Академический Документы
Профессиональный Документы
Культура Документы
Vapor and Liquid Equilibrium of Ethanol-Water System
Загружено:
AsilahОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Vapor and Liquid Equilibrium of Ethanol-Water System
Загружено:
AsilahАвторское право:
Доступные форматы
Chemical Engineering Laboratory Report
Heriot-Watt University Malaysia Campus
Stage 2
Chemical Engineering Laboratory Report
Name Liaw Li Ling
Group Number 3
Experiment Title Vapor and Liquid Equilibrium
Date of 30th January 2018
Experiment
Demonstrator Dr. Carynn
Date Created 2-Apr-18 Page 1 of 5
Chemical Engineering Laboratory Report
1. Aims & Objectives
The aim of this experiment is to study the binary system of ethanol-water
system at the atmospheric pressure. A certain amount of the mixture of ethanol
and water is initially fed into the evaporator. As the heat is switched on, the mixture
is heated up and started to boil. The heater consists of temperature controller to
make sure that the temperature will not exceed the set point value and it will turn
off as the temperature is exceeded. As the liquid mixture is boiled, the liquid is
changed into the vapor phase and the vapor is rose from the evaporator into the
condenser. The vapor is then condensed and turned into liquid phase again. The
phase equilibrium between vapor and liquid is reached at a certain point.
Moreover, the samples of vapor and liquid is collected and used to determine their
composition such as weightage and refractive index when the temperature is
constant at certain point.
The objective of this experiment is to construct an equilibrium curve for the
ethanol-water system at atmospheric pressure.
2. Derived Results & Discussion
Component A Component B
Name Ethanol Distilled Water
Density (g/mL) 0.789 1
Molecular Weight
46.07 18.02
(g/mol)
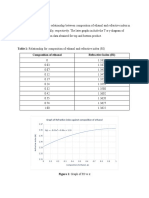
Table 1: Properties of the components used.
Mol Refractive
Volume of A (mL) Volume of B (mL)
Fraction Index (RI)
0 10 0.000 1.330
1 9 0.033 1.336
2 8 0.072 1.342
3 7 0.117 1.347
4 6 0.171 1.351
5 5 0.236 1.356
6 4 0.316 1.358
7 3 0.419 1.361
8 2 0.552 1.362
9 1 0.735 1.363
10 0 1.000 1.364
Table 2: Calibration Data
Table 2 represents the calibration data for ethanol and distilled water with certain
volume. Refractive index of the ethanol-water mixture is determined. Refractive index
is important to determine the composition of ethanol.
Date Created 2-Apr-18 Page 2 of 5
Chemical Engineering Laboratory Report
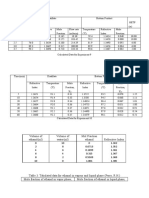
Vol. of Composition Refractive
Vol. of Temperature
Pressure Distilled (wt%) Index (RI)
Ethanol
(bar) Water
(L) Liquid Vapor Liquid Vapor Liquid Vapor
(L)
1 0 2.5 99.1 88.2 0.00 0.00 1.335 1.338
1 0.3 2.5 91.4 85.1 4.76 4.81 1.333 1.347
1 0.5 2.5 91.1 86.0 7.76 7.81 1.334 1.344
1 0.9 2.5 86.6 84.2 13.35 13.58 1.335 1.358
1 1.6 2.5 83.7 82.1 22.08 22.41 1.339 1.359
1 2.5 2.5 82.1 80.5 31.70 32.05 1.344 1.359
1 2.0 0.3 77.6 77.0 97.56 97.85 1.355 1.359
1 2.5 0.5 78.4 77.4 82.40 82.40 1.358 1.358
1 2.5 0.9 79.4 78.3 62.68 62.73 1.358 1.359
1 2.5 1.6 80.6 79.0 44.15 44.21 1.357 1.359
1 2.5 2.0 81.2 80.0 37.75 37.86 1.356 1.360
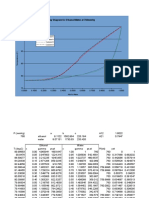
Table 3: Data For Equilibrium Curve at Atmospheric Pressure.
