Академический Документы
Профессиональный Документы
Культура Документы
Chapter II
Загружено:
FanialiahsaniОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Chapter II
Загружено:
FanialiahsaniАвторское право:
Доступные форматы
http://www.kidney-international.
org review
& 2011 International Society of Nephrology
Combination inhibition of the renin–angiotensin
system: is more better?
Michelle W. Krause1, Vivian A. Fonseca2 and Sudhir V. Shah1
1
Division of Nephrology, Department of Internal Medicine, University of Arkansas for Medical Sciences, Central Arkansas Veterans
Healthcare System, Little Rock, Arkansas, USA and 2Department of Internal Medicine, Tulane University, New Orleans, Louisiana, USA
Angiotensin-converting enzyme inhibitors or angiotensin II A wealth of research and publications has ensued in the last
receptor blockers are considered the standard of care for few decades describing the beneficial effects of inhibition of
treatment of cardiovascular disease and chronic kidney the renin–angiotensin system in cardiovascular disease and
disease. Combination therapy with both angiotensin- chronic kidney disease (CKD). Angiotensin-converting en-
converting enzyme inhibitors and angiotensin II receptor zyme (ACE) inhibition has improved survival and reduced
blockers effectively inhibits the renin–angiotensin system morbidity in hypertension, congestive heart failure (CHF),
as well as potentiates the vasodilatory effects of bradykinin. acute myocardial infarction, and halts progression in CKD.
It has been advocated that this dual blockade approach Angiotensin receptor blockers (ARBs) have been shown to be
theoretically should result in improved clinical outcomes in equally efficacious to ACE inhibition in both cardiovascular
both cardiovascular disease and chronic kidney disease. disease and CKD populations. ACE inhibitors or ARBs are
Clinical trial evidence for the use of combination therapy with considered the standard of care for treatment of hypertension
angiotensin-converting enzyme inhibitors and angiotensin II in diabetes mellitus and CKD, reducing proteinuria and CKD
receptor blockers in cardiovascular disease has provided progression, and improving outcomes in CHF and acute
conflicting results in hypertension, congestive heart failure, myocardial infarction. As both of these classes of medications
and ischemic heart disease. Clinical trial evidence to support were used in both cardiovascular disease and CKD, the theory
combination therapy with angiotensin-converting enzyme that ‘more is better’ ensued and dual blockade of the
inhibitors and angiotensin II receptor blockers in chronic renin–angiotensin system was advocated by many leading
kidney disease has largely been based on proteinuria physicians. This review serves as an appraisal on the clinical
reduction as a surrogate marker for clinically meaningful evidence of combination ACE inhibition and angiotensin
outcomes. Recent large-scale randomized clinical trials have receptor blockade (combination therapy) in both cardiovas-
not been able to validate protection in halting progression cular disease and CKD.
in chronic kidney disease with a dual blockade approach. The belief of impurities in the blood causing hypertension
This review serves as an appraisal on the clinical evidence of dates back well over 2000 years in ancient Egypt and Asia.
combination angiotensin-converting enzyme inhibition and The association between hypertension, cardiovascular dis-
angiotensin II receptor blockade in both cardiovascular ease, and kidney disease was first made by Richard Bright1 in
disease and chronic kidney disease. 1836. Modern day hypertension was described by Frederick
Kidney International (2011) 80, 245–255; doi:10.1038/ki.2011.142; Mahomed2 in 1872 with primitive sphygmography. Devel-
published online 1 June 2011 opment of the blood pressure cuff by Riva-Rocci3 in 1896 and
KEYWORDS: ACE inhibitors; angiotensin; cardiovascular disease; auscultation of blood flow with such a device by Nikolai
chronic kidney disease Korotkoff4 in 1905 pioneered research into the physiological
mechanisms of hypertension.
The renin–angiotensin system was first described by
Tigerstedt and Bergman5 in 1898, with the observation that
infusion of a substance termed ‘renin’ isolated from rabbit
kidneys induced hypertension. The importance of the kidney
in modulating hypertension was shown by the work of Harry
Goldblatt et al.6 in 1934 who demonstrated that renal
ischemia resulted in an animal model of hypertension. This
Correspondence: Michelle W. Krause, Division of Nephrology, Department
of Internal Medicine, University of Arkansas for Medical Sciences, Central
model, termed ‘Goldblatt hypertension’, supported Tigerstedt
Arkansas Veterans Healthcare System, Little Rock, Arkansas 72205, USA. and Bergman’s previous description of a vasopressor
E-mail: krausemichellew@uams.edu substance released into the renal vein during periods of
Received 1 October 2010; revised 9 February 2011; accepted 22 March renal ischemia. Frey and Krant discovered kallirein in 1926,7
2011; published online 1 June 2011 and parallel groups of investigations led by Eduardo
Kidney International (2011) 80, 245–255 245
review MW Krause et al.: Combination inhibition of the renin–angiotensin system
Braun-Menendez and colleagues8 in Argentina and by Irving
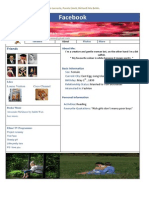
Angiotensinogen
Page and colleagues9 in Indianapolis in the 1930–1940s
demonstrated the pathway of renin activation to ‘hyperten- Renin Diect renin
ACE inhibitors
inhibitors
sin’ and ‘angiotonin’ by a renin activator. In 1956, Skeggs
et al.10 discovered ACE and by 1961 the terminology of
Angiotensin I
‘hypertensin’ and ‘angiotonin’ was changed to angiotensino-
gen as the renin substrate and angiotensin as the active Bradykinin ‘Angiotensin escape’
Chymase
metabolite.11 ACE Cathepsin G
The renin–angiotensin system was further characterized Angiotensin II
by efforts made by John Vane, Kevin Ng, Sergio Ferreira, Inactive
metabolites
Ervin Erdös, and Mick Bakhle and others who described Angiotensin II
receptor blockers
conversion of angiotensin I to angiotensin II in the
pulmonary circulation through ACE with bradykinin in- Angiotensin II receptor(s)
activation.12,13 A bradykinin-potentiating factor isolated
from pit viper venom (Bothrops jararac) resulted in ACE
Figure 1 | The renin-angiotensin system. ACE, angiotensin
inhibition and identified a novel mechanism in the treatment converting enzyme.
of hypertension.12 Collaboration with research scientists at
Squibb and other experts in the field at that time led to the
formulation of the first oral ACE inhibitor, captopril. Under of angiotensin II through non-ACE pathways.17 Therefore,
the direction of John Laragh and colleagues,14 a clinical trial combination therapy with inhibition of angiotensin II
for the treatment of hypertension with captopril was formation through ACE with ACE inhibitors and blockade
conducted in 1978 and led to Food and Drug Administration of angiotensin II binding to the AT receptors with ARBs
(FDA) approval of captopril for treatment of hypertension in provides a more complete blockade of the angiotensin effects.
1981. Drug development in the discovery of angiotensin II One caveat in combination therapy is that ACE inhibitors
receptor blockers and direct renin inhibitors occurred during and ARBs increase renin stimulation. Renin has been
the mid 1980s, but was limited by the use of peptide associated with increased risk for myocardial infarction and
compounds that had a short half-life and were not orally stroke,20 and therefore combination therapy is not ‘complete’
bioavailable. It was not until 1982 that a group of scientists at inhibition of the renin–angiotensin system (Figure 1). The
DuPont laboratories reviewed a US patent that described following serves as a review of the clinical evidence for
imidazole derivates that demonstrated angiotensin II recep- combination therapy in the treatment of hypertension, CHF,
tor-blocking activity.15 Such imidazole derivates were highly and ischemic heart disease.
