Академический Документы
Профессиональный Документы
Культура Документы
Relationship Between Pain and Tissue Damage Due To Thermal Radiation Stoll
Загружено:
nuvanОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Relationship Between Pain and Tissue Damage Due To Thermal Radiation Stoll
Загружено:
nuvanАвторское право:
Доступные форматы
Relationship between pain and tissue
damage due to thermal radiation
ALICE M. STOLL AND LEON C. GREENE
U.S. Naval Air Develokment Center, Aviation Medical Acceleration Lab-
oratory, Johnsville, Pennsylvania
S~I,L, ALICE M. AND LEON C. GREENE. Relationship between METHODSANDMATERIALS
luin and tissue damage due to thermal radiation. J. Appl. Physiol.
I4(3) 1373-382. 1g5g.-Sites on the volar surfaces of the fore- Except where noted, the techniques employed in this
arms of human subjects were blackened with India ink and study have been described in detail elsewhere (I, 5). In
exposed to thermal irradiances of from 50 to 400 mcal/cm2 brief, the experimental procedure consisted of exposing
sec. The exposure time and skin temperature at which threshold a blackened area on the volar surface of the forearm to
pain occurred, and which produced minimal blistering within radiation of known intensity for a time just sufficient to
24 hours, were noted. The thermal inertia (kpc) of the skin produce a threshold pain or threshold blister indicative
was shown to vary directly with the level of irradiance. The
of complete transepidermal necrosis occurring within 24
receptors effective in mediating the pain sensation were calcu-
lated to be at a depth of approximately 200 p and to have a hours of the burning episode. Skin temperature was
threshold of approximately 43.2’C. Tissue damage rates with measured continuously before, during and after each ex-
respect to temperature were derived empirically so that posure. Corrections were made for reflection effects
damage integrated over the time for which skin temperature which were measured for each exposure. Time was indi-
was elevated over the pain threshold was equated to unity. cated on the record automatically throughout and the
The substitution of the ratio of these rates with respect to report of pain threshold during the burn episode was
temperature for the stimulus ratio, in the prediction of the ob- noted on the record by means of a time signal.
served discriminable steps in pain sensation intensity, yielded For purposes of clarity, a schematic diagram of the
faithful reproduction of the just noticeable differences observed apparatus is shown in figure I. Referring to figure I, a
for pain through the range of this sensation.
typical experiment may be described as follows: the
desired irradiance was obtained from the 1000-w. pro-
jection lamp, PL, regulated by means of the variable
resistor, R, to give the appropriate intensity measured at
I N THE INVESTIGATION of the relationship between pain the aperture, A. A radiometer was mounted to measure
and tissue damage it has been pointed out that this rela- the irradiance at the exact site which the skin occupies
tionship is more complex than a simple causal one, and during exposures. The current through the lamp was
it has been suggested that the rate of inactivation of used to maintain a check on the stability of the radiation.
tissue proteins due to heat is probably intimately in- Following final adjustment of the radiation source, the
volved when pain is evoked by thermal stimulation radiometer was removed and a site on the volar surface
( I, 2). However, experiments concerned with protein in- of the forearm, blackened with India ink to assure ab-
activation rates in vitro and with dermal burns in animals sorption of the radiation at the skin surface, was placed
and man have involved constant temperature levels in against the aperture. At this time the opaque shutter,
the first instance and indirectly measured skin tempera- S, was closed, blocking the radiation from the lamp; the
tures (3, 4) in the second. The results, therefore, can be glass shutter GS, was closed, blocking radiation from the
applied only indirectly to the existing body of knowledge skin to the Golay detector, G. The record paper was
of pain and burns from thermal radiation. With the de- started in motion at the rate of 25 mm/set. and the
velopment of a technique for rapid measurement of skin temperature of the glass chopper, C, noted. The glass
temperature during exposure (5) it became possible to shutter, GS, was then lifted and a record of deflection
make direct comparisons of the skin temperature, thermal due to radiation from the skin before irradiation was ob-
irradiances and exposure times during the production
tained. At zero time, with respect to exposure, the opaque
of pain and burns (6). The present report embodies these
shutter, S, was lifted and the measured radiation per-
data and their analysis.
.--- . . -..-_ mitted to fall on the skin site while measurement of
kccived for publication September I 7, 1958. radiation from this site continued. If the exposure was
373
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (134.153.184.170) on October 12, 2018.
Copyright © 1959 American Physiological Society. All rights reserved.
374 ALICE M. STOLL AND LEON C. GREENE
where 0.94 is the emissivity of India ink. When the cali-
bration curve was not precisely linear, the expression
k(D T- DR> was modified to (kDT - k’DR) where k
refers to the calibration in degrees centigrade per milli-
meter (corrected for emissivity) at the point of measure-
ment of DT and k’ similarly refers to DR.
The area viewed by the Golay detector was just short
of that defined by the aperture, A, which was I .75 cm
in diameter. Thus, the skin temperature indicated by
the Golay detector was the average temperature over an
area about I .74 cm in diameter.
The distribution with respect to intensity of the inci-
dent radiation throughout the field was of importance
I in the determination of not only skin temperature but
also blister formation. Therefore, a radiometric device
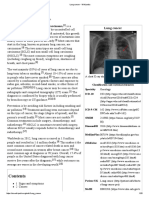
FIG. I. Irradiation and temperature-recording apparatus. was constructed for the purpose of measuring the even-
Thermal radiation source = projection lamp PL, in housing Z-Z, ness of the field. The instrument is shown schematically
power supplied from motor generator MG, current controlled
in figure 2. It consisted of a blackened receiver, 2 x 2.5
through resistance R and monitored through ammeter AMP
with shunt S. Optical system = lens system L1 and Lz, shutter S
mm,- soldered to a copper-constantan thermocouple and
and exposure aperture A. Temperature recording system = oriented perpendicular to the radiant flux. The reference
chopper (IO c.p.s.) C, Golay detector G, with glass shutter GS, junction was situated immediately behind the receiver
amplifier APF and Poly-Viso Sanborn recorder REC. and shielded by it from the radiation. Thin glass cover
plates were mounted on the face and back, thus pro-
to be terminated at pain threshold, the opaque shutter viding an instrument free from disturbances due to air
was dropped when the subject reported pain; if the ex- currents. This assembly was mounted on a worm-drive
posure was to be continued so as to produce blistering, which permitted the receiver and reference junction to
a time signal button was pressed when the subject re- be moved across the field for a distance of 2 cm in very
ported pain and the exposure continued for the predeter- small steps which were indicated on a millimeter scale
mined time, when the shutter, S, was dropped and the mounted above the worm-drive. The above components
exposure ended. Recording from the skin site was un- were mounted in a metal plate with a circular perimeter
interrupted, however, and cooling was measured for a which, in turn, was fitted into a second plate so that the
suitable time after termination of the exposure, at which inner plate and assembly could be turned through a full
time the glass shutter, GS, was interposed. With the circle. Thus, the receiver could be set and the radiation
glass shutter in place, the opaque shutter was then lifted measured at any point throughout the entire radiant
so that the radiation again fell on the skin and the con- field.