Vapour-Liquid Equilibrium Graph
120
Vapour Compostion Weightage (%)
100
80
60
40
20
0
0 20 40 60 80 100 120
Liquid Composition Weightage (%)
Graph 1: Vapour-Liquid Equilibrium Graph For Ethanol
Date Created 2-Apr-18 Page 3 of 5
Chemical Engineering Laboratory Report
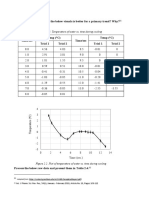
Temperature vs Vapour-Liquid
Equilibrium for Ethanol-Water System
120
100
Temperature (°C)
80
60
Liquid
Vapour
40
20
0
0 20 40 60 80 100 120
Liquid/Vapour Composition Weightage (%)
Graph 2: Vapour-Liquid Equilibrium Graph For Ethanol-Water System
In Thermodynamics, Vapour-liquid equilibrium (VLE) explains the distribution of
chemical species between the liquid phase and the gas phase. It is also a function of
total pressure of the conducted process. The concept of VLE is useful for chemical
engineers in fractional distillation design which is used to separate mixtures by
boiling and condensation process. This will eventually result in differences in
concentrations of components in liquid and vapour phases.
For this experiment, it is a mixture of ethanol and water where the system of this
mixture can be known as binary system of ethanol and water. For this experiment,
the graph of liquid and vapour composition weightage graph is plotted to determine
the equilibrium level of the system. As plotted, it shows a straight line which indicates
the vapour phase and the liquid phase of the ethanol is at equilibrium state.
The mole fraction for ethanol and water are determined by calculation by using
the density of each compound. The density of methanol is 0.789 g/cm³ while the
density of water is 1g/cm³. The mass of each compounds is then calculated with
density and volume used. The refractive index is then recorded at a constant interval
from both liquid and vapour samples to determine the composition of ethanol in liquid
and vapour phase.
Temperature-liquid-vapour graph is plotted (Graph 3) is plotted with the
liquid/vapour composition weightage. When the mole fraction of liquid ethanol
increases the mole fraction of vapor methanol increases as well.
Methanol is a volatile liquid. During the experiment, putting the ethanol in
the beaker without closing the beaker can lead to the leakage of the ethanol as
it vaporized slowly. The ethanol is added before the temperature heater is lower than
50°C. All this action is contributing to the error of this experiment where accurate
data is hard to achieve. The eyes were not perpendicular when recording the volume
of ethanol in the cylinder.
Date Created 2-Apr-18 Page 4 of 5
Chemical Engineering Laboratory Report
3. Summary of Findings
As the conclusion, this experiment is carried out successfully. The
relationship between the vapour and liquid at the normal pressure was
successfully determined. The objective of this experiment is to construct an
equilibrium curve for the ethanol-water system at atmospheric pressure. From
the result of this experiment, the composition of ethanol in vapor is higher
than the liquid. The composition of ethanol in vapor and liquid increases when
the volume of ethanol increases as well.
