Академический Документы
Профессиональный Документы
Культура Документы
Acid Dew Point
Загружено:
ankur20610 оценок0% нашли этот документ полезным (0 голосов)
58 просмотров1 страницаThe document provides equations to calculate the dew point temperature of several acids - sulfuric acid, sulfurous acid, hydrochloric acid, and nitric acid - given the partial pressures of water and the acid in question. It also gives an example calculation using the hydrochloric acid dew point equation and composition data from a flue gas stream, determining the dew point temperature is 45.6°C.
Исходное описание:
Calculate Acid Dew Point of Flue Gases
Авторское право
© © All Rights Reserved
Доступные форматы
XLSX, PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документThe document provides equations to calculate the dew point temperature of several acids - sulfuric acid, sulfurous acid, hydrochloric acid, and nitric acid - given the partial pressures of water and the acid in question. It also gives an example calculation using the hydrochloric acid dew point equation and composition data from a flue gas stream, determining the dew point temperature is 45.6°C.
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате XLSX, PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
58 просмотров1 страницаAcid Dew Point
Загружено:
ankur2061The document provides equations to calculate the dew point temperature of several acids - sulfuric acid, sulfurous acid, hydrochloric acid, and nitric acid - given the partial pressures of water and the acid in question. It also gives an example calculation using the hydrochloric acid dew point equation and composition data from a flue gas stream, determining the dew point temperature is 45.6°C.
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате XLSX, PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 1
Sulfuric acid (H2SO4) dew point: [5] [6]
(1)1000/T=1.7842−0.0269log10(PH2O)−0.1029log10(PSO3)+0.0329log10(PH2O)log10(PSO3)
or this equivalent form: [7] [8]
(2)1000/T=2.276−0.02943loge(PH2O)−0.0858loge(PSO3)+0.0062loge(PH2O)loge(PSO3)
Sulfurous acid (H2SO3) dew point: [9] [10]
(3)1000/T=3.9526−0.1863loge(PH2O)+0.000867loge(PSO2)+0.000913loge(PH2O)loge(PSO2)
Hydrochloric acid (HCl) dew point: [11] [12]
(4)1000/T=3.7368−0.1591loge(PH2O)−0.0326loge(PHCl)+0.00269loge(PH2O)loge(PHCl)
Nitric acid (HNO3) dew point: [13]
(5)1000/T=3.6614−0.1446loge(PH2O)−0.0827loge(PHNO3)+0.00756loge(PH2O)loge(PHNO3)
where:
T = The acid dew point temperature for the indicated acid, in kelvins
P = Partial pressure, in atmospheres for equation 1 and in mmHg for equations 2, 3, 4 and 5
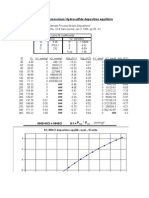
RIL Incinerator with HCl Acid Gas
Flue Gas Pressure: 752.50 mmHgA
Flue Gas Vol % Mole % Mol fr. Partial Pr,
mmHgA
CO2 5.79 5.79 0.0579 43.56975
H2O 8.17 8.17 0.0817 61.47925
O2 10.74 10.74 0.1074 80.8185
N2 75.29 75.29 0.7529 566.5573
HCl 0.01 0.01 0.0001 0.07525
100.00 100.00 1 752.5
T= 318.715 K
45.6 ⁰C
Вам также может понравиться
- Time Dependent Gas Leak Outflow Through HoleДокумент14 страницTime Dependent Gas Leak Outflow Through Holeankur2061Оценок пока нет
- Estimation of Saturated Liquid DensityДокумент3 страницыEstimation of Saturated Liquid Densityankur2061Оценок пока нет
- Chemical Process Design and Simulation: Aspen Plus and Aspen Hysys ApplicationsОт EverandChemical Process Design and Simulation: Aspen Plus and Aspen Hysys ApplicationsРейтинг: 2 из 5 звезд2/5 (1)
- Basic Surge Control System: FCV CoolerДокумент2 страницыBasic Surge Control System: FCV Coolerankur2061Оценок пока нет
- International Thermodynamic Tables of the Fluid State: Propylene (Propene)От EverandInternational Thermodynamic Tables of the Fluid State: Propylene (Propene)Оценок пока нет
- New CalculationДокумент9 страницNew CalculationDeepak Shakya100% (1)
- GRND LVL Conc of Unburned Flammable Gas Joe Wong CheckedДокумент1 страницаGRND LVL Conc of Unburned Flammable Gas Joe Wong Checkedankur2061Оценок пока нет
- Jet Venturi Fume ScrubbersДокумент7 страницJet Venturi Fume ScrubbersihllhmОценок пока нет
- Pilot Valve SizingДокумент4 страницыPilot Valve SizingJason ThomasОценок пока нет
- Acid Dew Point Calculation For CFB Boilers Parameters Formula Value UnitsДокумент2 страницыAcid Dew Point Calculation For CFB Boilers Parameters Formula Value UnitsUsman NaseemОценок пока нет