selective for the angiotensin II receptor, but required massive
doses for potency that the possibility of drug development Hypertension
was abandoned in several laboratories. It was not until a One of the early treatment trials in hypertension with
molecular change with the addition of a tetrazole group that combination therapy was published in 2000 by Azizi et al.,21
losartan was developed and ultimately approved as the first who reported that combination of losartan and enalapril
ARB by the FDA in 1995. Finally, aliskiren, the first oral direct compared with monotherapy with either agent alone had a
inhibitor of renin, was developed and approved for treatment greater diastolic blood pressure lowering at study clinic visits
of primary hypertension by the FDA in 2007. but did not show a significant difference in the 24-h mean
ambulatory blood pressure. A series of other trials of
DUAL BLOCKADE OF THE RENIN–ANGIOTENSIN SYSTEM AND combination therapy for hypertension were published
CARDIOVASCULAR DISEASE between 2000 and 2005 but were limited by small sample
Rationale sizes, lack of pre-defined hard clinical end points, use of non-
There are good theoretical reasons why complete inhibition generalizable subject populations, short durations of follow-
of the renin–angiotensin system should result in improved up, or suboptimal drug dosing regimens.22–24 In 2001, Weir
clinical outcomes in cardiovascular disease. In addition to et al.25 reported in 6465 hypertensive subjects that the most
blood pressure regulation and cardiac remodeling, angioten- significant blood pressure-lowering effects were seen with
sin has been implicated in inflammation, oxidative stress, candesartan and diuretics, followed by candesartan and
atherosclerosis, and has proliferative effects through the AT2 calcium antagonists, candesartan and b-blockers, and the
receptor.16–19 ACE inhibitors block the conversion of least effective combination was with candesartan and ACE
angiotensin I to angiotensin II and additionally inhibit inhibitors. This trial confirmed the results of the AURA
kininase II, potentiating the beneficial vasodilatory effects of (Atacand Under Real-life Aspects) study that candesartan was
bradykinin and nitric oxide resulting in blood pressure an effective anti-hypertensive agent in primary hypertension;
lowering.16–18 However, with ACE inhibitors alone, there is however, when added on to other classes of antihypertensive
incomplete inhibition of the renin–angiotensin system medications, combination of ACE inhibitors and ARBs had
because of ‘angiotensin escape’, which refers to production the weakest effect.26 The AMAZE (A Multicenter trial using
246 Kidney International (2011) 80, 245–255
MW Krause et al.: Combination inhibition of the renin–angiotensin system review
Atacand and Zestril versus zestril to Evaluate the effects on the study population that was normotensive contributed to
lowering blood pressure) trials included two randomized, excess syncopal and hypotensive events and likely increased
multi-centered, double-blind controlled investigations com- the cardiovascular events in this trial. This is in keeping with
paring the combination of lisinopril and candesartan versus previous studies, which have suggested that reducing blood
maximizing the dose of lisinopril alone.27 The results of the pressure is associated with a J-curve in which lower blood
studies provided conflicting data with one of the cohorts pressures are associated with increased cardiovascular
demonstrating that combination therapy was superior, events.30,31 In all practically, enrolling normotensive subjects
whereas the second cohort could not corroborate the in a dual anti-hypertensive clinical trial targeted at drug
same results. dosing rather than at blood pressure should have raised
A meta-analysis published in 2005 by Doulton et al.28 concern. Despite criticisms of the trial design, the results of
summarized the evidence of combination therapy as the ONTARGET trial resulted in leading investigators along
compared with monotherapy for the treatment of hyperten- with the Canadian Hypertensive Education Program and the
sion. A series of 14 publications met the author’s inclusion Canadian Heart and Stroke Foundation in 2009 to strongly
criteria of hypertensive subjects, having blood pressure advise discontinuation of combination therapy in the
control as an outcome measure and requiring that the trial treatment of hypertensive patients.32,33
design include randomization. The results showed that blood
pressure reductions were modest with combination therapy Congestive heart failure
as compared with monotherapy and achieved statistical If there is a lack of superiority established thus far with the
significance; however, the authors concluded that the impact use of combination therapy in hypertension (Table 1) or in a
on clinical outcomes was likely to be minor and therefore high-risk cardiovascular population, are there other cardio-
were unable to recommend combination therapy over other vascular disease states such as CHF or myocardial infarction
drug regimens. in which combination therapy may be effective? Several
The ONTARGET (The ONgoing Telmisartan Alone and in clinical trials have been performed to address these popula-
combination with Ramipril Global End point Trial) trial was tions, including RESOLVD (Randomized Evaluation of
a landmark trial of combination therapy in high-risk subjects Strategies for Left Ventricular Dysfunction), Val-HeFT
with cardiovascular disease or diabetes mellitus without (VALsartan Heart Failure Trial), and the CHARM (Cande-
CHF.29 A total of 25,620 subjects underwent randomization sartan in Heart failure: Assessment of Reduction in Mortality
into three double-blind treatment arms of ramipril (10 mg and morbidity) studies.34–38 RESOLVD was a pilot study
daily), telmisartan (80 mg daily), or the combination of published in 1999 to study the effects of candesartan and
telmisartan and ramipril daily. The primary outcomes of this enalapril as compared with candesartan alone or enalapril
investigation were twofold: first to determine non-inferiority alone in symptomatic subjects with NYHA (New York Heart
of telmisartan compared with ramipril and second to Association) Class II–IV CHF with a reduced ejection
investigate whether combination therapy was superior to fraction.31 The authors concluded that there was a benefit
ramipril alone in a composite end point of death from of combination therapy on left ventricular remodeling based
cardiovascular causes, myocardial infarction, stroke, or on measurements of left ventricular systolic and diastolic
hospitalization for heart failure. Approximately 69% of those volumes but lacked meaningful clinical end points in the
enrolled in the study had baseline primary hypertension. All study. The Val-HeFT trial was a multi-centered randomized
three treatment groups demonstrated effective lowering of double-blind placebo-controlled trial of 5010 subjects with
blood pressure that was persistent throughout the study, and CHF as defined by NYHA Class II–IV with reduced ejection
in the combination group, there was a greater reduction in fraction.32 Valsartan was compared with standard CHF
blood pressure as compared with the single treatment arms. therapy, with the majority of subjects receiving ACE
Surprisingly, the beneficial effect of blood pressure lowering inhibitors. The results revealed that there was no significant
did not result in greater protection that has been shown in benefit with valsartan for mortality (relative risk 1.02 (0.88,
other cardiovascular studies. For the composite outcomes of 1.18)); however, a benefit was seen in the composite end
death from cardiovascular causes, myocardial infarction, points of mortality and morbidity (relative risk 0.87 (0.77,
stroke, or hospitalization for heart failure, combination 0.97)). Further evidence suggested benefit for the use of
therapy did not demonstrate a significant benefit over combination therapy for treatment of CHF with the
monotherapy (relative risk 0.98 (0.90, 1.07)). On review of publication of the CHARM studies (Table 2). The CHARM
the adverse events, combination therapy was associated with studies were a series of three clinical trials evaluating the
significantly greater episodes of hypotensive symptoms, effects of candesartan on subjects with CHF intolerant to
syncopal events, diarrhea, kidney impairment, and hyperka- ACE inhibitors (CHARM-Alternative trial),36 on subjects
lemia. The increased risk of these adverse events and the lack currently on ACE inhibitors with a preserved ejection
of benefit on the clinical composite outcomes of this study fraction (CHARM-Preserved trial),37 and on subjects cur-
led investigators to conclude that combination therapy is not rently on ACE inhibitors with a reduced ejection fraction
warranted for this patient population. Criticisms of this study (CHARM-Added trial).38 On the basis of these study results,
validly point out that using combination therapy for 30% of Practice Guidelines from both Europe and the United States
Kidney International (2011) 80, 245–255 247
review MW Krause et al.: Combination inhibition of the renin–angiotensin system
Table 1 | Clinical trials for dual therapy with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in
the treatment of hypertension
Study Design Inclusion criteria Sample size Intervention Outcome Results
21
Azizi et al. Double-blind, Essential 177 Losartan and 24-h Ambulatory BP Ambulatory BP: no difference
randomized hypertension enalapril and clinic BP Clinic BP: versus losartan 3.2
( 0.7, 5.7) versus enalapril 4.0
( 1.5, 6.4)
Stergiou et al.22 Cross-over DBP4100 mm Hg 20 Valsartan and 24-h Ambulatory BP SBP: 6.8± 9.7
benazepril DBP: 4.9±6.8
Weir et al.23 Open label, Hypertensive 88 Valsartan and Change in BP SBP: 2.4
randomized pilot African Americans benazepril DBP: 1.7
(P-value NS)
Morgan et al.24 Double-blind Systolic 23 Candesartan and 24-h Ambulatory BP versus candesartan alone 6.4
cross-over hypertension in the lisinopril versus (P=0.0003)
elderly over the age monotherapy versus lisinopril alone 3.3
of 65 years (P=0.003)
Weir et al.25 Open label Essential 6465 Candesartan and Change in trough SBP: 16.4
clinical experience hypertension or other ACE inhibitors sitting BP DBP: 10.4
isolated systolic P-value o0.05
hypertension
Schulte et al.26 Open label Primary 4531 Candesartan and Change in sitting clinic DBP: 14.9
non-randomized hypertension other ACE inhibitors BP
27
Izzo et al. Double-blind, Essential Cohort 1: 537 Candesartan and Mean change in BP Cohort 1: 2.39 ( 3.86, 0.92)
randomized hypertension Cohort 2: 555 lisinopril Cohort 2: 1.21 ( 2.57, 0.16)
28
Doulton et al. Meta-analysis HTN subjects with 434 ACE inhibitor 24-h Ambulatory and versus ACE inhibitor alone
BP as outcome and ARB clinic blood pressure ambulatory BP: 4.7 ( 6.50, 2.87)
variable and Clinic BP: 3.8 ( 6.74, 0.94)
randomization versus ARB alone ambulatory
BP: 3.8 ( 5.29, 2.35)
Clinic BP: 3.7 ( 6.92, 0.39)
ONTARGET Double-blind, High-risk vascular 25,620 Telmisartan and CV death, myocardial versus ramipril
Investigators29 randomized disease or diabetes ramipril infarction, stroke, SBP: 2.4
mellitus with hospitalization for CHF DBP: 1.4
preserved ejection
fraction
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; CHF, congestive heart failure; CV, cardiovascular; DBP, diastolic
blood pressure; HTN, hypertension; NS, non-significant; SBP, systolic blood pressure.
