Академический Документы
Профессиональный Документы
Культура Документы
Assignment 1 CHE502/594 Reaction Engineering 1 Due Date: Monday (14 OF MAY 2018)
Загружено:
nazirulОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Assignment 1 CHE502/594 Reaction Engineering 1 Due Date: Monday (14 OF MAY 2018)
Загружено:
nazirulАвторское право:
Доступные форматы
ASSIGNMENT 1
CHE502/594
REACTION ENGINEERING 1
DUE DATE: MONDAY (14TH OF MAY 2018)
QUESTION 1 (PO2, CO1, C3)
An experiment of irreversible isothermal reaction A → B was carried out in a constant volume
batch reactor at 350 K. Results obtained for this experiment is presented in Table 1.
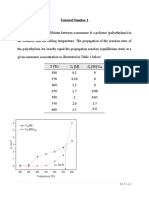
Table 1. Concentration versus time data
Time (min) Concentration, CA (mol/L)
0 4.0
5 2.3256
10 1.1025
15 0.3306
20 0.01
a) By using finite difference method, determine the reaction order and the reaction rate
constant.
b) The experiment above was repeated at temperature of 450K and the reaction rate
constant obtained was 0.3 (unit is depends on answer in (a)). Calculate the activation
energy, Ea and pre-exponential factor, A or k0.
Given that, gas constant, R =8.314 J/mol∙K.
QUESTION 2 (PO2, CO2, C3)
The stoichiometric reaction of reactants A and B is as follows:
A+B C+D
The liquid-phase reaction is first order with respect to A and B (elementary reaction). The
rate constant, k, for this reaction is 0.0017 m3/kmol∙min at 200oC with an activation energy,
E, of 47,000 J/mol. The initial entering concentrations of A and B are 2.0 and 6.5 kmol/m 3,
respectively.
a) Express the rate law for the rate of disappearance of A, -rA, in terms of concentration.
b) Set up a stoichiometric table for this reaction and express -rA in terms of conversion,
X A.
c) Calculate the initial rate of reaction (XA =0) at 200oC and 25oC.
d) Determine the rate of reaction at 90% conversion at 25oC.
Вам также может понравиться
- Reaction Engineering I-Problem Sheet IIДокумент7 страницReaction Engineering I-Problem Sheet IISimay AydoganОценок пока нет
- Tutorial 2Документ2 страницыTutorial 2EreenОценок пока нет
- Set 2 SonДокумент4 страницыSet 2 SonJerson Mendoza CОценок пока нет
- Tutorial 2 - Questions PDFДокумент2 страницыTutorial 2 - Questions PDFRaymond KakalaОценок пока нет
- Assignment Reaction EngineeringДокумент6 страницAssignment Reaction Engineeringnur hidayatiОценок пока нет
- Assignment 2 PDFДокумент1 страницаAssignment 2 PDFRam Lakhan MeenaОценок пока нет
- Tutorial For Chapter 1Документ3 страницыTutorial For Chapter 1Thurgah VshinyОценок пока нет
- Exam I Sem I 2011 12 Cheng 323Документ7 страницExam I Sem I 2011 12 Cheng 323Faisal MumtazОценок пока нет
- First Midterm, 1st Semester - Eve, SolutionДокумент4 страницыFirst Midterm, 1st Semester - Eve, Solutionحاتم غيدان خلفОценок пока нет
- 3 - Prob PFR 11-12 23-35 English-1Документ4 страницы3 - Prob PFR 11-12 23-35 English-1Biniyam haileОценок пока нет
- Tutorial 5 Reaction EngineeringДокумент1 страницаTutorial 5 Reaction EngineeringSurendra Louis Dupuis NaikerОценок пока нет
- Tutorial 1Документ1 страницаTutorial 1Aisyah ShaariОценок пока нет
- Tutorial 3Документ2 страницыTutorial 3Aisyah ShaariОценок пока нет
- CHE3044F, 2013: Reactor Design 1: TUTORIAL 3Документ3 страницыCHE3044F, 2013: Reactor Design 1: TUTORIAL 3nmhatityeОценок пока нет
