously or intramuscularly it takes 10 to 15
nylephrine 10% eye drops had been used. Details have also been minutes to act; subcutaneous and intramuscular injec-
Phenylephrine Bitartrate (rINNM); Bitartrato de fenilefrina; Phé- published on a series of 32 patients who had systemic cardiovas-
nyléphrine, Bitartrate de; Phenylephrine Tartrate (BANM); Phe- tions are effective for up to about 1 hour and up to
cular reactions, including fatal myocardial infarctions, after the about 2 hours, respectively. Intravenous injections are
nylephrini Bitartras; Tartrato ácido de fenilefrina. use of phenylephrine 10% solutions in the eye.5 Severe cardio-
Фенилэфрина Битартрат vascular adverse reactions have also been reported to the use of effective for about 20 minutes.
C 9 H 13 NO 2 ,C 4 H 6 O 6 = 317.3. phenylephrine as topical 10% ocular6 or 0.25% nasal7 pledgets. Systemic absorption follows topical application.
C AS — 13998-27-1. Although the incidence of such reactions seems low,8 the use of
ATC — C01C A06; R01AA04; R01AB01; R01BA03; lower concentrations1,5 and caution in susceptible patients such Uses and Administration
S01FB01; S01GA05. as those with cardiovascular disorders or the elderly,5 have been Phenylephrine hydrochloride is a sympathomimetic
ATC Vet — QC01C A06; QR01AA04; QR01AB01; advocated. A reduction in the eye-drop volume has been found
to produce adequate mydriasis and may reduce systemic absorp- (p.1408) with mainly direct effects on adrenergic re-
QR01BA03; QS01FB01; QS01GA05. ceptors. It has mainly alpha-adrenergic activity and is
tion and the risk of adverse cardiovascular effects.9,10
Pharmacopoeias. In US. 1. Borromeo-McGrail V, et al. Systemic hypertension following without significant stimulating effects on the CNS at
USP 31 (Phenylephrine Bitartrate). A white or almost white ocular administration of 10% phenylephrine in the neonate.
powder or colourless crystals. Freely soluble in water. pH of a Pediatrics 1973; 51: 1032–6.
usual doses. Its pressor activity is weaker than that of
10% solution in water is between 3.0 and 4.0. Store in airtight 2. Baldwin FJ, Morley AP. Intraoperative pulmonary oedema in a noradrenaline (p.1360) but of longer duration. After in-
child following systemic absorption of phenylephrine eyedrops. jection it produces peripheral vasoconstriction and in-
containers. Protect from light. Br J Anaesth 2002; 88: 440–2.
3. Vaughan RW. Ventricular arrhythmias after topical vasocon- creased arterial pressure; it also causes reflex bradycar-
Phenylephrine Hydrochloride (BANM, rINNM) strictors. Anesth Analg 1973; 52: 161–5. dia. It reduces blood flow to the skin and to the kidneys.
4. Lai Y-K. Adverse effect of intraoperative phenylephrine 10%:
Fenilefrin Hidroklorür; Fenilefrin-hidroklorid; Fenilefrino hidro- case report. Br J Ophthalmol 1989; 73: 468–9. Phenylephrine and its salts are most commonly used,
chloridas; Fenylefrin hydrochlorid; Fenylefrinhydroklorid; Fenyle- 5. Fraunfelder FT, Scafidi AF. Possible adverse effects from topi- either topically or by mouth, for the symptomatic relief
cal ocular 10% phenylephrine. Am J Ophthalmol 1978; 85:
fryny chlorowodorek; Fenyyliefriinihydrokloridi; Hidrocloruro de 447–53. of nasal congestion (p.1548). They are frequently in-
fenilefrina; Mesatonum; Metaoxedrini Chloridum; Phényléphrine, 6. Fraunfelder FW, et al. Adverse systemic effects from pledgets cluded in preparations intended for the relief of cough
chlorhydrate de; Phenylephrini hydrochloridum. of topical ocular phenylephrine 10%. Am J Ophthalmol 2002;
134: 624–5. and cold symptoms. For nasal congestion, a 0.25 to 1%
Фенилэфрина Гидрохлорид 7. Hecker RB, et al. Myocardial ischemia and stunning induced by solution may be instilled as nasal drops or a spray into
C 9 H 13 NO 2 ,HCl = 203.7. topical intranasal phenylephrine pledgets. Mil Med 1997; 162:
832–5. each nostril every 4 hours as required, or pheny-
C AS — 61-76-7. 8. Brown MM, et al. Lack of side effects from topically adminis- lephrine hydrochloride may be given in usual oral dos-
ATC — C01C A06; R01AA04; R01AB01; R01BA03; tered 10% phenylephrine eyedrops: a controlled study. Arch
S01FB01; S01GA05. Ophthalmol 1980; 98: 487–9. es of 10 mg every four hours (up to a maximum of
ATC Vet — QC01C A06; QR01AA04; QR01AB01; 9. Craig EW, Griffiths PG. Effect on mydriasis of modifying the 60 mg daily) or 12 mg up to four times daily.