4. Appendix
Volume Mol Mol total Mol Fraction Refractive Index Weightage
Ethanol Water Ethanol Water Ethanol Water Liquid Vapor Liquid,x Vapor,y
0 2.5 0 138.7347 138.7347 0.00 1.00 1.335 1.338 0.00 0.00
0.3 2.5 5.137834 138.7347 143.8726 0.04 0.96 1.333 1.347 4.76 4.81
0.5 2.5 8.563056 138.7347 147.2978 0.06 0.94 1.334 1.344 7.76 7.81
0.9 2.5 15.4135 138.7347 154.1482 0.10 0.90 1.335 1.358 13.35 13.58
1.6 2.5 27.40178 138.7347 166.1365 0.16 0.84 1.339 1.359 22.08 22.41
2.5 2.5 42.81528 138.7347 181.55 0.24 0.76 1.344 1.359 31.70 32.05
2.5 0.3 42.81528 16.64817 59.46345 0.72 0.28 1.355 1.359 97.56 97.85
2.5 0.5 42.81528 27.74695 70.56223 0.61 0.39 1.358 1.358 82.40 82.40
2.5 0.9 42.81528 49.94451 92.75979 0.46 0.54 1.358 1.359 62.68 62.73
2.5 1.6 42.81528 88.79023 131.6055 0.33 0.67 1.357 1.359 44.15 44.21
2.5 2 42.81528 110.9878 153.8031 0.28 0.72 1.356 1.36 37.75 37.86
Date Created 2-Apr-18 Page 5 of 5
Вам также может понравиться
- Government Publications: Key PapersОт EverandGovernment Publications: Key PapersBernard M. FryОценок пока нет
- R Efracti Ve Index VS Compositi On Based On EthanolДокумент10 страницR Efracti Ve Index VS Compositi On Based On EthanolChemineer JanОценок пока нет
- Environmental Analysis and Technology for the Refining IndustryОт EverandEnvironmental Analysis and Technology for the Refining IndustryОценок пока нет
- VLE Curve PlotДокумент12 страницVLE Curve PlotNipun RustagiОценок пока нет
- CombinepdfДокумент17 страницCombinepdfNaresh GanisonОценок пока нет
- Results: Test Tube Volume of Ethanol (ML) Volume of Deionized-Water (ML) Mole Fraction of Ethanol Refractive Index (RI)Документ4 страницыResults: Test Tube Volume of Ethanol (ML) Volume of Deionized-Water (ML) Mole Fraction of Ethanol Refractive Index (RI)magОценок пока нет
- Distillation Lab ReportДокумент6 страницDistillation Lab ReportSОценок пока нет
- DistillationДокумент8 страницDistillationfarahalsayed64Оценок пока нет
- Binary System Thermo DyncamixДокумент19 страницBinary System Thermo DyncamixJohn Fritz FestejoОценок пока нет
- Lab Report CPP Exp 5Документ11 страницLab Report CPP Exp 5Salihah AbdullahОценок пока нет
- Batch Distillation: Camila Carvajal Paula Gutiérrez Sojo Karen RomeroДокумент12 страницBatch Distillation: Camila Carvajal Paula Gutiérrez Sojo Karen RomeroCamila CarvajalОценок пока нет
- Isobaric Vapor Liquid Equilibria of The Water 1-Propanol System at 30, 60, and 100 KpaДокумент5 страницIsobaric Vapor Liquid Equilibria of The Water 1-Propanol System at 30, 60, and 100 KpaRafael HenriqueОценок пока нет
- Task1: Prepare A Refractive Index (RI) Calibration Curve For Ethanol-Water Mixture With Varying MoleДокумент2 страницыTask1: Prepare A Refractive Index (RI) Calibration Curve For Ethanol-Water Mixture With Varying MoleKhar Lok LimОценок пока нет
- Experiment 3: Ternary Phase Diagram (Liquid-Liquid Extraction)Документ15 страницExperiment 3: Ternary Phase Diagram (Liquid-Liquid Extraction)Noor Nasuha Noor AriffinОценок пока нет
- TPP Exp 1Документ14 страницTPP Exp 1Ffmohamad NAdОценок пока нет
- Calculations and GraphsДокумент6 страницCalculations and GraphschaitanyaОценок пока нет
- CPP Exp 5Документ10 страницCPP Exp 5izzrilОценок пока нет
