Академический Документы
Профессиональный Документы
Культура Документы
1 Thermochem
Загружено:
Von Joby RomeroИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
1 Thermochem
Загружено:
Von Joby RomeroАвторское право:
Доступные форматы
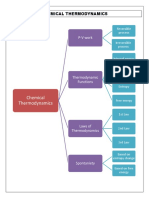
THERMOCHEMISTRY System
- the portion of the universe selected for a
Thermochemistry thermodynamic study.
a branch of thermodynamics which concerns with the
measurement, analysis & interpretation of heat that Surroundings
is involved in physical & chemical reactions. - any part of the universe outside the system.
Energy
- ability to do work or to transfer heat. surroundings
system
- unit is Joule (J)
1 J = 1 kg-m2/s2
1 calorie = 4.184 J boundary
Work, W Types of Systems:
- the movement of an object against some force. type example
- directed energy change resulting from a process. can exchange mass &
- 1 L-atm = 101.32 J energy with its glass w/ water,
open
Consider a cylinder with a movable piston, surroundings tree
-P V
W = -P can exchange only
* compression of gas energy with its
closed thermometer
force surroundings but not
mass
cannot exchange
mass & energy with
isolated styrofor ice box
gas h its surroundings
Vi Vf Sign Convention for Heat & Work:
Vi > Vf
ΔV = Vf – Vi = (-) negative W (+)
W = -PΔV = (+) positive
*If work done on the system, W is positive
system
* expansion of gas
q (+) syste q (-)
m
h
W (-)
force gas
Endothermic process
- a process at which heat is absorbed or enters the system
Vi Vf
Vi < Vf Exothermic process
ΔV = Vf – Vi = (+) positive - a process at which heat is released or exits the system
W = -PΔV = (-) negative
*If work done by the system, W is negative Internal Energy, E
- the total energy possessed by a system
Heat, q - the change in internal energy, E can be also
-the flow of energy from a body at higher defined as: E = q + w.
temperature to one at a lower temperature when Enthalpy, H
they are placed in thermal contact. - a quantity defined as the sum of internal energy, E
and PV. (H = E + PV)
note:
thermochemistry mike filomeno
Gen Chem 213 Page 1 of 4
- enthalpy (as well as internal energy) cannot be
measured directly, but it is actually the change in Isothermal Process (temperature is constant)
enthalpy, H which can be measured. E = q + w
H = E + P
P V E = 0 (for constant temp. process)
0=q+w
for processes involving solids and liquids: q = -w
H = E
- since the volumes of solids & liquids does not change Adiabatic Process (no heat enters or leaves the
significantly in the process system)
E = q + w
for process involving gases: q = 0 for adiabatic process
using ideal gas equation, PV = nRT E = w
PV = nRT
H = E + nRT Exercises:
where n = # moles of gaseous products - # moles of A. Determine whether the following are open, closed or
gaseous reactants. isolated system
1. “coleman” water jug
2. pressure cooker in use
State & Path Functions 3. burning wood
4. rice cooker in use
State Functions 5. flat iron
- are properties that are determined by the state of the 6. student inside a classroom
system, regardless of how that condition was
achieved. B. For the following processes, calculate the change in
- the change in the magnitude of the state function internal energy of the system & determine whether
depends only on the initial & final states of the the process is endothermic or exothermic:
system & not on how the change is accomplished. 1. A balloon is heated by adding 670 J of heat. It
- examples are energy, pressure, volume, temperature expands, doing 332 J of work on the atmosphere.
& enthalpy 20C to 10
2. A 10.0 g sample of water is cooled from 20 10C
thereby losing approximately 420 J of heat.
Path Functions 3. A chemical reaction releases 8.65 kJ of heat & does
- properties that are determined via how the conditions no work on the surroundings
are achieved
- examples are work & heat C. The ΔE of the system is –45 J. If 105 J of heat is
being released, calculate w. Is the work done on or
Thermodynamics done by the system.
the study of energy and its transformations.
Calorimetry
First Law of Thermodynamics - the measurement of heat flow or heat changes as a
energy can be transformed from one form to result of a chemical reaction.
another, but cannot be created or destroyed - the apparatus used to measure heat flow is called
energy in the universe is constant calorimeter.
qsys + qsurr = 0 Types of Calorimeter:
qsys = -qsurr 1. Bomb Calorimeter- a device used to measure the
Isometric/Isochoric Process (volume is constant) heat of combustion. A bomb calorimeter is
E = q + w considered a constant volume system.