- Tut 8a Multiple RxnsДокумент21 страницаTut 8a Multiple RxnsMark Antony LevineОценок пока нет
- CHME 314 Lecture 14 Collection and Analysis of Rate Data 2Документ17 страницCHME 314 Lecture 14 Collection and Analysis of Rate Data 2AmroKashtОценок пока нет
- Assignment 1Документ2 страницыAssignment 1Muhd HafetzОценок пока нет
- Tute 1 PDFДокумент1 страницаTute 1 PDFRBОценок пока нет
- CEB2043 - Reaction Engineering I - Ch03 Rate Laws PDFДокумент25 страницCEB2043 - Reaction Engineering I - Ch03 Rate Laws PDFScorpion RoyalОценок пока нет
- For Student Test1 Version 3 SKKK1113 1112-1 PDFДокумент3 страницыFor Student Test1 Version 3 SKKK1113 1112-1 PDFDon Jer Bear FirdausОценок пока нет
- Lesson Plan Reaction Engineering 1Документ4 страницыLesson Plan Reaction Engineering 1EreenОценок пока нет
- Assignment 1 CHE594 April 2013Документ1 страницаAssignment 1 CHE594 April 2013riniz92Оценок пока нет
- Assignment 2 DR Azizul PDFДокумент4 страницыAssignment 2 DR Azizul PDFjinОценок пока нет
- L10 Nonelementary RxnsДокумент34 страницыL10 Nonelementary RxnsRama KrishnaОценок пока нет
- Chapter 3 - Rate Laws and StoichiometryДокумент32 страницыChapter 3 - Rate Laws and StoichiometryKai Faha LukumОценок пока нет
- KRD Chapter 2Документ39 страницKRD Chapter 2Reyhan97Оценок пока нет
- L16 Unsteady State and Reaction EngrДокумент25 страницL16 Unsteady State and Reaction EngrDaniel TemoltzinОценок пока нет
- Tutorial 4Документ3 страницыTutorial 4EreenОценок пока нет
- Tutorial 4Документ1 страницаTutorial 4Aisyah ShaariОценок пока нет
- Chemical Reaction Engineering Mole Balances: ContentДокумент29 страницChemical Reaction Engineering Mole Balances: ContentMhmad E. HerzallahОценок пока нет
- TRK1 2013 Chapt 2Документ14 страницTRK1 2013 Chapt 2Putri JulietaОценок пока нет
- CH 1. Kinematics of Particles 2016 - Part A (Rectilinear Motion) PDFДокумент36 страницCH 1. Kinematics of Particles 2016 - Part A (Rectilinear Motion) PDFOstaz SasaОценок пока нет
- Mec412 Chap 2 Particle PDFДокумент21 страницаMec412 Chap 2 Particle PDFhidayatullahОценок пока нет
- Tutorial 4Документ5 страницTutorial 4Aakash R RajwaniОценок пока нет
- Engineering Mechanic - Chapter 1Документ22 страницыEngineering Mechanic - Chapter 1NurzanM.JefryОценок пока нет
- Topic 1: Siti Wahidah Binti Puasa PHONE NO: 03-55436327 011-32338927 Reference: Fogler 4 Edition, Levenspeil 3 EditionДокумент35 страницTopic 1: Siti Wahidah Binti Puasa PHONE NO: 03-55436327 011-32338927 Reference: Fogler 4 Edition, Levenspeil 3 EditionJohnОценок пока нет
- Reaction Kinetics Sample ProblemsДокумент1 страницаReaction Kinetics Sample ProblemsBenedict MarzanОценок пока нет
- Lec 1 - Fundamental Concepts, Force VectorsДокумент66 страницLec 1 - Fundamental Concepts, Force VectorsMarian Galvez-LuisОценок пока нет
- Steady State Non-Isothermal Reactor DesignДокумент34 страницыSteady State Non-Isothermal Reactor DesignFaisal MumtazОценок пока нет
- Chemical Reaction Engineering (CHE 331A) Assignment-2 (2017-18-II)Документ2 страницыChemical Reaction Engineering (CHE 331A) Assignment-2 (2017-18-II)Anonymous rkAeZVSKОценок пока нет
- 1 Chapter 1-Mole BalancesДокумент21 страница1 Chapter 1-Mole BalancesKai Faha LukumОценок пока нет
- Lecture 2 - Chapter 1-Mole Balance PDFДокумент40 страницLecture 2 - Chapter 1-Mole Balance PDFNizam JumadiОценок пока нет