- Vessel Analytical Calculation For:: InputsДокумент2 страницыVessel Analytical Calculation For:: InputsmakamahamisuОценок пока нет
- Flare Stack - FinalДокумент7 страницFlare Stack - FinalIoana Popescu0% (1)
- Comparison DGF and IGF UnitsДокумент1 страницаComparison DGF and IGF Unitsankur2061Оценок пока нет
- Determine Compressor Settling-Out Conditions For Recycle Gas Loop DesignДокумент6 страницDetermine Compressor Settling-Out Conditions For Recycle Gas Loop DesignMoutushi BhowmikОценок пока нет
- Training CaseДокумент15 страницTraining CaseThái Xuân QuangОценок пока нет
- Simple ORC Model SQ110918Документ9 страницSimple ORC Model SQ110918radanpetricaОценок пока нет
- Ammonia Absorber CalculationДокумент5 страницAmmonia Absorber CalculationKvspavan KumarОценок пока нет
- Compressibility Z FactorДокумент14 страницCompressibility Z FactorosbertodiazОценок пока нет
- Bubble and Dew Point Calculations in Multicomponent and Multireactive MixturesДокумент9 страницBubble and Dew Point Calculations in Multicomponent and Multireactive MixturesJack CheeОценок пока нет
- Flare RadiationДокумент27 страницFlare RadiationgrabettyОценок пока нет
- Z - Peng RobinsonДокумент1 страницаZ - Peng RobinsonMuhammadTanzeeLUsmanОценок пока нет
- Placement Summary For The Year 2018 - 2019 Department of Chemical Engineering - UGДокумент5 страницPlacement Summary For The Year 2018 - 2019 Department of Chemical Engineering - UGBubbleОценок пока нет
- Dehydration Unit Sizing Chart - 12-13-13Документ1 страницаDehydration Unit Sizing Chart - 12-13-13Andres Crucetta100% (1)
- Process Calculation - Purge Gas CalculationДокумент1 страницаProcess Calculation - Purge Gas CalculationmakamahamisuОценок пока нет
- Gpa Standard 2145 09Документ4 страницыGpa Standard 2145 09xjaf01Оценок пока нет
- Boudouard ReactionДокумент5 страницBoudouard ReactionHailey17100% (1)
- Brill Beggs ZДокумент3 страницыBrill Beggs ZFariz AdriansyahОценок пока нет
- PSV Sizing - Specially For The Boiler CaseДокумент1 страницаPSV Sizing - Specially For The Boiler Casemaurya888Оценок пока нет
- Wu NH3 Salt DepositionsДокумент6 страницWu NH3 Salt DepositionsRSGatesОценок пока нет
- Two Phase Flow RegimeДокумент8 страницTwo Phase Flow RegimeNoman Abu-FarhaОценок пока нет
- Acid Dew Point Calculation SpreadsheetДокумент2 страницыAcid Dew Point Calculation Spreadsheetunknown8787100% (1)
- Shell K.O.drum SeparatorДокумент11 страницShell K.O.drum SeparatorChitu Ionut LaurentiuОценок пока нет
- Packed Fluid BedДокумент26 страницPacked Fluid BedgeogeogeoОценок пока нет
- Heatbalcalc: A Heat Balance Calculator For An Aluminum Heating ProcessДокумент35 страницHeatbalcalc: A Heat Balance Calculator For An Aluminum Heating ProcessRana BiswasОценок пока нет
- Spray Nozzles Total STDДокумент3 страницыSpray Nozzles Total STDDylan RamasamyОценок пока нет
- Gas Velocity CalculatorДокумент10 страницGas Velocity CalculatorTran Van ThanhОценок пока нет
- Two-Phase Flow Pressure DropДокумент4 страницыTwo-Phase Flow Pressure DropStevenMvuyana100% (1)
- Standard Practice For Calculating Heat Value, Compressibility Factor and Relative Density of Gaseous FuelsДокумент10 страницStandard Practice For Calculating Heat Value, Compressibility Factor and Relative Density of Gaseous FuelsChikkam Sathi Raju100% (1)
- PSV Sizing - Non Ideal GasesДокумент1 страницаPSV Sizing - Non Ideal GasesSaeid Rahimi MofradОценок пока нет
- Bubble and Dew PointДокумент6 страницBubble and Dew PointMawaddah Nur TambakОценок пока нет
- Basicflowmeasurement 150428100633 Conversion Gate02 PDFДокумент50 страницBasicflowmeasurement 150428100633 Conversion Gate02 PDFankur2061Оценок пока нет
- Peng Robinson MixturesДокумент1 страницаPeng Robinson MixturesdckristantoОценок пока нет
- Doc. Calculation Orifice PlateДокумент6 страницDoc. Calculation Orifice PlatejamesrickynОценок пока нет
- Flame ArresterДокумент2 страницыFlame ArresterAariz KhanОценок пока нет
- Study of Vapour Absorption System Using Waste Heat-F0283439Документ6 страницStudy of Vapour Absorption System Using Waste Heat-F0283439Anonymous NGXdt2BxОценок пока нет
- Critical Property CorrelationsДокумент16 страницCritical Property Correlations李天Оценок пока нет