tribution of the reflected visible and near infrared With this radiometer the radiation distribution was
radiation to the total deflection during the burning epi- mapped in a radial pattern at T-mm intervals throughout
sode was determined. With this measurement the experi- the field and the evenness of radiation was determined
mental record was complete. as indicated in figure 3. The maximum intensity oc-
Skin temperature at any moment throughout the ex- curred at about the center, a little below and to the left.
periment was obtained by referring the observed deflec-
tion to a calibration curve obtained from exposure of the
Golay detector to a series of black-body temperatures,
provided by a Leslie cube suitably mounted at the aper-
ture, A. Deflections recorded during irradiation of the
skin were corrected by subtracting the amount attribut-
able to reflection as measured above. Thus, the skin
temperature at any time, t, was expressed as:
T, = L + k CDT - DR>
where
FIG. 2. Scanning radiometer. A = glass back plate; B = metal
Ts = skin temperature (“C) frame support for thermo-receiver worm-drive, C, for adjusting
Tc = chopper temperature (“C) position of receiver D ; E = leads from receiver; F = millimeter
DT = total deflection in millimeters at time t rule for receiver position indication ; G = glass front plate; H =
DR = deflection in millimeters due to reflected radiation metal plate for rotating frame and receiver; Z = metal mounting
k = calibration constant = ‘C/mm deflection/o.94 plate.
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (134.153.184.170) on October 12, 2018.
Copyright © 1959 American Physiological Society. All rights reserved.
PAIN AND TISSUE DAMAGE IN THERMAL RADIATION 375
INTENSITY CONTOURS IN minus 32.5OC. These values were then substituted in the
IRRADIATED AREAt% of MAXItim equation which was solved for t :
t =- (aT> 2kw
I .13Q2
where
1.13 = 4a2/r
The value of kpc may vary widely under different physio-
logical conditions (7). As shown in table I, the average
kpc varied over a considerable range and increased with
each increase in level of irradiance. Therefore, in com-
puting t, the kpc value obtained during the actual meas-
urement was used in each instance and no data were
r I I I I I II 11 1
10 8 6 4 2 0 2 4 6 8 10 used in which the initial temperature was more than
MILLIMETERS =t I “C different from 32.5OC.
FIG. 3. Intensity distribution within irradiated field.
Table 2 shows, for each irradiance used (col. I), the
average values of the corrected exposure time and of the
Two cool spots occurred in the right hemisphere and skin temperature at which pain threshold occurred
may be attributed to the space between the coils of the (col. 2 and 3) and those of the exposure time and skin
source filament. The maximum deviation, however, was temperature which resulted in blisters (col. 4 and 5). It
6 % and the average intensity was g7 % of maximum. is seen that the skin temperature at pain threshold in the
This pattern was the most even that could be achieved nonburning episodes, i.e. those in which the exposure
with the apparatus and was used throughout the exneri- was terminated immediately at pain threshold, tends to
ment al irradiations. be higher at the higher radiation intensities. While a
slight increase was expected due to the greater intra-
EXl’ERIMENTAL RESULTS dermal gradient at the higher intensities, the values noted
here exceeded those anticipated. This trend is not clearly
Three subjects were used. Sites on the volar surface of
evident in the pain threshold skin temperatures meas-
the forearm were exposed to irradiances of IOO, I 50, 200,
ured during burning. However, these latter are fewer in
3oo and 400 meal/cm” sec. and the time required to pro-
number and are prone to greater error, due to the ne-
&KC threshold pain and to produce minimal blistering
cessity for reducing the sensitivity of the recording system
(i.e. several small vesicles appearing within 24 hours)
in order to include the greater spans from initial to final
was determined. A total of 257 exposures was made, 39
skin temperatures and also, probably, due to some dis-
of which were prolonged to produce burns; 19 of these
traction of the subject’s attention to the threshold point
were productive of blisters. All exposure times were
in anticipation of the intense pain expected during the
normalized to an initial skin temperature of 32.5OC
ensuing burn. In figure 4, the irradiances are plotted
which was the average initial temperature measured
against the corrected exposure times to pain threshold
direct1 y. Such adjustmen ts, when necessary, were made and to blister formation. The pain threshold data con-
t>v use of the equation rel ati ng exposure time, irradiance
form to the usual strength-duration curve as noted in
and rise in skin temperature during irradiation (6, 7) :
earlier studies (I) and the threshold blister curve
parallels this curve. The area between the curves repre-
4a2Q2t
kpc = -~ (I > sents the region of suprathreshold pain and reversible
T (AT)2
tissue damage. This region has been explored with
respect to pain sensation (I, 8) and it was found that
where
kpc = product of therm al conductivity, k, density, p, and TABLE I. Thermal Inertia (kpc) of Skin at Pain Threshold
-.__ -----.- -
specific heat, c, in mcal/cm4 “C sec.
(A7*) = rise in skin temperature in “C = final skin tempera- kpc (X10-j) caP/cm4/“C/sec.
ture - initial skin temperature Irradiance mc/cm2 sec.