- Distillation ColumnДокумент6 страницDistillation ColumnArif HanafiОценок пока нет
- Distillation Report Group1 - 4DДокумент8 страницDistillation Report Group1 - 4D2023471822Оценок пока нет
- Ethylene Glycol HeatДокумент6 страницEthylene Glycol HeatVlad BalanОценок пока нет
- Esterification of EthanolДокумент15 страницEsterification of EthanolSadia HasanОценок пока нет
- Lab ReportДокумент7 страницLab ReportBilal AhmadОценок пока нет
- Zeszyt 1Документ8 страницZeszyt 1juzekОценок пока нет
- Lab Report - Distillation of Bubble CapДокумент21 страницаLab Report - Distillation of Bubble Capratish100% (1)
- Result and CalculationДокумент13 страницResult and CalculationgerrykhangОценок пока нет
- Water SupplyДокумент12 страницWater SupplyyusufОценок пока нет
- Esterification of Ethanol in A Batch Reactor in Presence of H2SO4Документ17 страницEsterification of Ethanol in A Batch Reactor in Presence of H2SO4MD.Khairul EducationОценок пока нет
- Interactive TD 280: Compensation of Oxygen MeasurementsДокумент17 страницInteractive TD 280: Compensation of Oxygen MeasurementsHariОценок пока нет
- Report - Cation ExchangerДокумент5 страницReport - Cation ExchangerMai HoangОценок пока нет
- Annexure B - Sucrose Conversion TableДокумент21 страницаAnnexure B - Sucrose Conversion TableLim Chen Gin100% (1)
- Vapor Liquid Equilibrium Ethanol WaterДокумент13 страницVapor Liquid Equilibrium Ethanol Waterhao GamesОценок пока нет
- Neutralizer ChartДокумент1 страницаNeutralizer ChartJUAN FELIPE ORTIZ PARRAОценок пока нет
- Cover Modul 8 (3 Files Merged)Документ9 страницCover Modul 8 (3 Files Merged)LilyОценок пока нет
- Refractive Index Calibration CurvesДокумент11 страницRefractive Index Calibration CurvesNeereishОценок пока нет
- Result Analysis Report: Um D (0.9) : 22.692 131.667 D (0.1) : Um Um 2.038 D (0.5)Документ1 страницаResult Analysis Report: Um D (0.9) : 22.692 131.667 D (0.1) : Um Um 2.038 D (0.5)Baher SaidОценок пока нет
- Flotation collector testing results for xanthate and derivativesДокумент11 страницFlotation collector testing results for xanthate and derivativesgerardo orrantiaОценок пока нет
- Cortante Basal Y Espectro De Diseño Nec 2015 Ubicación S. Estructural Suelo Opcupación Z (%g) Fa Fd Fs η r TДокумент13 страницCortante Basal Y Espectro De Diseño Nec 2015 Ubicación S. Estructural Suelo Opcupación Z (%g) Fa Fd Fs η r TLizz VillacresОценок пока нет
- Energy BalanceДокумент12 страницEnergy BalanceZain Ul AbedinОценок пока нет
- Lam C Fermen PenjunДокумент4 страницыLam C Fermen PenjunFrans ArapentaОценок пока нет
- Data Pengamatan ST FixДокумент7 страницData Pengamatan ST FixULWI ALIATUR ROHMAH -Оценок пока нет
- Data and CalculationsДокумент4 страницыData and CalculationsÖznur DuranОценок пока нет
- T-X-Y Diagram: Fraction of EthanolДокумент2 страницыT-X-Y Diagram: Fraction of EthanolmichsantosОценок пока нет
- Real Liquid Mixtures - Part 2: Cab 2023 Chemical Thermodynamic Tutorial NineДокумент2 страницыReal Liquid Mixtures - Part 2: Cab 2023 Chemical Thermodynamic Tutorial NineNaveed AhmadОценок пока нет
- Kurva Standarisasi Metanol: ρ destilat (gr/ml)Документ6 страницKurva Standarisasi Metanol: ρ destilat (gr/ml)dddinzОценок пока нет