Table 2 | Summary of the results of the CHARM studies (The effect of candesartan with chronic heart failure on standard-of-
care congestive heart failure therapy)
CHARM studies Study population Sample size Intervention Outcome Results
CHARM-Alternative trial CHF with NYHA II–IV, 2028 Candesartan 32 mg Cardiovascular death or HR 0.70 (0.60, 0.81),
intolerant of ACE inhibitors daily versus placebo hospitalization for CHF P-value o0.0001
CHARM-Preserved trial CHF with NYHA II–IV, 3023 Candesartan 32 mg Cardiovascular death or HR 0.86 (0.74, 1.0),
preserved ejection fraction daily versus placebo hospitalization for CHF P-value=0.051
CHARM-Added trial CHF with NYHA II–IV, reduced 2548 Candesartan 32 mg Cardiovascular death or HR 0.85 (0.75, 0.96),
ejection fraction of p40% daily versus placebo hospitalization for CHF P-value=0.01
Abbreviations: ACE, angiotensin-converting enzyme inhibitor; CHARM, Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity; CHF, congestive
heart failure; HR, hazard ratio; NYHA, New York Heart Association.
See references Granger et al.36; Yusuf et al.37; and McMurray et al.38
suggest recommendations for the use of combination therapy Ischemic heart disease
in CHF. The European guidelines recommend that an ARB is Clinical trials examining the efficacy of combination therapy
indicated in CHF with ejection fraction p40% if there are as compared with monotherapy are limited in subjects with
CHF symptoms on optimal standard-of-care therapy ischemic heart disease. In 2003, the VALIANT (VALsartan in
with ACE inhibitors (class of recommendation 1, level of Acute myocardial INfarction) trial showed that in subjects
evidence A).39 The United States guidelines are slightly with acute myocardial infarction with left ventricular
different, and in the most recent 2009 update, recommenda- dysfunction, there was no difference in mortality or in the
tions suggest that an ARB added to an ACE inhibitor may be secondary end points of death by cardiovascular causes,
considered in symptomatic CHF but should not be used with recurrent myocardial infarction, or hospitalization for CHF
other aldosterone antagonists (class II recommendation, level in the combination of valsartan and captopril as compared
of evidence B).40 The difference in the two guidelines focused with captopril alone.41 There is a paucity of clinical trials
on the strength of the evidence for a lack of all-cause examining the effects of combination therapy for myocardial
mortality benefit demonstrated in the combination therapy infarction with preserved left ventricular function, and in a
treatment groups in the aforementioned clinical trials.35–38 recent systematic review, the only clinical trial that was able
248 Kidney International (2011) 80, 245–255
MW Krause et al.: Combination inhibition of the renin–angiotensin system review
to be included was the ONTARGET trial.29,42 The authors more effective than monotherapy in blood pressure lowering
reiterated the previously published ONTARGET study and reducing albuminuria. However, this trial was criticized
conclusions that there was no benefit for combination by the use of suboptimal drug doses that were not applicable
therapy on reducing the risk of mortality or other to clinical practice. In a subsequent trial, Rossing et al.52
cardiovascular outcomes for myocardial infarction with added candesartan to maximal doses of an ACE inhibitor in
preserved ejection fraction and that there may be harm with type 2 diabetic subjects and demonstrated that there was
the increased risk of adverse events in the combination additional blood pressure lowering and proteinuria reduction
therapy group. with combination therapy as compared with monotherapy.
An insignificant decline in glomerular filtration rate (GFR)
Summary of the evidence for dual blockade of the was also noted in the combination treatment group.
renin–angiotensin system and cardiovascular disease Although this trial used drug dosing that was more applicable
At this time, there is no compelling evidence to recommend to clinical practice, it was still limited by a small sample size
the use of combination therapy simply for the treatment of of 20 subjects and short duration of 8 weeks follow-up.
hypertension. Combination therapy does not change all- Similar results have been demonstrated for combination
cause mortality in CHF; however, clinical evidence supports therapy in type 1 diabetics. In a small clinical trial of 20
that combination therapy may be considered for those with subjects, the combination of benazepril and valsartan was
symptomatic CHF with reduced ejection fraction to reduce more effective in reducing blood pressure and albuminuria at
cardiovascular death and hospitalizations. There is no benefit 8 weeks compared with monotherapy and confirmed the
of combination therapy for acute myocardial infarction with CALM study results.53 However, it is noteworthy that there
reduced or preserved left ventricular function. was a significant decline in the GFR in the combination
group of 10 ml/min per 1.73 m2 as compared with the
DUAL BLOCKADE OF THE RENIN–ANGIOTENSIN SYSTEM AND monotherapy groups that was reversible once therapy was
CKD discontinued. These studies were again criticized for using
Rationale suboptimal dosing regimens for both ACE inhibitors and
Blockade of the renin–angiotensin system with either ACE ARBs. Jacobsen et al.54 reported results of a small clinical trial
inhibitors or ARBs has been shown in numerous studies to in type 1 diabetics with hypertension and nephropathy, who
improve outcomes in CKD beyond their effects on lowering had a maximum dose of irbesartan added to maximal doses
systemic blood pressure. Although the precise mechanism is of enalapril. At 8 weeks, there were significant reductions in
not known, the additional beneficial effects have been blood pressure and in proteinuria excretion in the combina-
attributed to preferentially lowering intra-glomerular pres- tion therapy group than in the enalapril only group; however,
sure through effects on the efferent arteriole resulting in long-term follow-up was not reported.
reduction in proteinuria, blocking of mediators of inflam- A meta-analysis of the clinical trials for combination
mation, inhibiting oxidative stress, and eventually preventing therapy in diabetic subjects was published in 2007 by
fibrosis and renal scarring.43–45 Dual blockade of the Jennings et al. 55 This analysis included 10 trials with 156
renin–angiotensin system with ACE inhibitors and ARBs subjects receiving combination therapy. The results demon-
has been postulated to improve clinical outcomes in CKD strated that combination therapy was more effective in
superior to monotherapy by greater reduction in proteinuria, reducing proteinuria; however, there was a decrease in
which is a risk factor for CKD progression and end-stage estimated GFR (eGFR) and a trend toward increased
kidney disease (ESKD). The remainder of this review focuses creatinine in the combination group. The authors concluded
on the clinical evidence of combination therapy in both that recommendation for widespread use of combination
diabetic and non-diabetic kidney disease. therapy for diabetic nephropathy is limited by using
proteinuria as a surrogate marker for kidney outcomes, and
Diabetic kidney disease lack of hard clinical end points such as morality or
Diabetes mellitus is the leading cause of CKD and ESKD in cardiovascular events in the active treatment groups. In
the United States. Numerous randomized clinical trials have attempts to address issues of short follow-up times and small
demonstrated the benefit of ACE inhibitors or ARBs in sample sizes, the CALM II trial and the IMPROVE
albuminuria reduction and in slowing CKD progression in (Irbesartan in the Management of PRoteinuric patients at
diabetic nephropathy.46–50 The concept of dual blockade of high risk for Vascular Events) trial were performed to
the renin–angiotensin system with combination therapy was examine the effects of combination therapy on albuminuria
advanced in the late 1990s and early 2000s, with several small reduction and blood pressure control over a longer treatment
clinical trials suggesting renoprotection in diabetics with both time56 and in larger populations.57 In the CALM II trial, the
microalbuminuria and overt nephropathy. One of the largest combination of candesartan and lisinopril was compared
of these early clinical trials was performed by Mogensen with uptitration of lisinopril alone in hypertensive diabetic
et al.51 with the publication of the CALM (The Candesartan subjects. In contrast to the CALM study, at the end of 12
And Lisinopril Microalbuminuria) study. At 12 weeks, months, there was no significant difference in blood pressure
combination therapy with candesartan and lisinopril was lowering or albuminuria excretion in the combination group
Kidney International (2011) 80, 245–255 249
review MW Krause et al.: Combination inhibition of the renin–angiotensin system
as compared with lisinopril alone. In the IMPROVE trial, 405 consideration in the statistical analysis, then there was no
hypertensive subjects with microalbuminuria at high cardi- significant difference in eGFR decline with combination
ovascular risk previously on ACE inhibitors were randomized therapy as compared with monotherapy. In addition,
to combination therapy with irbesartan and ramipril versus criticisms accurately state that the need for dialysis was
ramipril alone. No significant difference was demonstrated in largely for acute dialysis and not for progression to ESKD. If
the combination therapy as compared with ramipril, despite acute dialysis was excluded from study analyses, then there
lower blood pressures in the combination arm. In a was no difference in combination therapy as compared with
subanalysis of those with albumin excretion rates of monotherapy. However, it should be noted that although
X200 mg/min, there was a trend toward benefit with acute dialysis should not be included as events for dialysis
combination therapy as compared with ramipril, but this that implies ESKD, acute kidney injury requiring dialysis is
analysis was significantly underpowered to adequately not benign. Acute kidney injury is associated with significant
address the use of combination therapy in macroalbuminur- morbidity and mortality and even if kidney function
ia. These results mirrored previous hypertension studies that recovers, acute kidney injury is one of the greatest risk
combination therapy has not been proven to be superior to factors for the development of CKD and progression to
maximal doses of monotherapy,27 and raise the question that ESKD especially in an elderly population.66 Therefore, any
if blood pressure is similar in the treatment groups, is there a dialysis places an individual at risk, and exclusion of these
demonstrable renoprotective effect unique to ACE inhibitors events in attempts to justify the use of combination therapy for
and ARBs in CKD? This was brought into question with the CKD needs to be carefully considered. If one interprets the
publication of a meta-analysis by Casas et al.,58 in 2005, who ONTARGET trial with the flaws and criticisms for kidney
reported that there was no added benefit of ACE inhibitors or outcomes, then the best interpretation is that there is no
ARBs in doubling of the serum creatinine or progression to additional benefit with combination therapy as compared with
ESKD in diabetic subjects compared with other anti- monotherapy in high-risk cardiovascular and diabetic subjects.