-PV
W = -P 2. Open-type Calorimeter- a device used to determine
V = 0 (since no change in volume) heat changes for non-combustion reactions (e.g. heat
W = 0 of solution, heat of neutralization, heat of dilution)
E = q - an open-type calorimeter is considered as a
constant pressure system
Isobaric Process (pressure is constant)
E = q + w
-PV
W = -P
E = q + (-P
(-PV)
E + PPV = q
Specific Heat & Heat Capacity
recall: E + P
PV = H
H = q
thermochemistry mike filomeno
Gen Chem 213 Page 2 of 4
Specific Heat, S the calorimeter assembly is 4.90 kJ/ oC. What is the
- amount of heat required to raise the temperature of heat of combustion of sucrose?
on gram of a substance by one degree Celcius. 3. A 1.567 gram sample of naphthalene, C 10H8(s), is
J/gC
- unit is J/g completely burned in a bomb calorimeter assembly
J/gC
- the specific heat capacity of water is 4.184 J/g and a temperature increase of 8.37 oC is noted. When
- if the amount of substance is one mole, it is called a 1.227-gram sample of thymol, C10H14O(s), is burned
molar heat capacity in the same calorimeter assembly, the temperature
increase is 6.12oC. If the heat of combustion of
Heat Capacity, C naphthalene is –5153.9 kJ/mole C 10H8, what is the
- the amount of heat required to raise the temperature heat of combustion of thymol?
of a given quantity of a substance by one degree
Celcius. Constant Pressure Calorimetry
J/C
- unit is J/ - the most common device used is the “coffee-cup”
calorimeter
qsys = qrxn + qmix + qcal
Measuring Heat Change qsys = 0 (since adiabatic)
0 = qrxn + qmix + qcal
C = mS where:
q = mS mSt qmix is the heat of the mixture
q = C C t qrxn can be qneut, qdil, qsoln, etc..
where t = tfinal – tinitial (change in temeperature)
qmix = mmixSmixt
Exercises: qcal = Ccalt
1. A piece of silver of mass 362 g has a heat capacity of qrxn = -qmix –qcal
J/C. What is the specific heat of silver.
85.7 J/ qrxn = - mmixSmix t - Ccal t
2. A 6.22 kg piece of Cu metal is heated from 20.5 C to
324.3 C. Calculate the heat absorbed by the metal. Exercises:
J/gC)
(SCu = 0.385 J/g 1. A quantity of 100 mL of 0.500 M HCl is mixed with
3. Calculate the amount of heat liberated (in kJ) from 100 mL of 0.500 M NaOH in a constant pressure
366 g of mercury when it cools from 77.0 C to 12.0 calorimeter that has a heat capacity of 335 J/ J/C. The
C. (SHg = 0.140 J/g-K) initial temperature of the HCl and NaOH solution is
the same, 22.50 C, and the final temperature of the
Constant Volume Calorimetry mixed solution is 24.90 C. Calculate the heat
- the device used is the bomb calorimeter change for the neutralization reaction:
NaOH(aq) + HCl(aq) NaCl(aq) + H2O(l)
qsys = qwater + qbomb + qrxn Assume that the densities and specific heats of the
but qcal = qwater + qbomb solutions are the same as for water (1.00 g/mL &
qsys = qcal + qrxn J/gC, respectively).
4.184 J/g
qsys = 0 (since adiabatic) 2. When 50.0 mL of 0.100 M AgNO 3 and 50.0 mL of
0 = qcal + qrxn 0.100 M HCl are mixed in a constant pressure
qrxn = -qcal calorimeter that has a heat capacity of 405 J/ J/C. The
temperature of the mixture increases from 22.30 22.30C to
qcal = Ccal t 23.11C. The temperature increase is caused by this
23.11
where: reaction:
Ccal – heat capacity of calorimeter AgNO3(aq) + HCl(aq) AgCl(s) + HNO3(aq)
t – change in temperature Calculate H for this reaction, assuming that the
combined solution has a mass of 100.0 g and a
Exercises: specific heat capacity of 4.184 J/gJ/gC.
1. A quantity of 1.922 g of methanol (CH 3OH) was
burned in a constant volume bomb calorimeter.