- Table 2: Refractive Index For The Varying Mol Percentage of Methanol Ethanol Mol % Refractive Index Volume of Ethanol (ML)Документ5 страницTable 2: Refractive Index For The Varying Mol Percentage of Methanol Ethanol Mol % Refractive Index Volume of Ethanol (ML)brittanyОценок пока нет
- Determination of Enthalpy of Combustion Using Bomb CalorimeterДокумент6 страницDetermination of Enthalpy of Combustion Using Bomb CalorimeterMuhammad Hazim TararОценок пока нет
- Test 1 PDFДокумент5 страницTest 1 PDFFibo ForexОценок пока нет
- SPE 148269 EOS Modelling For Two Oils With High Concentration of Organic Sulphur - Case StudyДокумент11 страницSPE 148269 EOS Modelling For Two Oils With High Concentration of Organic Sulphur - Case StudyMuezzElerebyОценок пока нет
- Temperature vs Time Graph Better for Cooling TrendДокумент2 страницыTemperature vs Time Graph Better for Cooling TrendThương ĐơОценок пока нет
- Ethane-Propane Separation Process SimulationДокумент6 страницEthane-Propane Separation Process Simulationsalman hussainОценок пока нет
- Result Analysis Report: Um D (0.9) : 244.410 505.925 D (0.1) : Um Um 9.152 D (0.5)Документ1 страницаResult Analysis Report: Um D (0.9) : 244.410 505.925 D (0.1) : Um Um 9.152 D (0.5)Baher SaidОценок пока нет
- CaCO3 Particle Size Analysis ReportДокумент1 страницаCaCO3 Particle Size Analysis ReportBaher SaidОценок пока нет
- Ethylene Glycol HeatДокумент10 страницEthylene Glycol Heatdalton2004Оценок пока нет
- Isobaric Vapor-Liquid Equilibrium For The EtДокумент5 страницIsobaric Vapor-Liquid Equilibrium For The EtSergioSanabriaОценок пока нет
- Result Analysis Report: Um D (0.9) : 141.732 464.814 D (0.1) : Um Um 4.270 D (0.5)Документ1 страницаResult Analysis Report: Um D (0.9) : 141.732 464.814 D (0.1) : Um Um 4.270 D (0.5)Baher SaidОценок пока нет
- Thermodynamics of Hydrogen-Bonding Mixtures 2.GE, HE, and SE of 1-Propanol +n-HeptaneДокумент12 страницThermodynamics of Hydrogen-Bonding Mixtures 2.GE, HE, and SE of 1-Propanol +n-Heptanemurdanetap957Оценок пока нет
- Universiti Tunku Abdul Rahman (Utar) Faculty of Engineering and Green Technology (Fegt)Документ26 страницUniversiti Tunku Abdul Rahman (Utar) Faculty of Engineering and Green Technology (Fegt)khairulОценок пока нет
- Vapor Liquid ExperimentДокумент1 страницаVapor Liquid ExperimentantaraОценок пока нет
- Vapor Liquid Equilibrium and Raoults Law Solved ProblemsДокумент23 страницыVapor Liquid Equilibrium and Raoults Law Solved ProblemsGenius media networkОценок пока нет
- Experiment 9: Determination of Vapor-Liquid EquilibriumДокумент1 страницаExperiment 9: Determination of Vapor-Liquid EquilibriumAsilahОценок пока нет
- Txy Diagram For Ethanol/Water at 760MmhgДокумент2 страницыTxy Diagram For Ethanol/Water at 760MmhgAsilahОценок пока нет
- All The BestДокумент1 страницаAll The BestAsilahОценок пока нет
- Thank YouДокумент1 страницаThank YouAsilahОценок пока нет
- CHEM 254 Experiment 8 Phase Diagrams - Liquid Vapour Equilibrium For Two Component SolutionsДокумент6 страницCHEM 254 Experiment 8 Phase Diagrams - Liquid Vapour Equilibrium For Two Component SolutionsAsilahОценок пока нет
- Good MorningДокумент1 страницаGood MorningAsilahОценок пока нет
- Txy Diagram For Ethanol/Water at 760MmhgДокумент2 страницыTxy Diagram For Ethanol/Water at 760MmhgAsilahОценок пока нет