hypertensive medications independent of their blood pres- Another factor to consider is the generalizability of the
sure-lowering effects. ONTARGET trial to the diabetic population. Only 4% of the
Despite the lack of supporting clinical evidence of benefit diabetics enrolled in this trial had macroalbuminuria, and
with clinically meaningful outcomes, the use of combination evidence has suggested that this high-risk population is the
therapy for diabetic kidney disease became a widespread most likely to derive benefit from combination therapy.
practice among many nephrologists and other treating Clinical trial evidence is lacking at this time to fully answer
physicians in the community. Concerns regarding the safety how best to manage diabetics with overt nephropathy. There
of combination therapy arose with the publication of the is some evidence that suggests that in a small cohort of
ONTARGET study29 specifically with regard to kidney patients with nephrotic range proteinuria, targeted treatment
outcomes.59 Pre-specified analyses of the ONTARGET trial of proteinuria is beneficial. Ruggenenti et al. published their
were performed to determine the effects of combination experience using ‘remission clinics’ in the treatment of CKD.
therapy on a primary composite outcome of dialysis, In this cohort, one-third of the subjects with nephrotic range
doubling of serum creatinine, or death, and second by proteinuria were diabetic and were compared with matched
changes in surrogate markers of kidney disease by decline in historical controls of therapy targeted at blood pressure.67
eGFR and change in albuminuria as measured by urinary The remission clinics initiated therapy with ramipril, then
albumin-to-creatinine ratios. Despite a reduction in albumi- losartan, and finally verapamil titrated to maximal doses to
nuria, there was an increased number of primary events with reduce proteinuria excretion to o0.3 g/day. When analyzing
combination therapy (hazard ratio 1.09 (1.01, 1.18)). diabetic subjects as compared with non-diabetics, active
Combination therapy also increased the risk for dialysis or remission clinic treatment reduced 24 h proteinuria excretion
doubling of serum creatinine (hazard ratio 1.24 (1.01, 1.51)). by 50%, but only a small percentage of diabetics achieved
The authors concluded that there was no benefit of remission of o0.3 g/day. In addition, diabetics in remission
combination therapy on kidney outcomes, and in fact, clinics did not demonstrate protection in decline in eGFR as
combination therapy led to overall harm. The results of this did non-diabetic subjects, and there was no benefit in
trial led to widespread criticisms of the study design and diabetic subjects in the projection of reaching ESKD.
flaws in the interpretation of the results.60–62 Such criticisms Acceptable goal blood pressures were not achieved in diabetic
include a lack of standardization of creatinine, single subjects, and thus may have influenced the study results with
measurements of both creatinine and urinary albumin-to- this targeted proteinuria approach.
creatinine ratios at study visits, and the use of eGFR based on If one considers ONTARGET a failure for combination
the Modification of Diet in Renal Disease study equation that therapy acknowledging the criticisms, then why did it fail?
is not validated in populations with eGFR 460 ml/min per Was the perception of benefit of combination therapy
1.73 m2 or in older subjects that were included in this brought on by our interpretation of previous small, under-
trial.63,64 Moreover, the early decline in eGFR in combination powered, clinical trials in which proteinuria reduction was
therapy was an anticipated hemodynamic effect that has been used as a surrogate marker for CKD outcomes? There have
demonstrated in other clinical trials,65 and if taken into been numerous occasions in which a surrogate marker of a
250 Kidney International (2011) 80, 245–255
MW Krause et al.: Combination inhibition of the renin–angiotensin system review
Table 3 | Current clinical trials of combination therapy in diabetic nephropathy: NEPHRON-D study (Combination angiotensin
receptor blockers and angiotensin-converting enzyme inhibitor for the treatment of diabetic nephropathy), VALID trial
(Preventing ESRD in overt nephropathy of type 2 diabetes), and the ALTITUDE trial (Aliskiren in Type 2 Diabetes using
cardiorenal end points)
Clinical trial Study population Intervention Primary outcome
NEPHRON-D Type 2 diabetes mellitus with Losartan and lisinopril versus losartan Reduction of 30 ml/min per 1.73 m2 for those
macroalbuminuria alone with a eGFRX60 ml/min per 1.73m2
Reduction in 50% of eGFR for those with a
eGFR of o60 ml/min per 1.73 m2 at study initiation
Progression to ESKD
Death
VALID Type 2 diabetes mellitus with Benazepril and valsartan versus Progression to ESKD
macroalbuminuria 42000 mg/g benazepril or valsartan alone
ALTITUDE Type 2 diabetes mellitus and/or Aliskiren versus ACE inhibitors or Cardiovascular death
AgeX35 years angiotensin receptor blockers Resuscitated death
Microalbuminuria and eGFR Myocardial infarction
30–60 ml/min per 1.73 m2 Stroke
History of cardiovascular Hospitalization for CHF
disease with an eGFR ESKD
30–60 ml/min per 1.73 m2 Doubling of serum creatinine
Abbreviations: ACE, angiotensin-converting enzyme; CHF, congestive heart failure; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease. See references
Freid et al.74; Parving et al.75; and Dillon.76
disease does not correlate with clinical benefit especially in proteinuria may be a viable option for surrogacy for CKD
cardiovascular disease trials.68,69 At present, the FDA will only outcomes, but the evidence for this approach is not as strong
accept hard clinical end points for drug approval for the as in nephrotic syndromes. There are currently three ongoing
treatment of CKD, which includes the need for dialysis large randomized controlled treatment trials and include the
or kidney transplantation, doubling of serum creatinine, NEPHRON-D study (Combination ARBs and ACE inhibitor
cardiovascular disease events, or death. Proteinuria reduction for the treatment of diabetic nephropathy: NCT00555217),74
is not accepted at this time as a valid surrogate marker for the VALID trial (Preventing ESRD in Overt Nephropathy of
CKD progression. In addition, any clinical trial of CKD using Type 2 Diabetes: NCT00494715) (http://clinicaltrials.gov/ct2/
microalbuminuria as a surrogate marker of nephropathy is show/study/NCT00494715), and the ALTITUDE (Aliskiren
now in question with evidence to suggest that microalbumi- in Type 2 Diabetes using cardio-renal end points:
nuria may be considered as a marker of endothelial NCT00549757) trial75 (Table 3). Results of these trials will
dysfunction rather than nephropathy, there is a lack of likely provide further insights into the role of combination
correlation for reduction in microalbuminuria and clinical therapy in diabetic nephropathy.