volume of phenylephrine drops. Br J Ophthalmol 1991; 75:
QR01BA03; QS01FB01; QS01GA05. 222–3. In ophthalmology, phenylephrine hydrochloride is
10. Wheatcroft S, et al. Reduction in mydriatic drop size in prema- used as a mydriatic (p.1874) in concentrations of up to
NOTE. PHNL is a code approved by the BP 2008 for use on single
ture infants. Br J Ophthalmol 1993; 77: 364–5.
unit doses of eye drops containing phenylephrine hydrochloride 10%; generally solutions containing 2.5 or 10% are
where the individual container may be too small to bear all the Effects on the eyes. Acute and chronic conjunctivitis has been
reported1 after use of over-the-counter ophthalmic decongestant
used but systemic absorption can occur (see Effects on
appropriate labelling information. PHNCYC is a similar code
approved for eye drops containing phenylephrine hydrochloride preparations of phenylephrine, naphazoline, or tetryzoline. The the Cardiovascular System, above) and the 10%
and cyclopentolate hydrochloride. conjunctival inflammation took several weeks to resolve in some strength, in particular, should be used with caution. The
Вам также может понравиться
- Phenylephrine: 1568 Cough Suppressants Expectorants Mucolytics and Nasal DecongestantsДокумент2 страницыPhenylephrine: 1568 Cough Suppressants Expectorants Mucolytics and Nasal DecongestantsRanny LaidasuriОценок пока нет
- EpinephrineДокумент4 страницыEpinephrinegovind_soni_15Оценок пока нет
- Injection, OTC Nasal Solution:: Generic Name: Action: IndicationsДокумент8 страницInjection, OTC Nasal Solution:: Generic Name: Action: IndicationsRonald Anthony TobiasОценок пока нет
- S 019 LBLДокумент6 страницS 019 LBLRa'fat RaheemОценок пока нет
- Generic Name: Indications: Adverse EffectsДокумент6 страницGeneric Name: Indications: Adverse EffectsJaysellePuguonTabijeОценок пока нет
- A Drug Study On: PhenylephrineДокумент6 страницA Drug Study On: PhenylephrineAlexandrea MayОценок пока нет
- EpinephrineДокумент1 страницаEpinephrineRaymundo MalijanОценок пока нет
- DRUG STUDY FlupentixolДокумент1 страницаDRUG STUDY FlupentixolLeris Luigi VictorioОценок пока нет
- EpinephrineДокумент7 страницEpinephrineFlorence NightingaleОценок пока нет
- Ipratroprium Drug CardДокумент3 страницыIpratroprium Drug CardXiaoDuckyОценок пока нет
- Aerovent, Apovent Atronase, Ipraxa, Ipvent Rhinovent, Rinatecrinovagos, Atrovent, Atrovent HfaДокумент4 страницыAerovent, Apovent Atronase, Ipraxa, Ipvent Rhinovent, Rinatecrinovagos, Atrovent, Atrovent HfaGwyn RosalesОценок пока нет
- DuaventДокумент2 страницыDuaventKristine YoungОценок пока нет
- CRITICAL THINKING CASE SCENARIO - Pharmacology - Module 3Документ4 страницыCRITICAL THINKING CASE SCENARIO - Pharmacology - Module 3Windi Dawn SallevaОценок пока нет
- Refractory Anaphylaxis, Treatment Algorithm.2021Документ3 страницыRefractory Anaphylaxis, Treatment Algorithm.2021Patricia AndradeОценок пока нет
- FluphenazineДокумент2 страницыFluphenazineMega AnandaОценок пока нет
- Drug Study inДокумент3 страницыDrug Study inaycee0316Оценок пока нет
- Shared AirwayДокумент5 страницShared AirwayVictoria MedfordОценок пока нет
- South Davao DsДокумент2 страницыSouth Davao DsJaneenne Fe Nicole SilvanoОценок пока нет
- Adrenergic Drugs Affecting The Autonomic Nervous System Adrenergic Drugs Affecting The Autonomic Nervous SystemДокумент11 страницAdrenergic Drugs Affecting The Autonomic Nervous System Adrenergic Drugs Affecting The Autonomic Nervous SystemApple MaeОценок пока нет
- Indications and Dosages Contraindications and Cautions Adverse Reactions Nursing Considerations Patient TeachingДокумент9 страницIndications and Dosages Contraindications and Cautions Adverse Reactions Nursing Considerations Patient TeachingJeunice WeeОценок пока нет