Consequently, the temperature of the H2O rose by Thermochemical Equations
4.20C. If the quantity of H 2O surrounding the
4.20 - a balanced chemical equation that shows the
calorimeter was exactly 2000.0 g and the heat associated enthalpy change.
kJ/C, calculate
capacity of the calorimeter was 2.02 kJ/ ex:
the molar heat of combustion of methanol. 2H2(g) + O2(g) 2H2O(g) H = -483.6 kJ
2. The combustion of 1.01 grams of sucrose, C 12H22O11,
in a bomb calorimeter causes the water temperature
to rise from 24.92 to 28.33 oC. The heat capacity of
Enthalpy of Formation (or heat of formation), Hf
thermochemistry mike filomeno
Gen Chem 213 Page 3 of 4
- energy released or absorbed when 1 mole of a Hess’s Law (Indirect Method)
compound is formed from its elements. - if a reaction is carried out in a series of steps, H for
ex: the reaction will be equal to the sum of the enthalpy
N2(g) + 6H2(g) 2NH3(g) Hf = -46.19 kJ changes for the individual steps.
Enthalpy of Reaction (or heat of reaction), Hr S(s) + O2(g) SO2(g) ΔHr = -296.9 kJ
- energy released or absorbed as a result of a certain SO2(g) + ½O2(g) SO3(g) ΔHr = -98.3 kJ
reaction SO3(g) + H2O(l) H2SO4(l) ΔHr = -130.2 kJ
Hr = Hf products - Hf reactants
Net rxn : S(s) + 3/2O2(g) + H2O(l) H2SO4(l)
Standard State of A Substance ΔHr = (-296.9)+(-98.3)+(-130.2)
- pure form of a substance at atmospheric pressure (1 ΔHr = -525.4 kJ
atm) and temperature of 25 C.
Exercises:
Standard Enthalpy of Reaction, Hr 1. Given the following thermochemical equations:
- is the enthalpy change when all reactants & products H2S(g) + 3/2O2 H2O(l) + SO2(g) Hr = -562.6 kJ
are in their standard states. The superscript CS2(l) + 3O2(g) CO2(g) + 2SO2(g) Hr = -1075.2 kJ
indicates standard state conditions. Calculate the value of Hr for the reaction:
CS2(l) + 2H2O(l) CO2(g) + 2H2S(g)
2. Given the following thermochemical equations:
Guidelines in Using Thermochemical Equations: 4NH3(g) + 3O2(g) 2N2(g) + 6H2O(l) ΔHr=-1531 kJ
1. Enthalpy is an extensive property
N2O(g) + H2(g) N2(g) + H2O(l) ΔHr=-367.4 kJ
- H is dependent on the amount of reactant
H2(g) + ½O2(g) H2O(l) ΔHr=-285.9 kJ
consumed in the process.
ex: Calculate the value of ΔHΔHr for the reaction:
CH4(g) + 2O2(g) CO2(g) + 2H2O(l) H = -890 kJ 2NH3(g) + 3N2O(g) 4N2(g) + 3H2O(l)
2CH4(g) + 4O2(g) 2CO2(g) + 4H2O(l) H = -1780 kJ
2. The change in enthalpy of a reaction is equal in
magnitude but opposite in sign when the reaction is
reversed.
ex:
SO3(g) + H2O(l) H2SO4(l) Hr = -130.2 kJ
H2SO4(l) SO3(g) + H2O(l) Hr = 130.2 kJ
3. The enthalpy change for a reaction depends on the
state of the reactants & products.