outcomes in progressive kidney failure, there is spontaneous
resolution of microalbuminuria in up to one-third of Non-diabetic kidney disease
patients, and finally, microalbuminuria is markedly variable Non-diabetic kidney disease is believed to be a different
in patients on repeated measurements.70–72 In 2008, the entity as compared with diabetic kidney disease and may
National Kidney Foundation and the FDA led a scientific correspond with diseases that are more apt to respond to
workshop entitled ‘Proteinuria as a Surrogate Outcome in combination therapy. Several small clinical trials have
Chronic Kidney Disease’.73 The charge of the workshop was demonstrated the benefit of proteinuria reduction and blood
to address three areas: ‘1. the importance of proteinuria in pressure lowering with combination therapy in IgA nephro-
CKD, 2. evaluation of surrogacy in clinical trials, and pathy76,77 and in primary glomerular diseases.78,79 Further
3. evaluation of change in proteinuria as a surrogate outcome clinical evidence for the benefit of combination therapy in
in kidney disease progression in 3 broad clinical areas: early non-diabetic kidney disease occurred in 2003 with the
diabetic kidney disease, nephrotic syndrome, and disease publication of the COOPERATE (Combination treatment
with mild to moderate proteinuria’.73 The conclusions of the of angiotensin receptor blocker and angiotensin converting
scientific panel at this workshop was that there is insufficient enzyme inhibitor in non-diabetic renal disease) study.80 The
evidence that proteinuria reduction correlates with outcomes COOPERATE study was designed to evaluate the efficacy of
in CKD and should not be used as a primary end point in combination therapy with trandolapril and losartan as
clinical treatment trials for early diabetic nephropathy. There compared with monotherapy alone, and the authors
was support for the use of proteinuria reduction as a concluded that combination therapy was superior to
surrogate outcome in nephrotic syndromes especially if monotherapy by reducing CKD progression measured by
remission is achieved as this has been reliably associated with doubling of the serum creatinine or ESKD. However, in 2006,
improved CKD outcomes in past studies. Targeted protei- the COOPERATE trial study results came into question by a
nuria reduction in kidney diseases with mild-to-moderate letter to the Editor in the American Journal of Nephrology by
Kidney International (2011) 80, 245–255 251
review MW Krause et al.: Combination inhibition of the renin–angiotensin system
Anil Bidani with regard to blood pressure reporting,81 and DIRECT RENIN INHIBITION COMBINED WITH ACE INHIBITORS
additionally in preparation for a meta-analysis of combina- OR ARBs
tion therapy in CKD, Kunz et al.82 submitted a letter of As stated earlier, combination therapy does not result in
warning to The Lancet that suggested that further investiga- complete inhibition of the renin–angiotensin system. Renin
tion should be performed to validate the previously is a significant risk factor for cardiovascular disease and
published COOPERATE trial results. The Lancet responded kidney injury,20,90 and plasma renin activity increases with
to the letter of warning and in 2008 retracted the ACE inhibitor and ARB use. It may be that this ‘incomplete’
COOPERATE trial after investigation concluded that the blockade of the renin–angiotensin system with combination
scientific merits of the study followed gross misconduct. therapy has led to the lack of benefit seen thus far in
After retraction of the COOPERATE trial, two subsequent cardiovascular disease and CKD trials. After approval in
meta-analysis studies were published examining the effects of 2007, clinical trials were developed to study the effects of
combination therapy in CKD.83,84 Both meta-analyses aliskiren in combination with either ACE inhibitors or ARBs
demonstrated benefit in proteinuria reduction with combi- in cardiovascular disease and CKD. Recently, the results of
nation therapy; however, the inclusion of the trials was the ASPIRE (Aliskiren Study in Post-MI patients to Reduce
limited by small sample sizes, short-term follow-up, lack of rEmodeling) trial were presented at the American College of
demonstrable benefit for meaningful clinical end points such Cardiology in 2010. In this trial, aliskiren was added to either
as mortality, progression of CKD, or ESKD, and the authors ACE inhibitors or ARBs in subjects with acute myocardial
concluded that clinical evidence is lacking to support the use infarction and left ventricular dysfunction. The investigators
of combination therapy in the treatment of CKD. cited that there was no benefit of aliskiren on cardiovascular
In contrast to the results published in the meta-analyses for death, recurrent myocardial infarction, stroke, and resusci-
CKD, Ruggenenti et al.67 have demonstrated dramatic results tated sudden death.91 Little evidence is available for direct
with a targeted proteinuria reduction approach with combi- renin inhibition with CHF; however, an ongoing trial, the
nation therapy in their remission clinics for non-diabetic ATMOSPHERE trial (Efficacy and Safety of Aliskiren and
kidney disease. Approximately 30% of the non-diabetic Aliskiren/Enalapril Combination on Morbi-mortality in
subjects achieved remission proteinuria of p0.3 g/day. There Patients With Chronic Heart Failure: NCT00853658), is
was a significant reduction in the projection to ESKD in the evaluating the efficacy and safety of aliskiren and aliskiren/
subject’s lifetime from 72 to 28%, and the projected time to enalapril combination in patients with chronic heart
reach ESKD increased from 8 years to 29 years. Perhaps failure with a primary outcome of cardiovascular death or
targeting proteinuria rather than blood pressure is an optimal CHF hospitalization (http://clinicaltrials.gov/ct2/show/
approach in the management of high-risk CKD. NCT00853658). Similar to previous CKD trials, the AVOID
Future trials may be able to further provide us with (Aliskiren in the Evaluation of Proteinuria in Diabetes) trial
recommendations for combination therapy in non-diabetic has demonstrated efficacy for aliskiren on proteinuria
CKD. The HALT PKD (HALT Progression of Polycystic Kidney reduction in combination with losartan for diabetic nephro-
Disease: NCT00283686) trial is evaluating the effect of the pathy over a short time period but lacked clinical end
combination of lisinopril and telmisartan compared with points.92 The ongoing ALTITUDE trial75 will be provide
lisinopril alone in polycystic kidney disease, with outcomes further insights into the role of aliskiren in the treatment of
measured by change in total kidney volume by abdominal diabetic nephropathy.
magnetic resonance imaging for those with eGFR 460 ml/min
per 1.73 m2, or time to reach 50% reduction in baseline eGFR, CURRENT RECOMMENDATIONS AND FUTURE DIRECTIONS
ESKD, or death for those with eGFR 25–60 ml/min per 1.73 m2 FOR THE USE OF COMBINATION THERAPY
(ref. 85). The LIRICO (Long-term Impact of RAS Inhibition on On the basis of evidence to date, we have summarized
Cardiorenal Outcomes) trial is designed to evaluate the effects current recommendations in Table 4 (http://www.
of combination therapy on high-risk subjects with micro- uspreventiveservicestaskforce.org/3rduspstf/ratings.htm). We
albuminuria with a primary composite outcome of cardiovas- believe that proteinuria remission is a reasonable target for
cular death, coronary hearty disease, nonfatal stroke, those with CKD and those with macroalbuminuria should be
hospitalization for a cardiovascular event.86 considered a high-risk population that may benefit from
such an approach until further randomized clinical trial
Summary of the evidence for dual blockade of the evidence with meaningful clinical outcomes is available to
renin–angiotensin system and CKD guide our practice. Importantly, it should be emphasized
Large-scale randomized controlled outcome trials thus far that there have been no adequately powered completed
have failed to demonstrate superiority for combination clinical trials or ongoing clinical trials that will fully
therapy for halting progression of CKD. An argument should address cardiovascular outcomes with combination therapy
be made that targeting proteinuria rather than blood pressure with ACE inhibitors and angiotensin II receptor blockers
and using supra-therapeutic dosing regimens may have an in CKD.
advantage over our current practice patterns for combination At present, development of novel therapies for halting
therapy in CKD.67,87–89 progression in CKD requires extensive resources and
252 Kidney International (2011) 80, 245–255
MW Krause et al.: Combination inhibition of the renin–angiotensin system review
Table 4 | Summary of recommendations based on clinical evidence for the use of dual blockade of the renin–angiotensin
system with ace inhibitors and angiotensin receptor blockers for cardiovascular and chronic kidney disease
Cardiovascular disease
Hypertension
Level of evidence D: No clinical evidence that supports recommendation for combination therapy in hypertension
Congestive heart failure
Preserved ejection fraction
Level of evidence D: No clinical evidence that supports recommendation for combination therapy
Reduced ejection fraction
Level of evidence B: No clinical evidence that supports recommendation for combination therapy for all-cause mortality, consideration for
combination therapy to reduce hospitalization for congestive heart failure or reduce cardiovascular death
Ischemic heart disease
Preserved ejection fraction
Level of evidence D: No clinical evidence that supports recommendation for combination therapy
Reduced ejection fraction
Level of evidence D: No clinical evidence that supports recommendation for combination therapy
Chronic kidney disease
Diabetic kidney disease
Microalbuminuria
Level of evidence I: Limited clinical evidence that supports recommendation for combination therapy
Macroalbuminuria
Level of evidence I: Limited clinical evidence that supports recommendation for combination therapy and awaiting further clinical trial evidence
for guidance
Non-diabetic kidney disease
Proteinuria o500 mg/day
Level of evidence I: Limited clinical evidence that supports recommendation for combination therapy and awaiting further clinical trial evidence
Proteinuria X500 mg/day
Level of evidence C: Limited clinical evidence that supports recommendation for combination therapy but favors use while awaiting further
clinical trial evidence
Level of evidence based on the US Preventive Services Task Force. Level A: good scientific evidence suggests that the benefits of the clinical service substantially outweigh the
potential risks. Clinicians should discuss the service with eligible patients. Level B: at least fair scientific evidence suggests that the benefits of the clinical service outweigh the
potential risks. Clinicians should discuss the service with eligible patients. Level C: at least fair scientific evidence suggests that there are benefits provided by the clinical
service, but the balance between benefits and risks are too close for making general recommendations. Clinicians need not offer it unless there are individual considerations.
Level D: at least fair scientific evidence suggests that the risks of the clinical service outweigh potential benefits. Clinicians should not routinely offer the service to
asymptomatic patients. Level I: Scientific evidence is lacking, of poor quality, or conflicting, such that the risk versus benefit balance cannot be assessed. Clinicians should help
patients understand the uncertainty surrounding the clinical service.
large-scale randomized clinical trials lasting several years. As DISCLOSURE
VAF: conflicts to report: Research support (to Tulane): Grants
a result, proteinuria has often been used as a surrogate from Novo-Nordisk, Sanofi-Aventis, Eli Lilly, Pan American
marker to indicate potentially beneficial effects in halting Laboratories, Halozyme, Daiichi-Sankyo, NIH; Honoraria for
CKD progression. However, several examples exist that have Consulting and Lectures: Glaxo Smith Kline, Novartis, Takeda, Novo
demonstrated that reduction in proteinuria does not always Nordisk, Sanofi-Aventis, Eli Lilly, Daiichi Sankyo, Pan American
lead to long-term benefit in halting progression of kidney Laboratories, Xoma, NuMe Health. MWK and SVS declared no
competing interests.
disease. In contrast, there are other examples wherein
therapies had no effect on proteinuria but were effective in
REFERENCES
halting progression in CKD.93–95 Thus, we are in need of 1. Bright R. Tubular view of the morbid appearances in 100 cases
other biomarkers for CKD progression that are easy to connected with albuminous urine: with observations. Guy’s Hops Rep
measure and serve as acceptable surrogates for clinical 1836; 1: 380–400.