- Lecture 1 - Introduction of CREДокумент6 страницLecture 1 - Introduction of CRENizam JumadiОценок пока нет
- Chapter 2 - Conversion Reactor SizingДокумент26 страницChapter 2 - Conversion Reactor SizingKai Faha LukumОценок пока нет
- Chbe 6300 Graduate Kinetics and Reactor Design: Carsten Sievers 8/18/2020Документ18 страницChbe 6300 Graduate Kinetics and Reactor Design: Carsten Sievers 8/18/2020AnnОценок пока нет
- Chapter 3 Rev1 Rate Laws & StoichiometryДокумент35 страницChapter 3 Rev1 Rate Laws & StoichiometryHakashiMirudoОценок пока нет
- L12 Nonisothermal Reaction EngineeringДокумент24 страницыL12 Nonisothermal Reaction EngineeringShixia Xu100% (1)
- Lec 4 - Isothermal Reactor Design PDFДокумент39 страницLec 4 - Isothermal Reactor Design PDFMhmad E. HerzallahОценок пока нет
- Tutorial 5drtuhДокумент2 страницыTutorial 5drtuhFikrie MuhdОценок пока нет
- 4.collection and Analysis of Rate Data - CHAPTER 5Документ37 страниц4.collection and Analysis of Rate Data - CHAPTER 5Marsya FarahОценок пока нет
- CHM 152 Final Exam Review 1 Spring 2012 NEW KEYДокумент4 страницыCHM 152 Final Exam Review 1 Spring 2012 NEW KEYCaguioa Mark Anthony G.Оценок пока нет
- Tutorial Data AnalysisДокумент4 страницыTutorial Data Analysisshuhui383838Оценок пока нет
- L9 Reactor Design For Multiple RxnsДокумент21 страницаL9 Reactor Design For Multiple RxnsKarrar AlhsnawyОценок пока нет
- 1.multiple ReactionsДокумент58 страниц1.multiple ReactionsDianah NajeebОценок пока нет
- Chapter 3Документ14 страницChapter 3AmandaEdwinОценок пока нет
- hw3 - Che324Документ3 страницыhw3 - Che324Ahmed Ali0% (1)
- Chep 424 2ND Semester 2013 Quiz 1Документ1 страницаChep 424 2ND Semester 2013 Quiz 1Clarissa AlfaroОценок пока нет
- 1-8 Reaction Kinetics PDFДокумент8 страниц1-8 Reaction Kinetics PDFBerry101Оценок пока нет
- Tutorial Number 1jjkДокумент4 страницыTutorial Number 1jjkSaif JassimОценок пока нет
- TDS20-v2 1Документ8 страницTDS20-v2 1nazirulОценок пока нет
- Waste LabelДокумент4 страницыWaste LabelnazirulОценок пока нет
- Flow Source of Wastewater From Production (Repaired)Документ2 страницыFlow Source of Wastewater From Production (Repaired)nazirulОценок пока нет
- Aoa UpdateДокумент1 страницаAoa UpdatenazirulОценок пока нет
- Fault Tree Diagram R-101Документ8 страницFault Tree Diagram R-101nazirulОценок пока нет
- Shindo B SeriesДокумент42 страницыShindo B SeriesnazirulОценок пока нет
- Name: Student No.: Group: Experiment: Date Performed: Semester: Programme / Code: Submit ToДокумент1 страницаName: Student No.: Group: Experiment: Date Performed: Semester: Programme / Code: Submit TonazirulОценок пока нет
- Budgeting The ProjectДокумент68 страницBudgeting The ProjectnazirulОценок пока нет
- Control Valve: CPE501 Chemical Process ControlДокумент4 страницыControl Valve: CPE501 Chemical Process ControlnazirulОценок пока нет
- Reaction Assignment Multiphase ReactionДокумент4 страницыReaction Assignment Multiphase ReactionnazirulОценок пока нет
- chapters/C06/E6 34 02 05 PDFДокумент1 страницаchapters/C06/E6 34 02 05 PDFnazirulОценок пока нет
- Multiphase ReactionДокумент1 страницаMultiphase ReactionnazirulОценок пока нет
- Sales ProjectДокумент1 страницаSales ProjectnazirulОценок пока нет
- Business Plan Format KemusaДокумент1 страницаBusiness Plan Format KemusanazirulОценок пока нет
- CalcДокумент2 страницыCalcnazirulОценок пока нет