ex:
CH4(g) + 2O2(g) CO2(g) + 2H2O(l) Hr = -890 kJ
CH4(g) + 2O2(g) CO2(g) + 2H2O(g) Hr = -802 kJ
Measuring H
Direct Method
Hr = nHf(prod) -
-nHf(react)
Exercises:
1. Calculate Hr for the following:
a. C6H6(l) + 15/2O2(g) 6CO2(g) + 3H2O(l)
b. NH3(g) + HCl(g) NH4Cl(s)
c. 2H2S(g) + 3O2(g) 2H2O(l) + 2SO2(g)
2. Calculate the heat of formation of OSCl 2(l) in the
following reaction:
OSCl2(l) + H2O(l) SO2(g) + 2HCl(g) Hr = 10.3 kJ
thermochemistry mike filomeno
Gen Chem 213 Page 4 of 4
Вам также может понравиться
- Thermodynamics P 01 2Документ43 страницыThermodynamics P 01 2gamertm37Оценок пока нет
- CH2105 - ThermodynamicsДокумент12 страницCH2105 - ThermodynamicsJohnОценок пока нет
- Introduction To Energy BalanceДокумент25 страницIntroduction To Energy Balancenhalieza1067Оценок пока нет
- Module 1Документ82 страницыModule 1ALL TIME STUDYОценок пока нет
- Chapter 2Документ28 страницChapter 2Siti Hajar Mohd PodziОценок пока нет
- Thermodynamic Revision 2021 Part 1Документ70 страницThermodynamic Revision 2021 Part 1Rawda AliОценок пока нет
- Chapter 2Документ125 страницChapter 2annaОценок пока нет
- Thermodynamics Module 1Документ12 страницThermodynamics Module 1Kirtan KumarОценок пока нет
- CE 2102 Reviewer MidtermДокумент3 страницыCE 2102 Reviewer MidtermIrish SalayОценок пока нет
- CHM432 Fundamental Physical Chemistry: ThermodynamicsДокумент97 страницCHM432 Fundamental Physical Chemistry: ThermodynamicsPriscyyОценок пока нет
- 2nd 1Документ4 страницы2nd 1Le ScienceОценок пока нет
- Thermodynamics (Part 2)Документ16 страницThermodynamics (Part 2)Yt SparkОценок пока нет
- Thermo ChemДокумент65 страницThermo ChemalinОценок пока нет
- SKF3013 Lecture 1aДокумент24 страницыSKF3013 Lecture 1aAsmaanieycb AsmaanieycbОценок пока нет
- FALLSEM2021-22 BCHY101L TH VL2021220106627 Reference Material II 16-09-2021 EC Module 1 - MARДокумент56 страницFALLSEM2021-22 BCHY101L TH VL2021220106627 Reference Material II 16-09-2021 EC Module 1 - MARHarsh AgarwalОценок пока нет
- Apni Kaksha ThermodynamicsДокумент27 страницApni Kaksha ThermodynamicsVcОценок пока нет
- Thermodynamics Mind MapДокумент8 страницThermodynamics Mind Mapzahida0515Оценок пока нет
- 0 Intro ThermodynamicsДокумент16 страниц0 Intro ThermodynamicsmoazamaakbarОценок пока нет
- Thermodynamic Potentials Phase Tran. Low Temp MaterialДокумент26 страницThermodynamic Potentials Phase Tran. Low Temp Materialsrinu reddyОценок пока нет
- Scope: State and Path Functions RevisitedДокумент4 страницыScope: State and Path Functions RevisitedChandler ManlongatОценок пока нет
- Engineering Chem Mod1Документ59 страницEngineering Chem Mod1sikkaОценок пока нет
- Science Second QuarterДокумент8 страницScience Second QuarterSaint DionysiusОценок пока нет
- Thermodynamics-EC Module 1Документ28 страницThermodynamics-EC Module 1pranavaravind19demoОценок пока нет
- 7 Thermodynamics 2023Документ18 страниц7 Thermodynamics 2023jagannathanОценок пока нет
- Thermodynamics WorksheetДокумент13 страницThermodynamics WorksheetHudsun HornetОценок пока нет
- Thermodynamics Synopsis and FormulaДокумент9 страницThermodynamics Synopsis and FormulaRushwin VaishnavОценок пока нет
- Chemical ThermodynamicsДокумент20 страницChemical ThermodynamicsShantanu KadamОценок пока нет
- SKF3013 Physical Chemistry I: Prof. Dr. Ramli Ibrahim Dr. Norlaili Abu BakarДокумент24 страницыSKF3013 Physical Chemistry I: Prof. Dr. Ramli Ibrahim Dr. Norlaili Abu BakarAisha NajihaОценок пока нет
- Lecture - 2 - 1st - Law of ThermodynamicsДокумент21 страницаLecture - 2 - 1st - Law of ThermodynamicsahmedОценок пока нет