- Emergency DrugsДокумент19 страницEmergency DrugsMean Elepaño50% (2)
- Use During Pregnancy and LactationДокумент2 страницыUse During Pregnancy and LactationYdha FpОценок пока нет
- Drugs For Eye and Ear DisordersДокумент4 страницыDrugs For Eye and Ear DisordersApple MaeОценок пока нет
- Br. J. Anaesth.-2012-Perks-562-71 - Anesthesia and EpilepsyДокумент10 страницBr. J. Anaesth.-2012-Perks-562-71 - Anesthesia and EpilepsyGustavo Viveros MОценок пока нет
- BOMATДокумент1 страницаBOMATDian NovitasariОценок пока нет
- CefuroximeДокумент11 страницCefuroximeAlmira Ballesteros CestonaОценок пока нет
- Pethidine HydrochlorideДокумент3 страницыPethidine HydrochlorideIcee SinlapasertОценок пока нет
- OptalginДокумент2 страницыOptalginanon_813207394Оценок пока нет
- AdrenalineДокумент13 страницAdrenalineMobahil AhmadОценок пока нет
- Drug Study (Epinephrine, Lidocaine, Diazepam)Документ6 страницDrug Study (Epinephrine, Lidocaine, Diazepam)Abigaile Operiano100% (2)
- Brufen Cold: Film-Coated TabletsДокумент1 страницаBrufen Cold: Film-Coated TabletsGirish ThakkarОценок пока нет
- PharmaДокумент20 страницPharmaMary Roan RonatoОценок пока нет
- Doxophylline Prescribing InformationДокумент1 страницаDoxophylline Prescribing InformationMohammed shamiul ShahidОценок пока нет
- 24 Emergency DrugsДокумент7 страниц24 Emergency DrugsApple BelicanОценок пока нет
- Adrenergic NS 2Документ43 страницыAdrenergic NS 2Abdullah Muhammed khaleel HassanОценок пока нет
- Pharmacology SammuryДокумент4 страницыPharmacology Sammuryضبيان فرحانОценок пока нет
- Drugs StudyДокумент6 страницDrugs StudyMark_Rebibis_8528Оценок пока нет
- Paracetamol (Alvedon) Acetylcysteine Fluimucil KCL (Kalium Durule)Документ1 страницаParacetamol (Alvedon) Acetylcysteine Fluimucil KCL (Kalium Durule)Jesse James Advincula EdjecОценок пока нет
- Naprex Drug StudyДокумент3 страницыNaprex Drug StudyAngelica shane NavarroОценок пока нет
- Fluticasone Propionate Aqueous Nasal Spray in The Treatment of Nasal PolyposisДокумент7 страницFluticasone Propionate Aqueous Nasal Spray in The Treatment of Nasal PolyposisAnonymous 5K38SwLОценок пока нет
- Cabasis Drug StudyДокумент3 страницыCabasis Drug StudyNick James CabasisОценок пока нет
- PhenytoinДокумент5 страницPhenytoinRhawnie B. GibbsОценок пока нет
- DiphenhydramineДокумент9 страницDiphenhydramineFhey Bernadette BeltranОценок пока нет
- Drug File SupriyaДокумент36 страницDrug File SupriyaSalim MinjОценок пока нет
- Fluphenazine Hydrochloride Drug StudyДокумент4 страницыFluphenazine Hydrochloride Drug Studyshadow gonzalezОценок пока нет
- NifedipineДокумент3 страницыNifedipineNovi YulianaОценок пока нет
- Flupentixol Drug StudyДокумент7 страницFlupentixol Drug Studyshadow gonzalezОценок пока нет
- Drug StudyДокумент9 страницDrug StudyKate Chavez100% (3)
- EpinephrineДокумент1 страницаEpinephrineKathrina IoannouОценок пока нет
- HeparinДокумент4 страницыHeparinTri Purma SariОценок пока нет
- Diphenhydramine Drug TabulationДокумент2 страницыDiphenhydramine Drug TabulationMeriyah EdzyleОценок пока нет
- Epinephrine Drug StudyДокумент7 страницEpinephrine Drug StudyJhoy Iris SarangayaОценок пока нет
- Drug Study - Salbutamol + IpratropiumДокумент4 страницыDrug Study - Salbutamol + IpratropiumJade Mikaela0% (1)
- Drug StudyДокумент5 страницDrug StudyYape AiraОценок пока нет
- Pulmonary Drugs For Nursing PharmacologyДокумент1 страницаPulmonary Drugs For Nursing Pharmacologylhayes123475% (4)
- Anaphylaxis: A Practical GuideОт EverandAnaphylaxis: A Practical GuideAnne K. EllisОценок пока нет
