Академический Документы
Профессиональный Документы
Культура Документы
Jrrysn
Загружено:
Eureca ParraИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Jrrysn
Загружено:
Eureca ParraАвторское право:
Доступные форматы
Orpilla, Jerryson M.
BSChE-5
2.15. Eadie (1942) measured the initial reaction rate of hydrolysis of acetylcholine (substrate) by
dog serum (source of enzyme) in the absence and presence of prostigmine (inhibitor), 1.5 x 10-7
mol/L and obtained the following data:
Substrate Initial Reaction Rate Rate (mol/L-min)
Cs/r Cs/r
Concentration, Cs Absence of Presence of
(Uninhibited) (Inhibited)
(mol/L) Prostigmine Prostigmine
0.0032 0.111 0.059 0.028828829 0.05423729
0.0049 0.148 0.071 0.033108108 0.06901408
0.0062 0.143 0.091 0.043356643 0.06813187
0.008 0.166 0.111 0.048192771 0.07207207
0.0095 0.2 0.125 0.0475 0.076
a. Is prostigmine competitive or noncompetitive inhibitor?
b. Evaluate the Michaelis-Menten kinetic parameters in the presence of inhibitor by employing
the Langmuir Plot.
Answers:
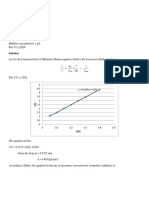
Langmuir Plot of Hydrolysis of Acetylcholine
0.09
0.08 y = 2.9883x + 0.0489
0.07
0.06
0.05 y = 3.3133x + 0.0191
Cs/r 0.04
0.03
0.02
-KMI -KM
0.01
0
-0.02 -0.015 -0.01 -0.005 0 0.005 0.01 0.015
-0.01
Cs
Without Inhibitor With Inhibitor
a. Based from the graph, the values of Km with and without the presence of inhibitor is not
equal. Therefore, prostigmine is a competitive inhibitor.
1|Biochemical Engineering 10/02/2017
Orpilla, Jerryson M. BSChE-5
b. Langmuir Equation (With inhibitor):
Cs 1 K
Cs m
r rmax rmax
y 2.9883x 0.0489
1
2.9883
rmax
mol
rmax 0.3346
L min
K mi
0.0489
rmax
mol
K mi 0.01636
L
2.19. The initial rate of reaction for the enzymatic cleavage of deoxyguanosine triphosphate was
measured as a function of initial substrate concentration as follows (Kornberg et al., J.BioI.Chern.,
233, 159, 1958):
Substrate Concentration Initial Reaction Rate (µmol/L-
1/Cs 1/r
(µmol/L) min)
6.7 0.3 0.149254 3.333333
3.5 0.25 0.285714 4
1.7 0.16 0.588235 6.25
a. Calculate the Michaelis-Menten constants of the above reaction.
b. When the inhibitor was added, the initial reaction rate was decreased as follows:
Initial Reaction
Substrate Concentration
Inhibitor Rate (µmol/L- 1/r
(µmol/L)
(µmol/L) min)
6.7 146 0.11 9.090909
3.5 146 0.08 12.5
1.7 146 0.06 16.66667
Is this competitive inhibition or noncompetitive inhibition? Justify your answer by showing the
effect of the inhibitor graphically. [Contributed by Professor Gary F. Bennett, The University of
Toledo, Toledo, OH]
2|Biochemical Engineering 10/02/2017
Orpilla, Jerryson M. BSChE-5
Answers:
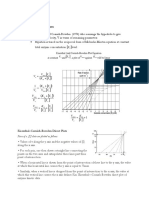
Lineweaver-Burk Plot of Enzymatic Cleave
of Deoxyguanosine Triphosphate
y = 6.7758x + 2.2168
7
6
5
4
1/r
3
2
1
0
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7
1/Cs
a. Lineweaver-Burk Equation
1 Km 1 1
r rmax Cs rmax
y 6.7758 x 2.2168
1
2.2168
rmax
mol
rmax 0.451
L min
Km
6.7758
rmax
mol
K m 3.0566
L
3|Biochemical Engineering 10/02/2017
Orpilla, Jerryson M. BSChE-5
b. Based from the graph, the values of Km with and without inhibitor are almost the same.