=
fl absorptivity of India-inked skin = 0.94 Average Range
Q = radiation intensity in Cal/cm2 set -..~._ -- ------
1 = time in seconds 100
96 75-I 17
150 I IO 89-142
Then, when the initial temperatu re w as differen t from 200 127 95-158
3oo ‘39 I15-168
‘32.5OC,
c . kpc was determ ined from the measured values
400 I59 144-181
and (AT) was set equal to the observed final temperature
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (134.153.184.170) on October 12, 2018.
Copyright © 1959 American Physiological Society. All rights reserved.
376 ALICE M. STOLL AND LEON C. GREENE
threshold pain occurs at a skin temperature of about Here are shown two heating curves at different irradi-
45OC and ceiling pain at about 6o”C, almost indepen- antes. The left ordinate indicates temperature and the
dently of the time required to attain these levels. Blister- right ordinate the corresponding rate of protein denatu-
ing, however, depends strongly on both temperature and ration as determined by Henriques and Moritz. In the
time. Thus, with an irradiance of IOO mcal/cm%ec., temperature curves ‘skin temperature’ refers to the sur-
threshold blisters were produced at a skin temperature face of the skin and ‘tissue temperature’ to the computed
of 52.g°C attained after 33.8 seconds of exposure; temperature at a depth of 80 p below the surface, or
whereas, at 300 mcal/cm2sec., threshold blistering oc- approximately the basal layer. The damage integral is
curred at a skin temperature of 56.7OC attained after obtained by plotting the rate of denaturation corre-
7.8 seconds of exposure, while heating the skin to 53OC sponding to the tissue temperature against the time at
with radiation of this higher intensity produced only a which the temperature occurred during the exposure and
transient erythema. On the other hand, even slight pro- then, by planimetry, measuring the area under the
longation of the exposure, such that the peak skin tem- resultant curve. Thus, it is seen that for the production
perature exceeds the values corresponding to threshold of threshold blisters in these instances, the damage
blisters by as little as I “C, results in a full blister, i.e. integral, 0, was 0.15 and 0.27, or about two-tenths of
a single blister involving the entire irradiated area formed the value obtained by Henriques and Moritz for the
within a few hours after the exposure and often raised to same injury using constant temperatures.
a height of 3-5 mm within 24 hours. These data, which Table 3 shows irradiances, temperatures and the
are represented by the third curve (fig. 4), resulted from corresponding average values of Q, as computed by the
overshooting the desired end point and are not intended method above for the threshold blisters. The full blister
to delineate the lower limit of full blistering. data are also shown, but are fragmentary and are in-
Henriques and Moritz (3, 4), using constant tempera- cluded only incidentally since they do not delineate the
ture levels, have derived the equation below for the lower limit of exposure time for full blisters. Not in-
evaluation of tissue damage, whereby damage is related cluded in this table, but worthy of mention as an illustra-
to the rate of protein denaturation and the exposure time tion of the great importance of initial skin temperature,
at a given temperature: is one burning episode at 150 mcal/cm2sec. which re-
sulted in an Q value of 2.0. This exposure was made for
a time (22.15 sec.) expected to produce blistering with
an initial skin temperature of about 32.o”C but, instead,
where produced a white burn with immediate destruction of
62 = tissue damage TABLE 2. Irradiance, Time and Tmjerature for Pain Threshold and
P = integration constant Blister Production
AE = energy of inactivation ----- ~.-
-- -~.___-- -~-
R = gas constant Pain Threshold
Pain Threshold
TI* = tissue temperature Controls Threshold Blister Full Blister
Radia- (During Burning)
(Nonburn)
t = exposure time tion
tensi ty
In-
meal/
cm2 sec. No .
I, 1 Ts,
Thus, the symbol Q was chosen to represent damage. It of
sec. “C
obs.
was defined as the integral of the damage rate (&/dt)
for the exposure time noted and was given the value of 100 6 /12.4;45.8: r2 i33.852.g I 37k53.4
49 ‘3.5145.’
I .o when complete transepidermal necrosis in pig skin, 125 3’ I0.1145.3 I I I I
or blistering in human skin, was produced. Using the 150 67 7-845-9
200 22 5.5 46.5
damage rates determined by these authors for various
300 36 Q-9,47- I
tissue temperatures, the computation of Q was applied to
400 ‘3
the present experimental data as illustrated in figure 5.
mc/Cm*/sec fiti-
fi
Fig. 5
lc
--
56-
do
701
RADIATION
t
= 300 mc/cmVsec dt
x lo-’
x10-=
RADIATION = 100 mc/cm%ec 16
FIG. 4. Irradiance and ex- 2.5
time to threshold pain -5
posure
and blistering.
-4
FIG. 5. Temperatures at skin
surface and those calculated at
-3
80 p beneath surface during ther-
mal exposure, and resultant /
-2 a=0 27
damage integrated at damage Threshold
Blister
rates from Henriques’ study.
-I
cc--J, !\ -0
I I, I I I I I, I, I, 1, t * I C&--l ’
4 8 12 16 20 24 28 32 36 0 IO 20 30 40 50 s,ec set
TIME (SECONDS) TIME
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (134.153.184.170) on October 12, 2018.
Copyright © 1959 American Physiological Society. All rights reserved.
PAIN AND TISSUE DAMAGE IN THERMAL RADIATION 377
TAHLK 3. h-radiance, 0 bserved Temperatures and Damage rate at which heat is conducted away from the surface.