- ThermodynamicsДокумент24 страницыThermodynamicsRaju SinghОценок пока нет
- Chemical Thermodynamics: 1 Semester-Module 5Документ8 страницChemical Thermodynamics: 1 Semester-Module 5Irish Mae LunaОценок пока нет
- Materi KD TermodinamikaДокумент75 страницMateri KD TermodinamikaMuhammad Quthbil IrsyadОценок пока нет
- Chapter4-Lecture No.2Документ11 страницChapter4-Lecture No.2Mohammad SaleemОценок пока нет
- Topic 3 and 4-ThermodynamicsДокумент95 страницTopic 3 and 4-ThermodynamicsNOR AZAM BIN ENDOT / FSОценок пока нет
- Chapter 8Документ37 страницChapter 8yobingewatcher06Оценок пока нет
- ME3100 TD First Law SystemДокумент11 страницME3100 TD First Law SystemddhhhdpnОценок пока нет
- Prof. Dr. Ramli Ibrahim Dr. Norlaili Abu BakarДокумент24 страницыProf. Dr. Ramli Ibrahim Dr. Norlaili Abu BakarNurshuhada NordinОценок пока нет
- 2Документ160 страниц2Abel KuhsutОценок пока нет
- Lec3 - First LawДокумент7 страницLec3 - First LawKaryl CoronelОценок пока нет
- Lec1 PostДокумент8 страницLec1 PostAt InОценок пока нет
- The First Law of Thermodynamics1Документ50 страницThe First Law of Thermodynamics1Beshir Heyru MohammedОценок пока нет
- ThermochemistryДокумент12 страницThermochemistrynsuperticiosoОценок пока нет
- Thermodynamics: Ashwani Tyagi Sir (Code: ATJEE)Документ24 страницыThermodynamics: Ashwani Tyagi Sir (Code: ATJEE)Vaibhav bhardwajОценок пока нет
- 1st Law of ThermodynamicsДокумент3 страницы1st Law of ThermodynamicschitooОценок пока нет
- 1 Law ThermodynamicДокумент33 страницы1 Law ThermodynamicismehairyОценок пока нет
- The First Law of Thermodynamics - 230727 - 113816Документ41 страницаThe First Law of Thermodynamics - 230727 - 113816Tshiamo MotaungОценок пока нет
- ThermodynamicsДокумент47 страницThermodynamicsGamesPickerОценок пока нет
- 2.1 - Work, Heat and The First LawДокумент63 страницы2.1 - Work, Heat and The First LawHONG XIAОценок пока нет
- System and Surroundings: - SystemДокумент19 страницSystem and Surroundings: - SystemVighnesh ManojОценок пока нет
- ExergyДокумент27 страницExergyniralnaikОценок пока нет
- Energy and Energy TransferДокумент20 страницEnergy and Energy TransferNIRUPAN KARKIОценок пока нет
- Lecture 23 Chapter 6Документ25 страницLecture 23 Chapter 6John ChristnardОценок пока нет
- Plus 1 - Chemistry ThermodynamicsДокумент12 страницPlus 1 - Chemistry Thermodynamicssivaranjini S.VОценок пока нет
- Hsslive XI CH 5 Chemistry Notes by AkДокумент11 страницHsslive XI CH 5 Chemistry Notes by AkkundrapupОценок пока нет
- CH 1 CHMДокумент53 страницыCH 1 CHMzuОценок пока нет
- Materials Engineering Science Mesc. 5025 Instructor: Herve MarandДокумент10 страницMaterials Engineering Science Mesc. 5025 Instructor: Herve MarandFa HedaiatОценок пока нет
- Lecture 2Документ21 страницаLecture 2Anonymous BW2VsFifi9Оценок пока нет
- This PDF Is The Sample PDF Taken From Our Comprehensive Study Material For IIT-JEE Main & AdvancedДокумент13 страницThis PDF Is The Sample PDF Taken From Our Comprehensive Study Material For IIT-JEE Main & AdvancedGod is every whereОценок пока нет
- AE 630 Aero Engineering ThermodynamicsДокумент103 страницыAE 630 Aero Engineering Thermodynamicsaeronautical rajasОценок пока нет
- 1 - GasesДокумент5 страниц1 - GasesVon Joby RomeroОценок пока нет
- 3 Chemical EquilibriaДокумент3 страницы3 Chemical EquilibriaVon Joby RomeroОценок пока нет
- 4 Acids&BasesДокумент3 страницы4 Acids&BasesVon Joby RomeroОценок пока нет
- Wine MakingДокумент5 страницWine MakingVon Joby RomeroОценок пока нет
- Cologne Making 1Документ5 страницCologne Making 1Von Joby RomeroОценок пока нет