Moreover, the y-intercepts, rmax of the Lineweaver-Burk for both inhibited and uninhibited are
not equal. Therefore, it is a noncompetitive inhibition.
Lineweaver-Burk Plot of Enzymatic Cleavage of
Deoxyguanosine Triphosphate
18
16 y = 16.68x + 7.0637
14
12
10
y = 6.7758x + 2.2168
1/r 8
6
4
2
0
-0.6 -0.4 -0.2 -2 0 0.2 0.4 0.6 0.8
1/Cs
Without inhibitor WIth inhibitor
4|Biochemical Engineering 10/02/2017
Вам также может понравиться
- Introductory Titrimetric and Gravimetric Analysis: The Commonwealth and International Library: Chemistry DivisionОт EverandIntroductory Titrimetric and Gravimetric Analysis: The Commonwealth and International Library: Chemistry DivisionОценок пока нет
- C H O + A O + B NH C C H NO + D H O+eCO: InstructionsДокумент4 страницыC H O + A O + B NH C C H NO + D H O+eCO: InstructionsJohn Paul Jandayan33% (3)
- GABAYNO PS Enzyme KineticsДокумент8 страницGABAYNO PS Enzyme KineticsJenny Llanes100% (1)
- Cebu Institute of Technology - University Chemical Engineering DepartmentДокумент7 страницCebu Institute of Technology - University Chemical Engineering DepartmentEd Ryan RualesОценок пока нет
- Palaganas Hw2 Che 197Документ5 страницPalaganas Hw2 Che 197Elah Palaganas100% (1)
- Flocculation and ClarifiersДокумент17 страницFlocculation and ClarifiersOnelОценок пока нет
- Energy Balance Around The Conveyor WasherДокумент5 страницEnergy Balance Around The Conveyor WasherlucasОценок пока нет
- Tutorial 5Документ6 страницTutorial 5hsiegler2Оценок пока нет
- BIochem AssДокумент5 страницBIochem AssCheng PasionОценок пока нет
- Foggler Elements of Chemical Reaction Engineering 3rd Edition 442 449 PDFДокумент8 страницFoggler Elements of Chemical Reaction Engineering 3rd Edition 442 449 PDFJuanОценок пока нет
- Experiment No. 7 Measurement of Reaction ConversionДокумент8 страницExperiment No. 7 Measurement of Reaction ConversionHoneylet Recaña TayactacОценок пока нет
- Sample Problem #14Документ7 страницSample Problem #14DozdiОценок пока нет
- DK5739 CH8Документ25 страницDK5739 CH8Özer Ökten100% (1)
- SedimentationДокумент9 страницSedimentationAutumn JohnsonОценок пока нет
- Test 1 Sample QuestionДокумент7 страницTest 1 Sample QuestionAnonymous GsiB6EMGОценок пока нет
- Final-Front Pages-2011 PME CodeДокумент10 страницFinal-Front Pages-2011 PME CodeJoseph R. F. DavidОценок пока нет
- Enzymes ProbsДокумент21 страницаEnzymes ProbsAnonymous sVNvV7Q100% (1)
- Tutorial 2Документ3 страницыTutorial 2Syakirin SpearsОценок пока нет
- Chloro BenzeneДокумент24 страницыChloro BenzeneAleem AhmedОценок пока нет
- Plant Design Project FinalДокумент221 страницаPlant Design Project FinalYasser AshourОценок пока нет
- Review Chemical Process Industries PDFДокумент64 страницыReview Chemical Process Industries PDFAkerdОценок пока нет
- Chapter 5 AdsorptionДокумент46 страницChapter 5 AdsorptionSyahmiОценок пока нет