Thus, the flow of heat through the surface layers, i.e. the
Skin Temp., “C India ink and the epidermis, must be enhanced, effecting
Threshold Blister
(I, mcal,/c-m2 sec. Full Blister f2
-_ sz a change in the value of kpc as derived from the observed
T, Tt quantities, irradiance, surface temperature and expo-
-__--.-- (- 1-----__I ---1
sure time. It is well known that the thermal conductivity
100 52.9 52.3 0.15 0.20
of water and certain aqueous solutions (in direct con-
150 54-o 53.2 0. ‘7 0.54
300 56.7 55.2 0.27 0.91 trast to most materials) increases with an increase in
40” 59.’ 57-2 0.83 temperature. It may be that the engorged tissue under-
lying the epidermis behaves in the same manner with
respect to thermal conductivity, effecting a real increase
the epidermis, completely bypassing blister formation in k of this tissue and further enhancing heat loss from
and deeply involving the dermis. On analysis of the the surface. As the level of irradiance is increased, higher
record it was found that the initial skin temperature had temperatures are produced within the same exposure
been ~~~+5°C, considerably higher than expected; there- time so that this k may attain increasingly high values,
fort, Ke exposure of 22. I 5 seconds was equivalent to an thus contributing to increasingly high values for kpc.
exposure of 24.0 seconds if the initial skin temperature From the data on pain threshold presented in table 2
had tIeen 32.5”C. Also, the final skin temperature was it is possible to estimate the firing temperature and the
58.2”C, whereas a skin temperature of 55OC (table I) average depth of the pain receptors, assuming that the
attained at this radiation intensity is sufficient to produce receptors fire at some definite temperature, from the
a full blister. Thus, the importance of skin temperature, mathematical formulations for heat transfer as used by
both initial and final, cannot be overemphasized in this Henriques (3). Although this equation was derived for
type of heating, in which far different histological results use in the unsteady state when the surface is immedi-
can be achieved with the same intensity-time dosages ately brought to and maintained at a constant tempera-
when the initial temperature is only I “C or so different. ture, it is equally valid when the surface temperature,
Empirically it is found from the accumulated data although not constant, is known at every instant in time,
that, because of the similarity in form of the curves for the radiation is nonpenetrating, and heat transfer in-
threshold pain and threshold blisters, the time to pro- ward is conductive. Thus, the receptor temperature at
duce blistering may be predicted simply by multiplying the time pain threshold is reached is the same at all
the exposure time to pain threshold by a constant, in this irradiances and may be expressed as:
instance 2.52. However, the value of the constant, just
as the exposure time itself, depends strongly upon the
initial skin temperature. Nevertheless, in any standard- 02
ized irradiation procedure a factor such as this one may
be determined approximately from a very few experi-
ments and thereafter serve as a useful rule-of-thumb where
throughout the range of irradiances to be used. The
TR = temperature of receptors (“C)
fundamental significance of the relationship shown here
Ts = skin temperature (“C) at pain threshold
is treated in the later part of the discussion which follows. 7-O = initial skin temperature at start of radiation
t = time to pain threshold
DISCUSSION
Y=
D
-
k where D is the depth of the receptors in cm;
Considering first the data on kpc values presented in 2 -
table I, it is seen that the value of kpc increases with ir- d
k/pc =
PC
thermal diffusivity where k is the conductivity
radiance and reaches a maximum of about 160 X IO-~
(Cal/cm sec. “C); p is the density (gm/cc) and c is the
ca12/cm4/‘C/sec. at the 400 mc/cm2 sec. level. It has specific heat (cal/gm “C)
been pointed out (9) that, for exposure times of less than
20 seconds the kpc of normal living skin was about go-100 and
x 10-5, while, on prolonged heating, this value in-
A
s
creased to as much as 400 X IO-~, which was at-
2 4t
trihuted to local passive vasodilation. However, in the - e--y2 dy = probability integral.
d = 0
measurements cited, the irradiance was not more than
55 mc/cm2sec. and the increase in skin temperature
(forearm) was about 7OC in 20 seconds, therefore, well Then, assuming as a first approximation that the
below pain threshold and well below the temperature thermal diffusivity of the skin from the surface inward is
elevation at pain threshold (I 2.5OC or greater) experi- a constant, the observed values for T,, & and t at each
enced in the present study. Since the epidermis is avas- irradiance are introduced and the equation solved for
cular it is unlikely that p and c of the surface vary greatly Tn through a series of increasing values for 7 until TR
during irradiation; however, the blood content of the is found to be the same at all irradiances. The value for
underlying tissue must certainly increase, affecting the y found by this method was 0.45 and that of TR, the
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (134.153.184.170) on October 12, 2018.
Copyright © 1959 American Physiological Society. All rights reserved.
378 ALICE M. STOLL AND LEON C. GREENE
pain receptor temperature at pain threshold, was not likely to affect the computation significantly. Of the
43.2 & 0.4”C throughout the range of irradiances em- other factors, certainly p and c cannot be far different
ployed. In order to solve for D in the equation from I .o. Thus, the figure of least confidence is the value
of k; the fact that this has a large effect is obvious. If,
D to circumvent the uncertainties of applying porcine skin
0.45 = y = ~
k constants, the value for thermal diffusivity, as deter-
2 - mined by Hensel (I 3), for the most superficial ‘layer’ of
^J PC
human skin (o to 0.26 mm) is used, the depth becomes
it becomes necessary to adopt reasonable values for k, p
and c. Dealing first with the most superficial 1ayer, epi-
dermis, a value of 0.0005 ca .l cm/cm2 sec.OC has been
determined for k of porcine epidermis (I o), which may
be expected to be quite similar to human epidermis. Al- where
though Henriques worked with an assumed value of
0.8 gm/cc for p, such data as are available in the litera- k/w = 4 x 10-1
ture (I I) indicate that the epidermal density is probably
I. I -I .2. Drawing again upon the measured values for considerably less than 240 p and considerably greater
porcine epidermis (8), c is 0.86 cal/gm “C. Using these than 120 ,u.
values then, From these data it would appear that the effective
pain receptors are subepidermal, as contended by
Bishop (14)) Woollard (I 5) and others, although
k
D =0.4.5 2 - Weddell (I 6) and others contend that intra-epidermal
(40 PC
free nerve endings may mediate pain. Buettner ( I 7) lo-
cated the pain receptors at a depth of about 0.1 mm and
=Od5
(2jL.I;-;o:.*6) assigned them an ‘average
of 44.8 =t 0.5’C. However,
pain threshold
the pain threshold
temperature’
to which
= 0.0202 cm or 200 p
he referred was measured for ‘unbearable’ pain rather
than for the least perceptible pain which was used in
Estimates of epidermal thickness (12) in various regions this study. Thus, the value of 43.2 of 0.4OC as deter-
of the bodv ra .nge from 500-120 D but indicate that mined here is a more appropriate ‘threshold’ tempera-
200 p is much too thick for epidermis on the forearm. ture. It is noteworthy with respect to this computed
Therefore, it follows that y is composed of at least two firing temperature that, in a subsequent study (18) by
similar terms, or one of the authors (LG), the skin temperature measured
at pain threshold was 43.7OC when heating was carried
out very slowly, so that pain threshold was attained in
about 2g minutes at an irradiance of 22 mcal/cm2sec.