2. Mahomed FA. The physiology and clinical use of the sphygmograph.
efficacy both to the regulatory agencies and to the nephrology Med Times Gaz 1872; 1: 62–64.
community. Recent studies have indicated that the urinary 3. Riva-Rocci S. Un sfigmomanometro nuovo. Gazetta Medica di Torino
biomarkers for acute kidney injury, namely neutrophil 1896; 47: 981–986.
4. Korotkoff NS. On methods of studying blood pressure [in Russian]. Bull
gelatinase-associated lipocalin, kidney injury molecule, and Imperial Mil Med Acad 1905; 11: 365–367.
liver fatty acid-binding protein may also be useful to predict 5. Tigerstedt R, Bergman PG. Niere Und Kreislauf. Skand Arch Physiol 1898;
progression in CKD.96–101 There is a need to urgently obtain 8: 223–227.
6. Goldblatt H, Lynch J, Hanzal RF et al. Studies on experimental
robust data, which would evaluate the utility of the known hypertension. The production of persistent elevation of systolic blood
and yet undiscovered biomarkers for predicting CKD pressure by means of renal ischaemia. J Exp Med 1934; 59: 347–379.
7. Werle E. Discovery of the most important kallikreins and kallikrein
progression that would serve as acceptable surrogates for inhibitors. Erdos EG (eds). Bradykinin, Kallidin, and Kallikrein Handbook of
drug development rather than awaiting the results of large- Experimental Pharmacology. Springer Verlag: Berlin, 1970, XXV 1–6.
scale randomized clinical trials that in the recent past have 8. Leloir LF, Munoz JM, Braun-Menendez E et al. La secretion de la renina y
la formacion de hipertensina. Rev Soc Arg Biol 1940; 16: 75–80.
not provided us with solid evidence to change our current 9. Kohlstaedt KG, Helmer OM, Page IH. Activation of renin by blood
practice in the treatment of progressive CKD. colloids. Proc Soc Exp Biol Med 1938; 39: 214–215.
Kidney International (2011) 80, 245–255 253
review MW Krause et al.: Combination inhibition of the renin–angiotensin system
10. Skeggs LT, Dorer FE, Kahn JR et al. Experimental renal hypertension: the 35. Cohn JN, Tognoni G, for the Valsartan Heart Failure Trial Investigators. A
discovery of the renin-angiotensin system. Soffer R (eds). Biochemical randomized trial of the angiotensin-receptor blocker valsartan in
Regulation of Blood Pressure. John Wiley & Sons: Hoboken, 1981: 3–38. chronic heart failure. N Engl J Med 2001; 345: 1667–1675.
11. Tacquini Jr AC, Taquini AC. The renin-angiotensin system in 36. Granger CB, McMurray JJ, Yusuf S, for the CHARM Investigators. Effects
hypertension. Am Heart J 1961; 62: 558–564. of candesartan in patients with chronic heart failure and reduced left-
12. Ferreira SH, Greene LJ, Alabaster VA et al. Activity of various fractions of ventricular systolic function intolerant to angiotensin-converting-
bradykinin potentiating factor against angiotensin I converting enzyme. enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003; 362:
Nature 1970; 225: 379–380. 772–776.
13. Erdös EG, Yang HY. An enzyme in microsomal fraction of kidney that 37. Yusuf S, Pfeffer MA, Swedberg K et al. Effects of candesartan in patients
inactivates bradykinin. Life Sci 1967; 6: 569–574. with chronic heart failure and preserved left-ventricular ejection
14. Case DB, Atlas SA, Laragh JH et al. Clinical experience with blockade of fraction: the CHARM-Preserved Trial. Lancet 2003; 362: 777–781.
the renin-angiotensin-aldosterone system by an oral converting enzyme 38. McMurray JJ, Östergren J, Swedberg K et al. Effects of candesartan in
inhibitor (SQ 14,225, captopril) in hypertensive patients. Prog Cardiovasc patients with chronic heart failure and reduced left-ventricular systolic
Dis 1978; 21: 195–206. function taking angiotensin-converting-enzyme inhibitors: the CHARM-
15. Yoshiyasu F, Kishimoto S, Nishikawa K. Hypotensive imidazole Added trial. Lancet 2003; 362: 767–771.
derivatives. US Patent 1982; 4: 340–598. 39. Dickstein K, Cohen-Solal A, Filippatos G, for the Task Force for Diagnosis
16. Swedberg K, Eneroth P, Kjekshus J, and the CONSENSUS Trial Study and Treatment of Acute and Chronic Heart Failure 2008 of European
Group. Hormones regulating cardiovascular function in patients with Society of Cardiology. ESC Guidelines for the diagnosis and treatment of
severe congestive heart failure and their relation to mortality. Circulation acute and chronic heart failure 2008: the Task Force for the Diagnosis
1990; 82: 1730–1736. and Treatment of Acute and Chronic Heart Failure 2008 of the European
17. Unger T. The role of the renin-angiotensin system in the development of Society of Cardiology. Developed in collaboration with the Heart Failure
cardiovascular disease. Am J Cardiol 2002; 89: 3A–9A. Association of the ESC (HFA) and endorsed by the European Society of
18. Schieffer B, Bünte C, Witte J et al. Comparative effects of AT-1 Intensive Care Medicine (ESICM). Eur Heart J 2008; 29: 2388–2442.
antagonism and angiotensin-converting enzyme inhibition on markers 40. Jessep M, Abraham WT, Casey DE, for the 2005 Heart Failure Writing
of inflammation and platelet aggregation in patients with coronary Committee. 2009 Focused Update: ACCF/AHA Guidelines for the
artery disease. J Am Coll Cardiol 2004; 44: 362–368. Diagnosis and Management of Heart Failure in Adults. A Report of the
19. Unger T, Stoppelhaar M. Rationale for double renin-angiotensin- American College of Cardiology Foundation/American Heart Association
aldosterone-system blockade. Am J Cardiol 2007; 100: 25J–31J. Task Force on Practice Guidelines: developed in collaboration with the
20. Brunner HR, Laragh JH, Baer L et al. Essential hypertension: renin and International Society for Heart and Lung Transplantation. Circulation
aldosterone, heart attack, and stroke. N Engl J Med 1972; 286: 441–449. 2009; 119: 1972–2016.
21. Azizi M, Linhart A, Alexander J et al. Pilot study of combined blockade of 41. Pfeffer MA, McMurray J, Velazquez EJ, for the Valsartan in acute
the renin-angiotensin system in essential hypertensive patients. myocardial infarction trial investigators. Valsartan, captopril, or both in
Hypertension 2000; 18: 1139–1147. myocardial infarction complicated by heart failure, left ventricular
22. Stergiou GS, Skeva II, Baibas NM et al. Additive hypotensive effect of dysfunction, or both. N Engl J Med 2003; 349: 1893–1906.
angiotensin-converting enzyme inhibition and angiotensin-receptor 42. Baker WL, Coleman CI, Kluger J et al. Systematic review: comparative
antagonism in essential hypertension. Cardiovasc Pharmacol 2000; 35: effectiveness of angiotensin-converting enzyme inhibitors or
937–941. angiotensin II-receptor blockers for ischemic heart disease. Ann Intern
23. Weir MR, Smith DH, Neutel JM et al. Valsartan alone or with a diuretic or Med 2009; 151: 861–871.
ACE inhibitor as treatment for African American hypertensives: relation 43. Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies.
to salt intake. Am J Hypertens 2001; 14: 665–671. N Engl J Med 1998; 339: 1448–1456.
24. Morgan T, Anderson A, Bertrem D et al. Effect of candesartan and 44. Ruiz-Ortega M, Lorenzo O, Suzuki Y et al. Pro-inflammatory actions of
lisinopril alone and in combination on blood pressure and angiotensins. Curr Opin Nephrol Hypertens 2001; 10: 321–329.
microalbuminuria. J Renin Angiotensin Aldosterone Syst 2004; 5: 64–74. 45. Remuzzi G, Perico N, Macia M et al. The role of renin-angiotensin-
25. Weir MR, Weber MA, Neutel JM, for the ACTION Study Investigators. aldosterone system in the progression of chronic kidney disease. Kidney
Efficacy of candesartan cilexetil as add-on therapy in hypertensive Int 2005; 68: S57–S65.
patients uncontrolled on background therapy: a clinical experience trial. 46. Lewis EJ Hunsicker LG, Bain RP et al. The effect of angiotensin-
Am J Hypertens 2001; 14: 567–572. converting enzyme inhibition on diabetic nephropathy. The
26. Schulte KL, Fischer M, Meyer-Sabellek W. Efficacy and tolerability of Collaborative Study Group. N Engl J Med 1993; 329: 1456–1462.
candesartan cilexetil monotherapy or in combination with other 47. Heart Outcomes Prevention Evaluation Study Investigators. Effects of
antihypertensive drugs. Results of the AURA study. Clin Drug Invest 1999; ramipril on cardiovascular and microvascular outcomes in people with
18: 453–460. diabetes mellitus. Results of the HOPE Study and MICRO-HOPE Sub-
27. Izzo JL, Weinberg MS, Hainer JW et al. Antihypertensive efficacy of Study. Lancet 2000; 355: 253–259.
candesartan-lisinopril in combination vs up-titration of lisinopril: the 48. Lewis EJ, Hunsicker LG, Clarke WR, for the Collaborative Study Group.