- Heat Trqsfer Hsolved Problems: Differential Distillation Q/1Документ9 страницHeat Trqsfer Hsolved Problems: Differential Distillation Q/1soran najebОценок пока нет
- Advanced Thermodynamics: Note 6 Applications of Thermodynamics To Flow ProcessesДокумент24 страницыAdvanced Thermodynamics: Note 6 Applications of Thermodynamics To Flow ProcessesHasif D. MüllerОценок пока нет
- Energy Valorization of Biogas in Santa CatarinaДокумент10 страницEnergy Valorization of Biogas in Santa CatarinagrimdorlfОценок пока нет
- L3 Yields StoichiometryДокумент25 страницL3 Yields StoichiometryDewansh KaushikОценок пока нет
- Biochemical Engineering Enzyme KineticsДокумент5 страницBiochemical Engineering Enzyme KineticsLin Xian Xing33% (3)
- Industrial Waste in Textile IndustryДокумент3 страницыIndustrial Waste in Textile IndustryChetak YadavОценок пока нет
- Leaching - Solid - Liquid Extraction Lecture 1Документ40 страницLeaching - Solid - Liquid Extraction Lecture 1Tumisang Seodigeng100% (1)
- Acre RTD ProblemsДокумент10 страницAcre RTD ProblemsHassan Al-AraimiОценок пока нет
- CHAPTER 4 Energy BalanceДокумент35 страницCHAPTER 4 Energy BalanceZafirahAhmadFauziОценок пока нет
- Unit 1 MT1Документ4 страницыUnit 1 MT1pandianvijaybharathiОценок пока нет
- Production of Bio Plastic Through Food Waste Valor IzationДокумент20 страницProduction of Bio Plastic Through Food Waste Valor IzationÖzgür Demir100% (2)
- CHEE 321: Chemical Reaction Engineering: Module 3: Isothermal Reactor DesignДокумент16 страницCHEE 321: Chemical Reaction Engineering: Module 3: Isothermal Reactor DesignPranav NakhateОценок пока нет
- 06 - Preliminary Optioneering - Rye Mead Water Cycle Strategy Final Report 2009Документ10 страниц06 - Preliminary Optioneering - Rye Mead Water Cycle Strategy Final Report 2009Kiran RmОценок пока нет
- Solution: 0.05 Moisture (Feed in Tons/hr) 0.95 Dry 9.8 (Tons/hr Dry Solid) Fdry 124.45 P 114.65Документ7 страницSolution: 0.05 Moisture (Feed in Tons/hr) 0.95 Dry 9.8 (Tons/hr Dry Solid) Fdry 124.45 P 114.65api-3728602Оценок пока нет
- Liquid Liquid ExtractionДокумент36 страницLiquid Liquid ExtractionamirnimoОценок пока нет
- Open Pan EvaporatorДокумент5 страницOpen Pan EvaporatorsenthilОценок пока нет
- Physical Refining of Coconut OilДокумент6 страницPhysical Refining of Coconut OilHiếu LêОценок пока нет
- CSTR Revised For HandoutДокумент16 страницCSTR Revised For HandoutbagasОценок пока нет
- CH E 511A: Separation Processes and Introduction To Particulate Technology LeachingДокумент8 страницCH E 511A: Separation Processes and Introduction To Particulate Technology LeachingKhayie Victoriano100% (1)
- Reactor Selection CriteriaДокумент2 страницыReactor Selection CriteriaJunaid JohnsonОценок пока нет
- Liquid Urea-Formaldehyde Resin Manufacturing Industry-217599 - 4Документ65 страницLiquid Urea-Formaldehyde Resin Manufacturing Industry-217599 - 4Sanzar Rahman 1621555030Оценок пока нет
- Steady-State Non-Isothermal Reactor DesignДокумент25 страницSteady-State Non-Isothermal Reactor DesignSulabh JainОценок пока нет