- Synthesis of The Key Intermediate of Isavuconazonium SulfateДокумент19 страницSynthesis of The Key Intermediate of Isavuconazonium SulfateSam SonОценок пока нет
- A Survey On Channel Estimation and Practical Passive Beamforming Design For Intelligent Reflecting Surface Aided Wireless CommunicationsДокумент37 страницA Survey On Channel Estimation and Practical Passive Beamforming Design For Intelligent Reflecting Surface Aided Wireless CommunicationsSam SonОценок пока нет
- E1052 FullДокумент16 страницE1052 FullSam SonОценок пока нет
- United States Patent (19) : Dec. 15, 1987 11. Patent Number: 45 Date of PatentДокумент8 страницUnited States Patent (19) : Dec. 15, 1987 11. Patent Number: 45 Date of PatentSam SonОценок пока нет
- Human Mutation - 2020 - McCormick - Specifications of The ACMG AMP Standards and Guidelines For Mitochondrial DNA VariantДокумент30 страницHuman Mutation - 2020 - McCormick - Specifications of The ACMG AMP Standards and Guidelines For Mitochondrial DNA VariantSam SonОценок пока нет
- Analysis of Paracetamol - AgilentДокумент7 страницAnalysis of Paracetamol - AgilentSam SonОценок пока нет
- Analysis of Pharmaceuticals and Drug Related Impurities Using Agilent InstrumentationДокумент582 страницыAnalysis of Pharmaceuticals and Drug Related Impurities Using Agilent InstrumentationSam SonОценок пока нет
- Complexity of Coupled Human and Natural SystemsДокумент5 страницComplexity of Coupled Human and Natural SystemsSam SonОценок пока нет
- Corruption and Business EthicsДокумент21 страницаCorruption and Business EthicsSam SonОценок пока нет
- Chemo-Catalytic Esterification and Transesterification Over Organic Polymer-Based Catalysts For Biodiesel SynthesisДокумент14 страницChemo-Catalytic Esterification and Transesterification Over Organic Polymer-Based Catalysts For Biodiesel SynthesisSam SonОценок пока нет
- Final Report of The Amended Safety Assessment of Glyceryl LaurateДокумент40 страницFinal Report of The Amended Safety Assessment of Glyceryl LaurateSam SonОценок пока нет
- Parallel Accumulation For 100% Duty Cycle Trapped Ion Mobility-MassspectrometrДокумент8 страницParallel Accumulation For 100% Duty Cycle Trapped Ion Mobility-MassspectrometrSam SonОценок пока нет
- Eur J Immunol - 2021 - Fink - Immunity in Acute Myeloid Leukemia Where The Immune Response and Targeted Therapy MeetДокумент10 страницEur J Immunol - 2021 - Fink - Immunity in Acute Myeloid Leukemia Where The Immune Response and Targeted Therapy MeetSam SonОценок пока нет
- PLS-SEM: Prediction-Oriented Solutions For HRD ResearchersДокумент19 страницPLS-SEM: Prediction-Oriented Solutions For HRD ResearchersSam SonОценок пока нет
- IP India - Patent Process - Flow ChartДокумент1 страницаIP India - Patent Process - Flow ChartSam SonОценок пока нет
- Hypertrophic CardiomyopathyДокумент10 страницHypertrophic CardiomyopathySam SonОценок пока нет
- Piperaquine: A Resurgent Antimalarial DrugДокумент13 страницPiperaquine: A Resurgent Antimalarial DrugSam SonОценок пока нет
- 10 1080@03007995 2018 1425674 PDFДокумент35 страниц10 1080@03007995 2018 1425674 PDFSam SonОценок пока нет
- On English Translation of Infant Tuina Points in Traditional Chinese MedicineДокумент3 страницыOn English Translation of Infant Tuina Points in Traditional Chinese MedicineSam SonОценок пока нет
- Tine AДокумент6 страницTine AMemo AliОценок пока нет
- Cord Presentation and ProlapseДокумент15 страницCord Presentation and ProlapsechandanОценок пока нет
- Care of Patient On VentilatorДокумент18 страницCare of Patient On VentilatorJose Paul Rader100% (1)
- Current Concepts of Damage Control Resuscitation and Damage Control SurgeryДокумент52 страницыCurrent Concepts of Damage Control Resuscitation and Damage Control SurgerydewiswahyuОценок пока нет