- CHEM 254 Experiment 8 Phase Diagrams - Liquid Vapour Equilibrium For Two Component SolutionsДокумент6 страницCHEM 254 Experiment 8 Phase Diagrams - Liquid Vapour Equilibrium For Two Component SolutionsAsilahОценок пока нет
- CHEM 254 Experiment 8 Phase Diagrams - Liquid Vapour Equilibrium For Two Component SolutionsДокумент6 страницCHEM 254 Experiment 8 Phase Diagrams - Liquid Vapour Equilibrium For Two Component SolutionsAsilahОценок пока нет
- Multiphase T2Документ5 страницMultiphase T2AsilahОценок пока нет
- Packed column pressure drop and flooding experimentДокумент4 страницыPacked column pressure drop and flooding experimentSantosh ReddyОценок пока нет
- Packed column pressure drop and flooding experimentДокумент4 страницыPacked column pressure drop and flooding experimentSantosh ReddyОценок пока нет
- DuluxGroup Sustainability Report 2015Документ8 страницDuluxGroup Sustainability Report 2015AsilahОценок пока нет
- Thank YouДокумент1 страницаThank YouAsilahОценок пока нет
- Effect of Drying Parameters On Physiochemical and Sensory Properties of Fruit Powders Processed by PGSS-, Vacuum-And Spray-DryingДокумент10 страницEffect of Drying Parameters On Physiochemical and Sensory Properties of Fruit Powders Processed by PGSS-, Vacuum-And Spray-DryingAsilahОценок пока нет
- Environmental and Health Risks of Rubber Infill Rubber Crumb From Car Tyres As Infill On Artificial TurfДокумент7 страницEnvironmental and Health Risks of Rubber Infill Rubber Crumb From Car Tyres As Infill On Artificial TurfAsilahОценок пока нет
- F18XD2 Solutions 7: Eigenvalues and EigenvectorsДокумент10 страницF18XD2 Solutions 7: Eigenvalues and EigenvectorsAsilahОценок пока нет
- Recycling Waste Tyres To Products PDFДокумент69 страницRecycling Waste Tyres To Products PDFAsilahОценок пока нет
- F18XD2 Solutions 5: Systems of Linear EquationsДокумент5 страницF18XD2 Solutions 5: Systems of Linear EquationsAsilahОценок пока нет
- A0017 Crude OilДокумент9 страницA0017 Crude OilAsilahОценок пока нет
- A0017 Crude OilДокумент9 страницA0017 Crude OilAsilahОценок пока нет
- Geometry SolutionsДокумент5 страницGeometry SolutionsAsilahОценок пока нет
- Greenfields (1996)Документ10 страницGreenfields (1996)AsilahОценок пока нет
- Solutions 2Документ5 страницSolutions 2AsilahОценок пока нет
- Shin Et Al (2003)Документ8 страницShin Et Al (2003)AsilahОценок пока нет
- Tutorial 3Документ3 страницыTutorial 3AsilahОценок пока нет
- H352 V4Документ11 страницH352 V4AsilahОценок пока нет
- Appendix A Useful Formulæ For F1.8XD2: Standard Derivatives: Standard IntegralsДокумент3 страницыAppendix A Useful Formulæ For F1.8XD2: Standard Derivatives: Standard IntegralsAsilahОценок пока нет
- Lab 10Документ1 страницаLab 10AsilahОценок пока нет
- GARLOCK Gasket SheetsДокумент59 страницGARLOCK Gasket SheetsdanianishОценок пока нет
- Pile CapДокумент27 страницPile CapHafeel Ahamed Ashraf AliОценок пока нет
- Racor Oil Filtration Hydraulic Filter Cart 7768Документ2 страницыRacor Oil Filtration Hydraulic Filter Cart 7768sinter-musicОценок пока нет
- 012.0 - Cat-6060 - Central Greasing System - AttachmentДокумент50 страниц012.0 - Cat-6060 - Central Greasing System - AttachmentJorby Cuadros100% (2)