- CHE40 LT#2-Money-Time Relationship and EquivalenceДокумент1 страницаCHE40 LT#2-Money-Time Relationship and EquivalenceVon Joby Romero0% (1)
- Kundt's Tube ExperimentДокумент9 страницKundt's Tube ExperimentVon Joby Romero100% (5)
- Mapua Institute of Technology: School of Chemical Engineering and ChemistryДокумент12 страницMapua Institute of Technology: School of Chemical Engineering and ChemistryVon Joby RomeroОценок пока нет
- Slab-On-Grade Reinforcing DesignДокумент9 страницSlab-On-Grade Reinforcing DesignAdam GreenlawОценок пока нет
- TN H01-Hand Book For Design of Steel StructuresДокумент210 страницTN H01-Hand Book For Design of Steel StructuresEdward van Martino88% (8)
- Phyto-Mediated Synthesis of Zinc Oxide Nanoparticles of BerberisДокумент31 страницаPhyto-Mediated Synthesis of Zinc Oxide Nanoparticles of BerberisRabeea NasirОценок пока нет
- QM-I ManualДокумент87 страницQM-I ManualMuhammad Masoom AkhtarОценок пока нет
- Pharmacological Review On Terminalia ChebulaДокумент5 страницPharmacological Review On Terminalia ChebulaSri Sakthi SumananОценок пока нет
- Sist Iso 293 1996Документ8 страницSist Iso 293 1996rtplemat lemat100% (1)
- Xliil-On J. Brown. My: An Acetic Ferment Which Form CelluloseДокумент8 страницXliil-On J. Brown. My: An Acetic Ferment Which Form CelluloseFiqa SuccessОценок пока нет
- MasterSeries 880V Specification SheetДокумент4 страницыMasterSeries 880V Specification SheetFEBCOОценок пока нет
- Design and Analysis of Gas Turbine Combustion Chamber For Producer Gas AsДокумент5 страницDesign and Analysis of Gas Turbine Combustion Chamber For Producer Gas AsPhạm Công ÁnhОценок пока нет
- S - Amlo BesylateДокумент20 страницS - Amlo BesylateDrkrishnasarma pathyОценок пока нет
- (Martin Moeller, Krzysztof Matyjaszewski) PolymerДокумент1 052 страницы(Martin Moeller, Krzysztof Matyjaszewski) PolymerGrayОценок пока нет
- Spons Encyclopaedia of The Industrial Arts, Manufactures, and Commercial Products, Part 3Документ396 страницSpons Encyclopaedia of The Industrial Arts, Manufactures, and Commercial Products, Part 3Books for the lotОценок пока нет
- CorrosionДокумент9 страницCorrosionhesampirОценок пока нет
- Quiz Answer Key WeldingДокумент2 страницыQuiz Answer Key WeldingKawan kawankaanОценок пока нет
- Building Repairs and MaintenanceДокумент167 страницBuilding Repairs and MaintenanceAnshul Soni100% (1)
- Technical Sheet CEB Case Study USДокумент2 страницыTechnical Sheet CEB Case Study USluisgomezrojasОценок пока нет
- (William F Hosford) Materials For Engineers (BookFi) PDFДокумент298 страниц(William F Hosford) Materials For Engineers (BookFi) PDFgimgimoОценок пока нет
- STD PipingДокумент51 страницаSTD PipingRodrigo Iván Latorre AlmirallОценок пока нет
- Concrete Mix DesignДокумент12 страницConcrete Mix DesignWilliam ProvidoОценок пока нет
- MSDS Alpha PineneДокумент6 страницMSDS Alpha PineneAbdul RajabОценок пока нет
- VDG P201 EnglischДокумент15 страницVDG P201 EnglischGiacomo ZammattioОценок пока нет
- DNA Transposons PDFДокумент14 страницDNA Transposons PDFALОценок пока нет
- Cement Manufacturing Specifications Guide: RapidcureДокумент9 страницCement Manufacturing Specifications Guide: RapidcureHeramb TrifaleyОценок пока нет
- Bioinspiration and BiomimeticsДокумент7 страницBioinspiration and BiomimeticsAyush 100niОценок пока нет
- ES 5562-2006 - Sanitary WaresДокумент15 страницES 5562-2006 - Sanitary WaresPrima SatriaОценок пока нет
- (Donald Mackay) Multimedia Environmental ModelsДокумент273 страницы(Donald Mackay) Multimedia Environmental ModelsKim HiềnОценок пока нет
- Welding SymbolsДокумент53 страницыWelding SymbolsLâm Thanh100% (9)