- PSV Sizing2Документ3 страницыPSV Sizing2pavanОценок пока нет
- Estimate Subsonic Flare Tip Pressure Drop With Graph Derived CorrelationДокумент3 страницыEstimate Subsonic Flare Tip Pressure Drop With Graph Derived CorrelationbtjajadiОценок пока нет
- Tank Venting Capacity-Fire CaseДокумент1 страницаTank Venting Capacity-Fire CaseAjay TiwariОценок пока нет
- Makalah 4.19 EditedДокумент22 страницыMakalah 4.19 EditedRana Rezeki Najeges100% (1)
- 69-Chiara Gilardi Foster Wheeler Italiana-Relief-EДокумент16 страниц69-Chiara Gilardi Foster Wheeler Italiana-Relief-EKamil MarszałekОценок пока нет
- 1055 Crude Stailization Systems-SperoidsДокумент0 страниц1055 Crude Stailization Systems-SperoidsgshdavidОценок пока нет
- Steam VentДокумент4 страницыSteam VentShameer MajeedОценок пока нет
- Isobutane Butane Fractionator PDFДокумент7 страницIsobutane Butane Fractionator PDFhoustonmathОценок пока нет
- Theol Heat Transfer FluidДокумент3 страницыTheol Heat Transfer Fluidgautam_96948069Оценок пока нет
- Title of ExperimentДокумент16 страницTitle of ExperimentLi Xian YongОценок пока нет
- Vap. Pressure of Diphenylene OxideДокумент4 страницыVap. Pressure of Diphenylene OxidePratik WadherОценок пока нет
- Glycolysis PET WasteДокумент13 страницGlycolysis PET Wasteankur2061Оценок пока нет
- Instrument Process Datasheet ChecklistДокумент1 страницаInstrument Process Datasheet Checklistankur2061Оценок пока нет
- Protectoseal Emergency Vent Application Worksheet: Service ConditionsДокумент1 страницаProtectoseal Emergency Vent Application Worksheet: Service Conditionsankur2061Оценок пока нет
- Tank Steam Heating Overall HTC Heavy FOДокумент2 страницыTank Steam Heating Overall HTC Heavy FOankur2061Оценок пока нет
- High Purity Oxygen ( 99.5%) Production Using Vacuum Pressure Swing Adsorption (VPSA)Документ2 страницыHigh Purity Oxygen ( 99.5%) Production Using Vacuum Pressure Swing Adsorption (VPSA)ankur2061Оценок пока нет
- P&ID Check ListДокумент3 страницыP&ID Check Listankur2061Оценок пока нет
- Equipment Process Data Sheet ChecklistДокумент1 страницаEquipment Process Data Sheet Checklistankur2061100% (1)
- Characterization of C6 Plus HCs in Natural GasДокумент6 страницCharacterization of C6 Plus HCs in Natural Gasankur2061Оценок пока нет
- General Guiidelines For Precommissioning and Commissioning A Chemical Process PlantДокумент2 страницыGeneral Guiidelines For Precommissioning and Commissioning A Chemical Process Plantankur2061100% (1)
- PFD Check ListДокумент2 страницыPFD Check Listankur2061Оценок пока нет
- 6th Central Pay Commission Salary CalculatorДокумент15 страниц6th Central Pay Commission Salary Calculatorrakhonde100% (436)
- Centrifugal Compressor Calculations: Suction Discharge Input ParametersДокумент1 страницаCentrifugal Compressor Calculations: Suction Discharge Input Parametersankur20610% (1)
- Burner Management System PresentationДокумент63 страницыBurner Management System Presentationankur2061100% (1)
- Basicflowmeasurement 150428100633 Conversion Gate02 PDFДокумент50 страницBasicflowmeasurement 150428100633 Conversion Gate02 PDFankur2061Оценок пока нет
- Knowledge Sharing SeriesДокумент12 страницKnowledge Sharing Seriesankur2061Оценок пока нет
- Design of Hoppers Using Spreadsheet: Journal of Agricultural Engineering Research January 2010Документ7 страницDesign of Hoppers Using Spreadsheet: Journal of Agricultural Engineering Research January 2010ankur2061Оценок пока нет
- Machine Reliability in Parallel Operations: Re (%) 100 PF Liability PДокумент4 страницыMachine Reliability in Parallel Operations: Re (%) 100 PF Liability Pankur2061Оценок пока нет
- Designing and Testing of TurboexpanderДокумент44 страницыDesigning and Testing of Turboexpanderankur2061Оценок пока нет
- Heat Transfer and Energy Loss in Bitumen Batching SystemДокумент9 страницHeat Transfer and Energy Loss in Bitumen Batching Systemankur2061Оценок пока нет
- A Loop Thermosyphon For Asphalt Tank HeatingДокумент6 страницA Loop Thermosyphon For Asphalt Tank Heatingankur2061Оценок пока нет
- Design Guidelines For Shell N Tube Exchs For High Viscosity FluidsДокумент2 страницыDesign Guidelines For Shell N Tube Exchs For High Viscosity Fluidsankur2061Оценок пока нет
- Phases of Process DesignДокумент18 страницPhases of Process Designankur2061Оценок пока нет