- Distillation 13 22Документ2 страницыDistillation 13 22blueberrytimeОценок пока нет
- Soln Sa Adsorption PDFДокумент2 страницыSoln Sa Adsorption PDFRee ValeraОценок пока нет
- Gravimetry - ActivityДокумент1 страницаGravimetry - ActivityJolina Lastimoso MatamisОценок пока нет
- 04 Oxygen DemandДокумент19 страниц04 Oxygen DemandCharisma SubaОценок пока нет
- CHAPTER 11: Dynamic Behaviour & Stability of Closed-Loop Control SystemsДокумент69 страницCHAPTER 11: Dynamic Behaviour & Stability of Closed-Loop Control Systemshakita86Оценок пока нет
- Stoichiometry of Cell Growth and Product FormationДокумент2 страницыStoichiometry of Cell Growth and Product Formationmneilg0% (1)
- Acetone Water MIKДокумент1 страницаAcetone Water MIKFrancisAeronPabalanОценок пока нет
- AgitationДокумент3 страницыAgitationKuo SarongОценок пока нет
- Catalyst Characterization TechniquesДокумент8 страницCatalyst Characterization TechniquesDaniel DadzieОценок пока нет
- Biochemical Oxygen Demand (BOD) Chemical Oxygen Demand (COD)Документ35 страницBiochemical Oxygen Demand (BOD) Chemical Oxygen Demand (COD)wahyu hidayatОценок пока нет
- Bioche ProblemsДокумент5 страницBioche ProblemsTimothy Jones100% (1)
- Activity Number 2Документ9 страницActivity Number 2Mariella SingsonОценок пока нет
- JJДокумент4 страницыJJEureca ParraОценок пока нет
- BiochemprintfinalДокумент14 страницBiochemprintfinalJomhel CalluengОценок пока нет
- Adsorption - Solved ProblemsДокумент5 страницAdsorption - Solved Problemsshaik mohammed Arshad100% (1)
- Eisenthal-Cornish Transform: S S K V VДокумент1 страницаEisenthal-Cornish Transform: S S K V VEureca ParraОценок пока нет
- JJДокумент4 страницыJJEureca ParraОценок пока нет
- 2.10 A Substrate Is Decomposed in The Presence of An Enzyme According To The Michaelis-MentenДокумент2 страницы2.10 A Substrate Is Decomposed in The Presence of An Enzyme According To The Michaelis-MentenEureca ParraОценок пока нет
- Diluter A Clarifier B: Ai2 Bi2 Bi3 Ai1 Ao1 Bi1 Bo1 Ci1 MolassesДокумент3 страницыDiluter A Clarifier B: Ai2 Bi2 Bi3 Ai1 Ao1 Bi1 Bo1 Ci1 MolassesEureca ParraОценок пока нет
- Eureca Joy M. Parra: Anatomy of Beer Making ProcessДокумент1 страницаEureca Joy M. Parra: Anatomy of Beer Making ProcessEureca ParraОценок пока нет
- Microbial Metabolism: Biochemical DiversityДокумент60 страницMicrobial Metabolism: Biochemical DiversityAntonio SilvaОценок пока нет
- Activity Number 2Документ9 страницActivity Number 2Mariella SingsonОценок пока нет
- Enzymes: Dr. Dindin H. Mursyidin, M.SCДокумент29 страницEnzymes: Dr. Dindin H. Mursyidin, M.SCHalisa IndrianiОценок пока нет
- AP+Bio 048+Enzymes+Worksheet WLДокумент3 страницыAP+Bio 048+Enzymes+Worksheet WLPatricia Andrea Alexei FernandezОценок пока нет
- Energy and Enzymes IIДокумент22 страницыEnergy and Enzymes IIapi-418176886Оценок пока нет