- Gek105060 File0060Документ12 страницGek105060 File0060Mauricio GuanellaОценок пока нет
- Senior CapstoneДокумент6 страницSenior Capstoneapi-313278667Оценок пока нет
- Protocol Hybridization Capture of Dna Libraries Using Xgen Lockdown Probes and Reagents Version 3Документ16 страницProtocol Hybridization Capture of Dna Libraries Using Xgen Lockdown Probes and Reagents Version 3Rodger12Оценок пока нет
- Hero Found: The Greatest POW Escape of the Vietnam WarОт EverandHero Found: The Greatest POW Escape of the Vietnam WarРейтинг: 4 из 5 звезд4/5 (19)
- Dirt to Soil: One Family’s Journey into Regenerative AgricultureОт EverandDirt to Soil: One Family’s Journey into Regenerative AgricultureРейтинг: 5 из 5 звезд5/5 (125)
- The End of Craving: Recovering the Lost Wisdom of Eating WellОт EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellРейтинг: 4.5 из 5 звезд4.5/5 (82)
- Sully: The Untold Story Behind the Miracle on the HudsonОт EverandSully: The Untold Story Behind the Miracle on the HudsonРейтинг: 4 из 5 звезд4/5 (103)
- The Fabric of Civilization: How Textiles Made the WorldОт EverandThe Fabric of Civilization: How Textiles Made the WorldРейтинг: 4.5 из 5 звезд4.5/5 (58)
- Faster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestОт EverandFaster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestРейтинг: 4 из 5 звезд4/5 (28)
- The Future of Geography: How the Competition in Space Will Change Our WorldОт EverandThe Future of Geography: How the Competition in Space Will Change Our WorldРейтинг: 4 из 5 звезд4/5 (6)
- When the Heavens Went on Sale: The Misfits and Geniuses Racing to Put Space Within ReachОт EverandWhen the Heavens Went on Sale: The Misfits and Geniuses Racing to Put Space Within ReachРейтинг: 3.5 из 5 звезд3.5/5 (6)
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaОт EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaОценок пока нет
- ChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindОт EverandChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindОценок пока нет
- The Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyОт EverandThe Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyОценок пока нет
- The Technology Trap: Capital, Labor, and Power in the Age of AutomationОт EverandThe Technology Trap: Capital, Labor, and Power in the Age of AutomationРейтинг: 4.5 из 5 звезд4.5/5 (46)
- Pale Blue Dot: A Vision of the Human Future in SpaceОт EverandPale Blue Dot: A Vision of the Human Future in SpaceРейтинг: 4.5 из 5 звезд4.5/5 (588)
- Four Battlegrounds: Power in the Age of Artificial IntelligenceОт EverandFour Battlegrounds: Power in the Age of Artificial IntelligenceРейтинг: 5 из 5 звезд5/5 (5)
- Permaculture for the Rest of Us: Abundant Living on Less than an AcreОт EverandPermaculture for the Rest of Us: Abundant Living on Less than an AcreРейтинг: 4.5 из 5 звезд4.5/5 (33)
- The Book of the Moon: A Guide to Our Closest NeighborОт EverandThe Book of the Moon: A Guide to Our Closest NeighborРейтинг: 4.5 из 5 звезд4.5/5 (11)
- Restoration Agriculture: Real-World Permaculture for FarmersОт EverandRestoration Agriculture: Real-World Permaculture for FarmersРейтинг: 4.5 из 5 звезд4.5/5 (86)
- Project Management All-in-One For DummiesОт EverandProject Management All-in-One For DummiesРейтинг: 5 из 5 звезд5/5 (6)
- Reality+: Virtual Worlds and the Problems of PhilosophyОт EverandReality+: Virtual Worlds and the Problems of PhilosophyРейтинг: 4 из 5 звезд4/5 (24)
- Process Plant Equipment: Operation, Control, and ReliabilityОт EverandProcess Plant Equipment: Operation, Control, and ReliabilityРейтинг: 5 из 5 звезд5/5 (1)