AMAZE trials. J Clin Hypertens 2004; 6: 485–493. Renoprotective effect of the angiotensin-receptor antagonist irbesartan
28. Doulton TW, He FJ, MacGregor GA. Systematic review of combined in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;
angiotensin-converting enzyme inhibition and angiotensin receptor 345: 851–860.
blockade in hypertension. Hypertension 2005; 45: 880–886. 49. Brenner BM, Cooper ME, de Zeeuw D, for the RENAAL Study
29. The ONTARGET Investigators. Telmisartan, ramipril, or both in patients Investigators. Effects of losartan on renal and cardiovascular outcomes
at high risk for vascular events. N Engl J Med 2008; 358: 1547–1559. in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;
30. Berl T, Hunsicker LG, Lewis JB, for the Collaborative Study Group. 345: 861–869.
Impact of achieved blood pressure on cardiovascular outcomes in the 50. Parving HH, Lehnert H, Bröchner-Mortensen J, for the Irbesartan in
irbesartan diabetic nephropathy trial. J Am Soc Nephrol 2005; 16: patients with type 2 diabetes and microalbuminuria study group. The
2170–2179. effect of irbesartan on the development of diabetic nephropathy in
31. Thune JJ, Signorovitch J, Kober L et al. Effect of antecedent patients with type 2 diabetes. N Engl J Med 2001; 345: 870–878.
hypertension and follow-up blood pressure on outcomes after high-risk 51. Mogensen CE, Neldam S, Tikkanen I, for the CALM Study group.
myocardial infarction. Hypertension 2008; 51: 48–54. Randomised controlled trial of dual blockade of renin-angiotensin
32. Messerli FH. The sudden demise of dual renin-angiotensin system system in patients with hypertension, microalbuminuria, and non-
blockade or the soft science of the surrogate end point. J Am Coll insulin dependent diabetes: the Candesartan and Lisinopril
Cardiol 2009; 53: 468–470. Microalbuminuria (CALM) study. BMJ 2000; 321: 1440–1444.
33. Heart and Stroke Foundation. Guideline alert for blood pressure patients 52. Rossing K, Jacobsen P, Pietraszek L et al. Renoprotective effects of
as treatment combo (press release). Heart and Stroke Foundation, 2009; adding angiotensin II receptor blocker to maximal recommended doses
http://www.heartandstroke.com. of ACE inhibitor in diabetic nephropathy. Diabetes Care 2003; 26:
34. McKelvie RS, Yusuf S, Pericak D et al. Comparison of candesartan, 2268–2274.
enalapril, and their combination in congestive heart failure: Randomized 53. Jacobsen P, Andersen S, Jensen BR et al. Additive effect of ACE
Evaluation of Strategies for Left Ventricular Dysfunction (RESOLVD) Pilot inhibition and angiotensin II receptor blockade in type i diabetic
Study. Circulation 1999; 100: 1056–1064. patients with diabetic nephropathy. J Am Soc Nephrol 2003; l4: 992–999.
254 Kidney International (2011) 80, 245–255
MW Krause et al.: Combination inhibition of the renin–angiotensin system review
54. Jacobsen P, Andersen S, Rossing K et al. Dual blockade of the renin- 79. Rutkowski P, Tylicki L, Renke M et al. Low-dose dual blockade of the
angiotensin system versus maximal recommended dose of ACE renin-angiotensin system in patients with primary glomerulonephritis.
inhibition in diabetic nephropathy. Kidney Int 2003; 63: 1874–1880. Am J Kidney Dis 2004; 43: 260–268.
55. Jennings DL, Kalus JS, Coleman CI et al. Combination therapy with an 80. Nakao N, Yoshimura A, Morita H et al. Combination Treatment of
ACE inhibitor and an angiotensin receptor blocker for diabetic Angiotensin II Receptor Blocker and Angiotensin-Converting-Enzyme
nephropathy: a meta-analysis. Diabetes Med 2007; 24: 486–493. Inhibitor in Non-Diabetic Renal Disease (COOPERATE): a randomised
56. Andersen NH, Poulsen PL, Knudsen T et al. Long-term dual blockade controlled trial. Lancet 2003; 361: 117–124.
with candesartan and lisinopril in hypertensive patients with diabetes: 81. Bidani A. Controversy about COOPERATE ABPM trial data. Letter to the
the CALM II study. Diabetes Care 2005; 28: 273–277. Editor. Am J Nephrol 2006; 26: 629–632.
57. Bakris GL, Ruilope L, Locatelli F et al. Treatment of microalbuminuria in 82. Kunz R, Wolbers M, Glass T et al. The COOPERATE trial: a letter of
hypertensive subjects with elevated cardiovascular risk: results of the concern. Lancet 2008; 371: 1575.
IMPROVE trial. Kidney Int 2007; 72: 879–885. 83. Catapano F, Chiodini P, De Nicola L et al. Antiproteinuric response
58. Casas JP, Chua W, Loukogeorgakis S et al. Effect of inhibitors of the to dual blockade of the renin-angiotensin system in primary
renin-angiotensin system and other antihypertensive drugs on renal glomerulonephritis: meta-analysis and metaregression. Am J Kidney Dis
outcomes: systematic review and meta-analysis. Lancet 2005; 366: 2008; 52: 475–485.
2026–2033. 84. Kunz R, Friedrich C, Wolbers M et al. Meta-analysis: effect of
59. Mann J, Schmieder RE, McQueen M, on behalf of the ONTARGET monotherapy and combination therapy with inhibitors of the renin-
investigators. Renal outcomes with telmisartan, ramipril, or both, in angiotensin system on proteinuria in renal disease. Ann Intern Med 2008;
people at high vascular risk (the ONTARGET study): a multicentre, 148: 30–48.
randomized, double-blind, controlled trial. Lancet 2008; 372: 547–553. 85. Chapman AB, Torres VE, Perrone RD et al. The HALT Polycystic kidney
60. Parving HH, Brenner BM, McMurray JJ et al. Dual renin-angiotensin disease trials. Design and implementation. Clin J Am Soc Nephrol 2010; 5:
system blockade and kidney disease. J Am Coll Cardiol 2009; 54: 102–109.
278–279. 86. Maione A, Nicolucci A, Craig JC et al. LIRICO study group. Protocol of the
61. Abutaleb N. ONTARGET should not be over interpreted. Nephrol Dial Long-term Impact of RAS Inhibition on Cardiorenal Outcomes (LIRICO)
Transplant 2010; 25: 44–47. randomized trial. J Nephrol 2007; 20: 646–655.
62. Ruggenenti P, Remuzzi G. Proteinuria: Is the ONTARGET renal substudy 87. Berl T. Maximizing inhibition of the renin-angiotensin system with high
actually off target? Nat Rev Nephrol 2009; 5: 436–437. dose of converting enzyme inhibitors or angiotensin receptor blockers.
63. Poggio ED, Wang X, Greene T et al. Performance of the modification of Nephrol Dial Transplant 2008; 23: 2443–2447.
diet in renal disease and Cockcroft-Gault study equations in the 88. Hou FF, Xie D, Zhang X et al. Renoprotection of optimal antiproteinuric
estimation of GFR in health and in chronic kidney disease. J Am Soc doses (ROAD) study: a randomized controlled study of benezapril and
Nephrol 2005; 16: 459–466. losartan in chronic renal insufficiency. J Am Soc Nephrol 2007; 18:
64. Rule AD, Larson TS, Bergstralh EJ et al. Using serum creatinine to 1889–1898.
estimate glomerular filtration rate: accuracy in good health and in 89. Burgess E, Muirhead N, Rene De Cotret P. A double-blind randomized
chronic kidney disease. Ann Intern Med 2004; 141: 929–937. controlled trial of high dose candesartan cilexetil in proteinuria renal
65. Wright JT, Bakris G, Greene T, for the African American Study of Kidney disease results from SMART (Supra maximal atacand renal trial). J Am Soc
Disease and Hypertension Study Group. Effect of blood pressure Nephrol 2009; 20: 893–900.
lowering and antihypertensive drug class on progression of 90. Fisher N, Hollenberg NK. Renin inhibition: what are the therapeutic
hypertensive kidney disease. JAMA 2002; 288: 2421–2431. opportunities. J Am Soc Nephrol 2005; 16: 592–599.