- Autoscan P12 04 16 enДокумент12 страницAutoscan P12 04 16 enCouscoussiere GrandgazОценок пока нет
- LAB 2 Free N Force VibrationДокумент8 страницLAB 2 Free N Force Vibrationmohdanis53yahoocomОценок пока нет
- Kairos Thermo HF Natural Circulation Solar System for Domestic Hot Water ProductionДокумент4 страницыKairos Thermo HF Natural Circulation Solar System for Domestic Hot Water ProductionFILID MADОценок пока нет
- AERMOTOR Windmill Catalog Page50Документ1 страницаAERMOTOR Windmill Catalog Page50cristobal_tl2277Оценок пока нет
- Bearing Capacity of Rockspub101172495Документ13 страницBearing Capacity of Rockspub101172495Mehdi Mir100% (1)
- Wei Gong Lattice Tower Design of Offshore Wind Turbine Support Structures v0Документ135 страницWei Gong Lattice Tower Design of Offshore Wind Turbine Support Structures v0Sachin Sithik50% (2)
- Api Bottom Load ValveДокумент2 страницыApi Bottom Load ValveMạnh Ngô ĐứcОценок пока нет
- Pumps and SystemsДокумент5 страницPumps and SystemsDhi AdhiОценок пока нет
- Electronics 18 E0306Документ9 страницElectronics 18 E0306GiangDoОценок пока нет
- PAES 303-2000roller Chains and Sprockets For Agricultural Machines - SpecificДокумент30 страницPAES 303-2000roller Chains and Sprockets For Agricultural Machines - SpecificYanYan CustodioОценок пока нет
- Technical Recommendations For Choke Valve Specifications: ReferenceДокумент4 страницыTechnical Recommendations For Choke Valve Specifications: ReferencejowarОценок пока нет
- Structural Reliability FrameworkДокумент65 страницStructural Reliability FrameworkkhairurОценок пока нет
- Practice Problems - Breath Section PDFДокумент68 страницPractice Problems - Breath Section PDFnickОценок пока нет
- Salford Journal of Bridge Engineering Jan-18Документ24 страницыSalford Journal of Bridge Engineering Jan-18Swaminathan VivekananthamОценок пока нет
- Hydcat 2005Документ68 страницHydcat 2005Amit GunjalОценок пока нет
- VXN150 Vixion Radiator & Hose PDFДокумент1 страницаVXN150 Vixion Radiator & Hose PDFHarris Jum'aniandaОценок пока нет
- WinPet BrochureДокумент2 страницыWinPet BrochureTamil KumarОценок пока нет
- EmДокумент6 страницEmGonzalo Antonio Mamani PayeОценок пока нет
- Sachin Solanki CNC/IPC/13 Summary SupportДокумент61 страницаSachin Solanki CNC/IPC/13 Summary Supportshivam modanwalОценок пока нет
- Yfm35Fgx Yfm35Fax Yfm35Fgx Yfm35Fgx: (5UHL) (5UHM) (5UHN) (5UHP)Документ0 страницYfm35Fgx Yfm35Fax Yfm35Fgx Yfm35Fgx: (5UHL) (5UHM) (5UHN) (5UHP)Cristiano Marcelo Oliveira MelloОценок пока нет
- Vibrational Analysis of Machine Foundation ACI 351.3R-04 (FEA Method)Документ9 страницVibrational Analysis of Machine Foundation ACI 351.3R-04 (FEA Method)Randy CernaОценок пока нет
- Fluid Mechanics Test and Exam DetailsДокумент8 страницFluid Mechanics Test and Exam DetailsDiego FungОценок пока нет
- V2607-DI-T-E3B: Kubota 07 SeriesДокумент2 страницыV2607-DI-T-E3B: Kubota 07 SeriesRodrigoThuLokithoPkmz0% (1)
- Fluid Machinery Syllabus PDFДокумент3 страницыFluid Machinery Syllabus PDFBajajОценок пока нет
- Mini Fragment Implants and InstrumentsДокумент13 страницMini Fragment Implants and InstrumentsMarc KleinОценок пока нет
- Parts of A CarДокумент5 страницParts of A CarMaria MolinaОценок пока нет