Similarly, when the skin was heated to pain threshold
more rapidly and the subject was then permitted to ad-
just the irradiance to that just sufhcient to maintain
threshold pain sensation, the skin temperature so main-
D, = depth of epidermis tained was 43.8”C. Thus, in situations wherein the
Dd = depth of dermis temperature gradient from surface to receptor might be
k, p, c = constants for epidermis (as before) expected to be minimal, the computed receptor firing
k’, p’, c’ = constants for dermis = 0.00088, 1.2 and 0.77, re- temperature of 43.2 “C is closely approximated by the
spectively (8, IO).
measured surface temperature, affording considerable
support to the validity of the determination of the depth
Then, if IOO p is assumed for epidermal thickness from of the receptors as well as that of their firing tempera-
the above relationship, ture. The significance of the subepidermal location of
the pain receptors is that it is then theoretically possible
Dd = 141 p
to produce damage at the basal layer without pain, as
has been reported (4), provided that the temperature
and the average depth of the receptors would be about
240 p. It is recognized that this treatment of the data
TABLE 4. Irradiance and Exfosure Time for ‘Threshold’ Blisters
neglects certain considerations, e.g. the thickness of the
India ink (about IO r-l) and the gradient through it, Q, mcal/cm2 sec. t2 Predicted, sec. t2 Observed, sec.
possible interface effects at the epidermal-dermal junc- 100 54.2 33.8
tion and, also, the appropriateness of the use of constants 150 21. IO 20.8
determined for porcine tissues rather 200 ‘3.35 ‘3.4
than for hu man
300 7.58 7.8
tissues. However, the India ink layer is so th .in that it is 5*75 5.6
400
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (134.153.184.170) on October 12, 2018.
Copyright © 1959 American Physiological Society. All rights reserved.
PAIN AND TISSUE DAMAGE IN THERMAL RADIATION 379
\gradient through the skin is such that the basal layer is ble without producing complete transepidermal necrosis
maintained at an injurious temperature level while the in porcine tissue) was assumed with the expectation that
pain receptors are maintained at a subthreshold level. additional damage at a submaximal rate would proceed
Considering now the threshold blister data, it is possi- after the end of the exposure during the return of the
ble to apply the rate process equation to these and to skin temperature to the initial level. With the exception
predict the exposure time to blister formation applicable of the instance of the lowest intensity radiation used,
to the dynamic type of heating, used here in a manner which will be discussed later, the exposure times pre-
similar to that used by Henriques and Moritz for steady dicted in this manner were remarkably accurate
states. To arrive at an approximate exposure time pro- (table 4). H owever, despite the agreement in exposure
ductive of blistering, the procedure used was to deter- time, the values for Q (table 3) obtained by planimetry
mine the theoretical temperature rise during a given ex- from the observed data and the corresponding damage
posure to radiation intensity according to equation I, then rates were much smaller than the predicted values of
to find the corresponding tissue temperature at a depth from 0.5 to LO, except for the exposures at the highest
of 8o p, according to the method of Henriques and intensity (table 5, col. 8). The reasons for the discrepan-
Moritz, and assign the damage rate associated with each cies noted become clear on consideration of the data pre-
tissue temperature to the instant in time at which the sented in table 5. It should be recalled that the assump-
tcmpcrature was attained. Thus, a log plot of d&G&, the tions made in predicting the exposure time were: a) that
damage rate, with respect to t, the exposure time, was the temperature rise with respect to exposure time was
obtained, and the slope of this line was used to find the described adequately by equation I; 6) that the damage
time at which a given value for a, the total damage, performed during cooling would be approximately equal
could bc expected. Thus, to that performed during heating and c) that the damage
rate derived from the data of Henriques and Moritz,
I/ =
sc 2iyo3K(tz- to)- e2*303Jwl-t~)
t2 du
- dt =
t1 dt s t1
t2
Ce2.303K(+-to) dt
(3)
dfi/dt = PeAE”RTt
(,1=
1 2.303K
1 where
dit/dt = rate of damage
where 3.1 X log8 (constant)
P = sec.+
AE = 150,000 Cal/mole
$2 = total damage X = 2 (gas constant)
C-
&/& =
damage rate It = basal layer temperature (OK)
t1 = time of pain perception
I ‘2 =
time to blistering
to = the point on the curve log dQ/dt vs. t used in finding
accurately represented the relationship of darnage to
EC tissue temperature and time. Then, from table 4 it is
C = constant = dil/dt at t 0 (for convenience = unity). seen that the first assumption was not borne out, but re-
A‘ = slope of log dQ/dt vs. t sulted in tissue temperatures about 2OC lower than pre-
dicted. The second assumption was most nearly fulfilled
solving equation 3 for t2 at the higher intensities, as indicated roughly by the
length of time required for cooling of the skin down to
2 .303Kt0 + ln2.303K + 1nQ the pain threshold level and, more accurately, by the
12 =
2.303 EC graphical integration of damage during cooling. The
latter method (fig. 5) showed that about IO % of the
with the insertion of the desired value for Q, total dam- total damage was incurred during cooling at the lowest
age, provides the corresponding exposure time. As a first intensity, while about 35 % of the total damage occurred
approximation, Q = 0.5 (corresponding to Henriques’ during this phase at the highest intensity. It may be
and Moritz’ value for the longest exposure time permissi- noted here that this fact undoubtedly accounts for the
‘TAHI,E 5. Irradiance, Predicted and 0 bserved Temperatures and Damage
_-. -. _~-_.--.. --___--_ --~ _-.-_-__
Predicted Observed Damage Time
--
(1, mcal/cm2
sec. Above pain threshold
Ts, “C Tt, “C di!/dt at Tt Ts, “C Tt, “C di!/dt at Tt 51
Rise, sec. Fall, sec.