- Biology 155 - General Biology I Laboratory Supplement (PDFDrive)Документ78 страницBiology 155 - General Biology I Laboratory Supplement (PDFDrive)CrystalОценок пока нет
- Biochemistry MCQ Module 1Документ60 страницBiochemistry MCQ Module 1ZZ_14U100% (3)
- DocumentДокумент7 страницDocumentANIS NURALYSA AFFENDIОценок пока нет
- Sas4 Bio024Документ24 страницыSas4 Bio024Merlyn Limbaga CastroverdeОценок пока нет
- Vet 115 Quiz 4Документ14 страницVet 115 Quiz 4Chiku MteghaОценок пока нет
- Rogers & Gibon 2009Документ33 страницыRogers & Gibon 2009Ibrar HussainОценок пока нет
- BS 3Документ60 страницBS 3himanshu_agraОценок пока нет
- Unit One Enzymes: General PropertiesДокумент24 страницыUnit One Enzymes: General PropertiesHUAWEI HUAWEIОценок пока нет
- Biology Useful 3 Grade 11 Reference GuideДокумент77 страницBiology Useful 3 Grade 11 Reference GuideHassenMohОценок пока нет
- Solutions To End-of-Chapter Questions: Erland Stevens Medicinal Chemistry and Drug Discovery - Solutions 1Документ78 страницSolutions To End-of-Chapter Questions: Erland Stevens Medicinal Chemistry and Drug Discovery - Solutions 1Miguel AguilarОценок пока нет
- Lesson 6 InhibitorsДокумент12 страницLesson 6 Inhibitorstbrook2017Оценок пока нет
- Leep 516Документ16 страницLeep 516Priyesh Kumar SinghОценок пока нет
- Journal of Hazardous Materials: Devlina Das, D. Charumathi, Nilanjana DasДокумент12 страницJournal of Hazardous Materials: Devlina Das, D. Charumathi, Nilanjana DasMARIA PAULA PORRAS ARGUELLOОценок пока нет
- Enzymes PresДокумент34 страницыEnzymes PresRasmia SalamОценок пока нет
- C1 W14 EnzymesДокумент49 страницC1 W14 EnzymesJasmine Kaye CuizonОценок пока нет
- InhibiciónДокумент8 страницInhibiciónJUAN PABLO RUIZ CASTELLANOSОценок пока нет
- General Biology Exam 3 Study GuideДокумент14 страницGeneral Biology Exam 3 Study GuideSamantha LapierreОценок пока нет
- Chapter 3 Biology 11 Class Federal BoardДокумент18 страницChapter 3 Biology 11 Class Federal BoardMuhammad MustafaОценок пока нет
- Bioreactor: Chemical Reaction Engineering (CRE) Is TheДокумент25 страницBioreactor: Chemical Reaction Engineering (CRE) Is ThekhunafaОценок пока нет
- TugasДокумент23 страницыTugasAnisa Ramadhani Sahlan100% (1)
- 9701 w13 QP 42 2 PDFДокумент20 страниц9701 w13 QP 42 2 PDFNeural Spark Physics CieОценок пока нет
- Enzyme Regulation (Article) - Khan AcademyДокумент19 страницEnzyme Regulation (Article) - Khan Academyteam TSOTAREОценок пока нет
- Concepts in Biochemistry2Документ38 страницConcepts in Biochemistry2atefmaboodОценок пока нет
- Medicinal Chemistry: The Molecular Basis of Drug Discovery: Khan AcademyДокумент17 страницMedicinal Chemistry: The Molecular Basis of Drug Discovery: Khan AcademyRinta MoonОценок пока нет
- How Should Apples Be Prepared For A Fruit Salad? A Guided Inquiry Physical Chemistry ExperimentДокумент7 страницHow Should Apples Be Prepared For A Fruit Salad? A Guided Inquiry Physical Chemistry ExperimentDany PandaОценок пока нет