66. Ishani A, Xue JL, Himmelfarb J et al. Acute kidney injury increases risk of 91. Solomon S. Effect of the direct renin inhibitor aliskiren on left
ESRD among elderly. J Am Soc Nephrol 2009; 20: 223–228. ventricular remodeling following myocardial infarction with left
67. Ruggenenti P, Perticucci E, Cravedi P et al. Role of remission clinics ventricular dysfunction: ASPIRE. Am Coll Cardiol 2010 Abstract
in the longitudinal treatment of CKD. J Am Soc Nephrol 2008; 19: 3019–3010.
1213–1224. 92. Parving HH, Persson F, Lewis JB, for the AVOID study investigators.
68. Psaty BM, Lumley T. Surrogate end points and FDA approval. A tale of 2 Aliskiren combined with losartan in type 2 diabetes and nephropathy.
lipid-altering drugs. JAMA 2008; 299: 1474–1476. N Engl J Med 2008; 358: 2433–2446.
69. Fleming TR. Surrogate endpoints and FDA’s accelerated approval 93. Alfrey AC, Froment DH, Hammond WS. Role of iron in the tubulo-
process. Health Affairs 2005; 24: 67–78. interstitial injury in nephrotoxic serum nephritis. Kidney Int 1989; 36:
70. Khosla N, Sarafidis PA, Bakris GL. Microalbuminuria. Clin Lab Med 2006; 753–759.
26: 635–653. 94. Lin JL, Lin-Tan DT, Hsu KH et al. Environmental lead exposure and
71. Glassock RJ. Debate: CON position. Should microalbuminuria ever be progression of chronic renal disease in patients without diabetes.
considered as a renal endpoint in any clinical trial? Am J Nephrol 2010; N Engl J Med 2003; 348: 277–286.
31: 462–465. 95. Cho ME, Smith DC, Branton MH et al. Pirfenidone slows renal function
72. Weir MR, Bakris GL. Editorial perspective. Should microalbuminuria ever decline in patients with focal segmental glomerulosclerosis. Clin J Am
be considered as a renal endpoint in any clinical trial? Am J Nephrol Soc Nephrol 2007; 2: 906–913.
2010; 31: 469–470. 96. Waandeis F, Vaidya VS, van Goor H et al. Effect of renin-angiotensin-
73. Levey AS, Cattran D, Friedman A et al. Proteinuria as a surrogate aldosterone system inhibition, dietary sodium restriction, and/or
outcome in CKD: report of a scientific workshop sponsored by the diuretics on urinary kidney injury molecule 1 excretion in non diabetic
National Kidney Foundation and the US Food and Drug Administration. proteinuric kidney disease: a post hoc analysis of a randomized
Am J Kidney Dis 2009; 54: 205–226. controlled trial. Am J Kidney Dis 2009; 53: 16–25.
74. Freid L, Duckworth W, Zhang JH, for the VA Nephron-D Investigators. 97. Bolignano D, Lacquamiti A, Coppolino G et al. Neutrophil gelatinase-
Combination angiotensin receptor blockers and angiotensin converting associated lipocalin (NGAL) and progression of chronic kidney disease.
enzyme inhibitor for the treatment of diabetic nephropathy VA Clin J Am Soc Nephrol 2009; 4: 337–344.
Nephron-D study. Clin J Am Soc Nephrol 2008; 4: 361–368. 98. Bolignano D, Coppolino G, Campo S et al. Urinary neutrophil
75. Parving HH, Brenner BM, McMurray JJ et al. Aliskiren Trial in Type 2 gelatinase-associated lipocalin (NGAL) is associated with severity of
Diabetes Using Cardio-Renal Endpoints (ALTITUDE): rationale and study renal disease in proteinuric patients. Nephrol Dial Transplant 2008; 23:
design. Nephrol Dial Transplant 2009; 5: 1663–1671. 414–416.
76. Dillon JJ. Angiotensin-converting enzyme inhibitors and angiotensin 99. Perico N, Cattaneo D, Remuzzi G. Kidney injury molecule 1: in search of
receptor blockers for IgA nephropathy. Sem Nephrol 2004; 24: biomarkers of chronic tubulointerstitial damage and disease
218–224. progression. Am J Kidney Dis 2009; 53: 1–14.
77. Russo D, Minutolo R, Pisani A et al. Coadministration of losartan and 100. Kamijo A, Sugaya T, Hikawa A et al. Clinical evaluation of urinary
enalapril exerts additive antiproteinuric effect in IgA nephropathy. Am J excretion of liver-type fatty acid-binding protein as a marker for the
Kidney Dis 2001; 38: 18–25. monitoring of chronic kidney disease: a multicenter trial. J Lab Clin Med
78. Tylicki L, Rutkowski P, Renke M et al. Renoprotective effect of small 2005; 145: 125–133.
doses of losartan and enalapril in patients with primary 101. Kamijo-Ikemori A, Sugaya T, Kimura K. Urinary fatty acid binding protein
glomerulonephritis. Am J Nephrol 2002; 22: 356–362. in renal disease. Clin Chim Acta 2006; 374: 1–7.
Kidney International (2011) 80, 245–255 255
Reproduced with permission of the copyright owner. Further reproduction prohibited without permission.
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- Account Statement 250820 240920 PDFДокумент2 страницыAccount Statement 250820 240920 PDFUnknown100% (1)
- Toh MFS8B 98B 003-11114-3AG1 PDFДокумент92 страницыToh MFS8B 98B 003-11114-3AG1 PDFDmitry NemtsoffОценок пока нет
- Ci Thai RiceДокумент4 страницыCi Thai RiceMakkah Madina riceОценок пока нет
- CLG418 (Dcec) PM 201409022-EnДокумент1 143 страницыCLG418 (Dcec) PM 201409022-EnMauricio WijayaОценок пока нет
- Syllabus PDFДокумент3 страницыSyllabus PDFBibin Raj B SОценок пока нет
- Electronic Spin Inversion: A Danger To Your HealthДокумент4 страницыElectronic Spin Inversion: A Danger To Your Healthambertje12Оценок пока нет
- Aliping PDFДокумент54 страницыAliping PDFDirect LukeОценок пока нет
- 52 - JB CHP Trigen - V01Документ33 страницы52 - JB CHP Trigen - V01July E. Maldonado M.Оценок пока нет
- International Security Notes International Security NotesДокумент34 страницыInternational Security Notes International Security NotesBEeNaОценок пока нет
- Acc116 Dec 2022 - Q - Test 1Документ6 страницAcc116 Dec 2022 - Q - Test 12022825274100% (1)
- Surgery - 2020 With CorrectionДокумент70 страницSurgery - 2020 With CorrectionBaraa KassisОценок пока нет
- Brigade Product Catalogue Edition 20 EnglishДокумент88 страницBrigade Product Catalogue Edition 20 EnglishPelotudoPeloteroОценок пока нет
- ThorpeДокумент267 страницThorpezaeem73Оценок пока нет
- SABRE MK-3 CFT Gel SpecДокумент1 страницаSABRE MK-3 CFT Gel Specseregio12Оценок пока нет
- BJAS - Volume 5 - Issue Issue 1 Part (2) - Pages 275-281Документ7 страницBJAS - Volume 5 - Issue Issue 1 Part (2) - Pages 275-281Vengky UtamiОценок пока нет
- Student Management SystemДокумент232 страницыStudent Management Systemslu_mangal73% (37)
- Automatic Gearbox ZF 4HP 20Документ40 страницAutomatic Gearbox ZF 4HP 20Damien Jorgensen100% (3)
- HRMДокумент118 страницHRMKarthic KasiliaОценок пока нет
- This Study Resource Was: For The Next 6 ItemsДокумент9 страницThis Study Resource Was: For The Next 6 ItemsJames CastañedaОценок пока нет
- Open Source NetworkingДокумент226 страницOpen Source NetworkingyemenlinuxОценок пока нет
- Top 100 Chemical CompaniesДокумент11 страницTop 100 Chemical Companiestawhide_islamicОценок пока нет
- Project Management TY BSC ITДокумент57 страницProject Management TY BSC ITdarshan130275% (12)
- Smart Gas Leakage Detection With Monitoring and Automatic Safety SystemДокумент4 страницыSmart Gas Leakage Detection With Monitoring and Automatic Safety SystemYeasin Arafat FahadОценок пока нет
- Multimodal Essay FinalДокумент8 страницMultimodal Essay Finalapi-548929971Оценок пока нет
- 19c Upgrade Oracle Database Manually From 12C To 19CДокумент26 страниц19c Upgrade Oracle Database Manually From 12C To 19Cjanmarkowski23Оценок пока нет
- Facebook: Daisy BuchananДокумент5 страницFacebook: Daisy BuchananbelenrichardiОценок пока нет
- Chinese Paper Cutting Work SheetДокумент4 страницыChinese Paper Cutting Work Sheet黃梓Оценок пока нет
- Manual For Tacho Universal Edition 2006: Legal DisclaimerДокумент9 страницManual For Tacho Universal Edition 2006: Legal DisclaimerboirxОценок пока нет
- CiscoДокумент6 страницCiscoNatalia Kogan0% (2)
- Mahesh R Pujar: (Volume3, Issue2)Документ6 страницMahesh R Pujar: (Volume3, Issue2)Ignited MindsОценок пока нет