------ ----- -_____ --~ -~. --
100 55.5 54.9 I.42 X 10-l 52.9 52.3 2.4 X Iow2 0.15 20.1 14.0
150 56.5 55.5 I .8 X 10-l 54.0 53.2 4.4 x 10-2 0.17 ‘3.4 8-4
300 58.8 57.2 6.4 X 10-l 56.7 55.2 1.7 X 10-l 0.27 4.8 5-o
4oo 62.4 59.3 3-o 59-I 57.2 6.9 x IO-’ 0.83 3.4 3.5
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (134.153.184.170) on October 12, 2018.
Copyright © 1959 American Physiological Society. All rights reserved.
ALICE M. STOLL AND LEON C. GKI<ENE
common observation that less total heat is required to This finding leads to a consideration of the second
produce equivalent damage at high levels of radiation alternative, that of attributing the difference to inaccu-
intensity than at low levels. The third assumption, that racies in measurement of the surface temperatures or
the damage rate relationship is accurate, is borne out inappropriateness of method in obtaining tissue tem-
approximately at the highest intensity exposures, but peratures. The difference in damage rates at 45°C is
becomes progressively inapplicable at the lower intensi- equivalent to a temperature difference of almost 6°C. It
ties. Thus, the agreement, or lack of agreement, in pre- is not possible, even by accumulating all reasonable
dicted and observed exposure times reflects the degree to possible errors of measurement of both methods in addi-
which the necessary conditions were fulfilled. tion to those of the method of computation, to approach
In reconciling the experimental findings of this study a difference of 6OC, particularly at the lower levels of
with those obtained by conductive heating, at least three temperature where the greatest accuracy is achieved in
alternatives present themselves : either the damage rate both methods. Thus, an error in tissue temperature as
associated with each temperature is not accurate, or the great as even 2OC is inconceivable with the conductive
surface or tissue temperatures obtained by one or the method of heating where the heating is maintained for
other methods are not accurate, or there is a disconti- 2-3 hours. Similarly, in the radiative method, even if
nuity in the relationship between damage rate and other methods of computation of tissue temperature are
temperature which is obscured by the transition from used (7, Ig), the accumulated possible error does not
the steady state to the unsteady state conditions. approach 2OC. In fact, if the measured surface tempera-
Considering these alternatives in order, it was possible ture itself is used as the damage-effecting temperature,
to adjust the damage rate with respect to tissue tempera- the damage integral Q increases to only 0.28 at the IOO
ture empirically to yield Q = I .o for each level of radia- mcal/cm2sec. level and to 0.38 at the 150 meal cm2sec.
tion intensity. This adjustment resulted in a change of level, both far less than 1.0. Thus, it appears that the
slope of log dQ/dt vs. T, from 0.297 for the conductive discrepancy cannot be attributed to this source.
heat data to o. 166 for the radiative heating. The two The third alternative remains, i.e. there is a discon-
curves then intersected at 57.4OC, indicating that with tinuity in the damage rate function which is obscured
radiative heating the damage proceeded more rapidly at in passing from the steady to the unsteady temperature
lower temperatures and more slowly at temperatures state. In evaluating this possibility, careful consideration
above 57.4OC than it did with conductive heating. How- must be given to the special features applicable to each
ever, on substituting the new rates in the original equa- method of producing the tissue damage, as well as to
tion of the rate process and solving for the exposure time the handling of the experimental facts. Enumeration and
when the temperature is maintained constant, it was comparison of these features reveals that, in the con-
found that the exposure time at a tissue temperature of ductive method: I) experimental data are most de-
57.2OC was practically unaffected, while at 45OC the pendable at the relatively low-temperature, long-term
exposure time was reduced by a factor of about 60. exposures, i.e. up to a surface temperature of 5oOC.
FIG. 6. Comparison of damage 1.0
0
rates derived from conductive and 6
from radiative data. Solid circles 4
= damage rates at tissue temper-
atures such that bt = 1.0 at thres-
Rodiative Heoting,J/ 1
hold blistering of human skin with
IO"
radiative heating. Open circles =
damage rates at tissue tempera- t
tures such that dt = 1.0 with 4
conductive heating, according to E
a 2
Henriques. a
Fro. 7. Correlation of pain sen-
sation intensity with damage rate
and tissue temperature. Solid circles t i i 1
= damage rate at tissue tem-
perature indicated. Open circles =
damage rate corresponding to
basal layer skin temperature as- O-3
0
sociated with each level of pain 6
sensation plotted against the sen-
sation intensity measured in just
noticeable differences (JND)
throughout the range of discrim-
ination. IJCIO’4 I I ’ %
44 46 40 50 52 54 56 50 44 7 7 % :s2 52: 56 58
TEMPERATURE “C 0 2 6 IO I4 I8 22 JND
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (134.153.184.170) on October 12, 2018.
Copyright © 1959 American Physiological Society. All rights reserved.
PAIN AND TISSUE DAMAGE IN THERMAL RADIATION 381
However, the finding of a pain threshold at 47.5’- TABLE 6. Pain, Tissue Temperatures and DarnaS” Rates
Established Herein
48.5OC (4), with exposures of 40 minutes duration, is
puzzling, since direct skin temperature measurements, Observed Tissue Damage Ratios of Predicted
whether on blackened or unblackened skin, yield a pain Pain Temp., Rate, Stimulus Time to
Intensity, “C dQ/dt Intensity, Threshold
threshold of about 45OC when heating time to pain
threshold is not shorter than IO seconds.
JND s/so = dfj/dt/ Blister When
du/dt (1 512= I, see.
2) The inactivation energy value was derived from I 3.3x10-4 I .o 3300.0
45
the data pertaining to low temperatures only and was 2 4.7X IO-~ I -4 2130.0
applied subsequently to observations at higher temper- 4 46 9.7x 10-J 2.9 1030.0
8 4.2X 10~~ 12.4 228.0
a tures.