- 4ps of InnovationДокумент5 страниц4ps of InnovationAdrianProud100% (2)
- Resident Medical Officer KAmL4W116erДокумент4 страницыResident Medical Officer KAmL4W116erAman TyagiОценок пока нет
- Advanced Homoeo PracticeДокумент74 страницыAdvanced Homoeo Practiceom1kareshwari75% (4)
- Marieb ch11bДокумент28 страницMarieb ch11bapi-229554503Оценок пока нет
- ACL Reconstruction History and Current Concepts: Sean Hazzard, PA-C Massachusetts General Hospital Boston, MAДокумент7 страницACL Reconstruction History and Current Concepts: Sean Hazzard, PA-C Massachusetts General Hospital Boston, MAana starcevicОценок пока нет
- Ebook Chemical Engineering Journal Vol 155 Issues 1-2 2009 ISSN.1386-8947Документ19 страницEbook Chemical Engineering Journal Vol 155 Issues 1-2 2009 ISSN.1386-8947ariyasti100% (1)
- OBGYN Form 3 AnswersДокумент2 страницыOBGYN Form 3 AnswersGrace0% (1)
- Who's Who in NLPДокумент10 страницWho's Who in NLPjan_modaaltjeОценок пока нет
- Drug CardsДокумент4 страницыDrug CardsBrittany Lynn MyersОценок пока нет
- Cardex Diet:: Date/Time Treatment IVF Labs ProceduresДокумент1 страницаCardex Diet:: Date/Time Treatment IVF Labs ProceduresWeng RamojalОценок пока нет
- Advice and Safe Practice For MicropigmentationДокумент41 страницаAdvice and Safe Practice For MicropigmentationAlina PiscanuОценок пока нет
- Herbal Drugs Used in AsthmaДокумент24 страницыHerbal Drugs Used in AsthmaRajesh Dholpuria100% (2)
- Annoted Bibliography ObesityДокумент8 страницAnnoted Bibliography Obesityapi-451532782Оценок пока нет
- Desordenes Gastrointestinales Con La Edad 2019Документ20 страницDesordenes Gastrointestinales Con La Edad 2019Tabatha Velarde ValenciaОценок пока нет
- Mcqs 11Документ18 страницMcqs 11Muhammad JavedОценок пока нет
- Dialog EvolutionДокумент2 страницыDialog EvolutionMaxence Kouessi100% (2)
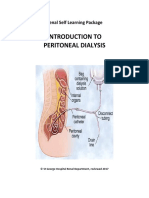
- Introduction To Peritoneal Dialysis: Renal Self Learning PackageДокумент12 страницIntroduction To Peritoneal Dialysis: Renal Self Learning PackageArun PatilОценок пока нет
- Bionator - ApuravaДокумент307 страницBionator - ApuravaApurava Singh100% (3)
- Myron Spector - Biomaterials-Based Tissue Engineering and Regenerative Medicine Solutions To Musculoskeletal ProblemsДокумент10 страницMyron Spector - Biomaterials-Based Tissue Engineering and Regenerative Medicine Solutions To Musculoskeletal ProblemsHutsDMОценок пока нет
- The Outcome Rating Scale ArticleДокумент24 страницыThe Outcome Rating Scale ArticleAdhie DarmawanОценок пока нет
- Master Diploma in Acupressure Therapy (M.D.Acu.) Attempt Any Ten Questions All Questions Carry Equal Marks Maximum Marks - 100Документ1 страницаMaster Diploma in Acupressure Therapy (M.D.Acu.) Attempt Any Ten Questions All Questions Carry Equal Marks Maximum Marks - 100shabanaОценок пока нет
- Dietary Diversity Score and Associated Factors Among High School Adolescent Girls in Gurage Zone, Southwest EthiopiaДокумент5 страницDietary Diversity Score and Associated Factors Among High School Adolescent Girls in Gurage Zone, Southwest EthiopiaFadhila NurrahmaОценок пока нет
- Five To 10-Year Followup of Open Partial Nephrectomy in A Solitary KidneyДокумент5 страницFive To 10-Year Followup of Open Partial Nephrectomy in A Solitary KidneyMihaela LitovcencoОценок пока нет
- Treatment of Class II MalocclusionДокумент162 страницыTreatment of Class II Malocclusiongoyal8492% (13)
- Diagnostic and Therapeutic Approaches To HepatocelДокумент5 страницDiagnostic and Therapeutic Approaches To HepatocelrahmaОценок пока нет
- Goat MilkДокумент39 страницGoat Milkinkpen56789Оценок пока нет