IO 48 8.4X IO-~ 25.4 I 18.0
7) At temperatures above 5o°C, the steady state was I2 I .8x IO-~ 56.0
54.5
never achieved and the exposure time noted did not ‘4 50 3.6x 10~~ 109.0 28.0
include the cooling time. The latter becomes progres- 16 8.3x 10-l 252 .o 12.0
18 I .5x 10-l 6.7
sively longer the higher the final temperature. Thus, the 53 456-o
20 3.0X 10-l 912 .o
3.3
observed time was shorter than the actual time through
21 56 4.7X 10-l 1430.0 2.1
which damage was inflicted and became progressively
so as the applied temperature level was elevated.
Similarly, with respect to the radiative method, it is rates at lower temperatures may possibly be indicative
found that: a) the measurements at the lowest radiation of higher temperature at the basal layer for the same sur-
intensities and, therefore, the longest times, are least face temperatures when heating is produced by radi-
critical and therefore technically most dependable. ation on blackened skin. The blackening may result in
!I) Temperature and time vary simultaneously greater
c localization of heat near the surface as compared
throughout the range of experimentation; therefore, with conductive heating where a flatter temperature
assumptions with respect to inactivation energy, etc. gradient to the interior might be expected. On the other
apply equally throughout and the steady state condition hand, it should be pointed out that the application of
does not apply at all. However, damage inflicted during cgraphical integration to the experimental data of
both heating and cooling is taken into account in the Henriques results in Q’s well over I .o and as much as I .5
determination of total damage. when damage during temperature fall is included.
c) In the original series, no experiments were per- It appears also that the change in rates of damage with
formed at levels of irradiation productive of peak temperature decelerates above the pain threshold tem-
temperatures less than 52 “C; therefore, inferences con- perature, rather than accelerating as might be expected.
cerning relatively low temperature exposures were ex- It is possible that this effect may be brought about
trapolative in nature. through alterations, chemical, physical, or both, in the
In order to overcome the latter deficiency and to pro- more superficial cells of the epidermis so that these cells
vide a common ground for direct comparison, a few then provide an insulating layer with respect to the
experiments were performed in which the radiative tech- deeper layers of the tissue. Fugitt (20) and Mixter (29,
nique was modified to provide a ‘steady state’ at rela- from considerations of Henriques’ and Moritz’ data,
tively low temperature. This end was accomplished by have suggested that a two-process system may be in-
producing a constant skin temperature reported as just volved in the relation of damage rates to temperature.
above the Pain threshold and maintained long enough to The present data neither support nor refute such a pos-
yield the desired end point of blistering. This procedure sibility. In this type of procedure, however, inferences
required adjustment of the radiation intensity after the with respect to intracellular mechanisms and molecular
initial heating to pain threshold (I o sec.) and resulted in changes must be considered as highly speculative at
an average temperature level of 46. I “C =t 0.5OC main- best. It would appear to be preferable to confine such
tained for 1050 seconds. The time required was much inferences to mechanisms in the physiological sense, each
longer than that predicted by extrapolation of the encompassing perhaps myriad chemical systems, and
damage rate curve appropriate to the higher temper- each related to a feature of the organism as a whole, in
ature burns (IOO sec.) and much smaller than that ob- the present instance, the production of pain and the
served by Henriques and Moritz with conductive heat- destruction of a portion of the integument. From this
ing at the same temperature (5000 sec.). However, the point of view it should be possible to show a relationship
application of the graphical integration method so that between these two phenomena, inasmuch as they have in
f-2 = I at the blister threshold provided a damage rate common the same stimulus, approximately the same
such that the log plot of the curve &/dt vs. T has a slope threshold and the same culmination in the sense that
of 0.421 and intersects the damage rate curve appro- sensation from the tissue stimulated ceases when the
priate to the higher temperature burns at about 5oOC. tissue is destroyed. It has been shown by Hardy, Wolff
Thus, all the data could be fitted to the composite and Goode11 (8) that there are 21 just noticeable differ-
damage rate curve shown in figure 6. The displacement ences (JND) in the discrimination of pain intensity.
of the curve for the present data toward higher damage It was possible also to correlate the temperature of the
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (134.153.184.170) on October 12, 2018.
Copyright © 1959 American Physiological Society. All rights reserved.
382 ALICE M. STOLL AND LEON C. GREENE
basal layer of the skin with JND’s of pain (22). Drawing intensity and, where S and So are expressed in terms of
upon these data and plotting the rate of injury, as de- the damage rate corresponding to the appropriate tissue
termined for the present thermal burn data (fig. 6), temperature as shown in table 6. Since it has been dem-
corresponding to the tissue temperature productive of onstrated here that at the same damage rates, threshold
a given level of pain sensation measured in JND’s, the blistering is produced within 24 hours when exposure
relationship shown in figure 7 was found. This relation- is such that Q = I, then it follows that under the same
ship indicates that pain intensity is linearly related to conditions the time to blistering at a constant pain in-
the log of the damage rate throughout the entire range tensity can also be predicted and is the reciprocal of the
of pain sensation. Previous attempts (I) to establish this damage rate obtained at the corresponding tissue tem-
correlation by use of the damage rates as determined perature (table 6, col. 5). Of more importance than the
by Henriques and Moritz failed at pain intensities above actual prediction of the stimulus duration, however, is
I 7 JND’s. The present data establish that pain intensity, the fact that the excellent correlation of the psycho-
like other sensations, quantitatively bears out the rela- physical data with the empirically derived physiological
damage rates constitutes strong support for the hy-
pothesis (I, 22) that noxious stimulation depends upon
P= the instantaneous rate at which certain reactions are
PO (I + K log S/So)
occurring, rather than upon the amount of effect or
extent of damage existing at any moment.
where P = pain intensity (JND), PO = threshold sensa-
tion, EC = (~/slope) of log &/dt vs. JND = constant = The authors express thanks to Mr. Richard J. Crosbie for the
6.4, S = stimulus intensity and So = threshold stimulus sol ution of equation 3.
REFERENCES
I. HARDY, J. D., I. JACOBS AND M. MEIXNER. 3. AppZ. Physiol. I 3. HENSEL, H. Arch. ges. Physiol. 252 : 146, 1950.
12: 725, 1953. I 4. BISHOP, G. H. 3. Invest. Dermat. I I : I 43, I 948.
2. LIPKIN, M., 0. BAILEY AND J. D. HARDY. 3. Appl. Physiol. 15. WOOLLARD, H. H. 3. Anat. 7 I : 54, I 936.
7 : 683, ‘955. I 6. WEDDELL, G. Brit. M. Bull. 3 : I 67, 1947.
3. HENRIQUES, F. C., JR. Arch. Pathol. 43 : 489, 1947. I 7. BUETTNER, K. 3. AppZ. Physiol. 3: 703, 1951.
4. MORITZ, A. R. AND F. C. HENRIQUES, JR. Am. 3. Path. 23: 18. GREENE, L. C., J. C. ALDEN AND J. D. HARDY. Fed. Proc.
695, I947- 17: 60, 1958.
5. HARDY, J. D. AND I. JACOBS. 3. &@. Physiol. 5 : 559, 1953. I g. HENDLER, E., R. J. CROSBIE AND J. D. HARDY. U.S. Naval
6. STOLL, A. M. AND J. D. HARDY. Fed. Proc. 15: 180, 1956. Air Materiel Center, Air Crew Equipment Laboratory,
7. BUETTNER, K. 3. AppZ. Physiol. 3 : 703, 1951. Phila., Pa. Report NAMC-ACEL-332, Ig March 1957.
8. HARDY, J. D., H. G. WOLFF AND H. GOODELL. 3. CZin. In- 20. FUGITT, C. H. Weapons Effects Division, Hq. Armed Forces
vest. 26: I 152, 1947. Special Weapons Project, Washington, D. C. Report AFSWP-
g. LIPKIN, M. AND J. D. HARDY. 3. AppZ. Physiol. 7 : 2 I 2, 1954. 606, 6 December 1955.
I o. HENRIQUES, F. C., ,JR. AND A. R. MORITZ. Am. 3. Path. 23 : 2 I. MIXTER, G. Univ. of Rochester, Atomic Energy Project,
53’9 ‘947. Rochester, N.Y. Report UR-316, 3 March I 954.
I I. LEIDER, M. AND C. M. BUNCKE. A.M.A. Arch. Dermat. & 22. HARDY, J. D., H. G. WOLFF AND H. GOODELL. Pain Sensa-
Syfh. 69: 563, Ig54. sations and Reactions. Baltimore : Williams & Wilkins, I 952,
I 2. SOUTHWOOD, W. F. W. Plast. & Reconstruct. Surg. I 5 : 423, I 955. P* 243.
Downloaded from www.physiology.org/journal/jappl by ${individualUser.givenNames} ${individualUser.surname} (134.153.184.170) on October 12, 2018.
Copyright © 1959 American Physiological Society. All rights reserved.
Вам также может понравиться
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (120)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Clinac Technical Reference Guide - Interlocks IncludedДокумент292 страницыClinac Technical Reference Guide - Interlocks Includedsenthilonco_2175338780% (5)
- ICRU 91 - Prescribing, Recording, and Reporting of Stereotactic Treatments With Small Photon Beams PDFДокумент160 страницICRU 91 - Prescribing, Recording, and Reporting of Stereotactic Treatments With Small Photon Beams PDFAnonymous pLhz3VCwОценок пока нет
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- C. S. Sureka, Christina Armpilia - Radiation Biology For Medical Physicists (2017, CRC Press, Taylor & Francis Group) - 148-163Документ16 страницC. S. Sureka, Christina Armpilia - Radiation Biology For Medical Physicists (2017, CRC Press, Taylor & Francis Group) - 148-163dwi riris indriyaniОценок пока нет
- Monte Carlo High Energy PDFДокумент64 страницыMonte Carlo High Energy PDFIrinaStiruОценок пока нет
- Brain Cancer ReportДокумент5 страницBrain Cancer ReportCrisantaCasliОценок пока нет
- Formula Sheet Engineering EconomicsДокумент1 страницаFormula Sheet Engineering EconomicsnuvanОценок пока нет
- Energies: Single-Solution-Based Vortex Search Strategy For Optimal Design of O Liquefaction ProcessesДокумент22 страницыEnergies: Single-Solution-Based Vortex Search Strategy For Optimal Design of O Liquefaction ProcessesnuvanОценок пока нет
- Extractive Metallurgy PicturesДокумент4 страницыExtractive Metallurgy PicturesnuvanОценок пока нет
- Sense and NonsenseThe Environmental Impacts of Exploration Marine Offshore Cape BretonДокумент17 страницSense and NonsenseThe Environmental Impacts of Exploration Marine Offshore Cape BretonnuvanОценок пока нет
- Canada Energy OverviewДокумент6 страницCanada Energy OverviewnuvanОценок пока нет
- ASPEN-HYSYS Simulation of Natural Gas PR PDFДокумент6 страницASPEN-HYSYS Simulation of Natural Gas PR PDFvlananlo100% (1)
- Processes: Simulation of Batch Slow Pyrolysis of Biomass Materials Using The Process-Flow-Diagram COCO SimulatorДокумент20 страницProcesses: Simulation of Batch Slow Pyrolysis of Biomass Materials Using The Process-Flow-Diagram COCO SimulatornuvanОценок пока нет
- Sensors 19 02548 PDFДокумент36 страницSensors 19 02548 PDFmohit narayanОценок пока нет
- Energies: Membrane-Assisted Removal of Hydrogen and Nitrogen From Synthetic Natural Gas For Energy-EДокумент18 страницEnergies: Membrane-Assisted Removal of Hydrogen and Nitrogen From Synthetic Natural Gas For Energy-EnuvanОценок пока нет
- Energies: Advanced and Intensified Seawater Flue Gas Desulfurization Processes: Recent Developments and ImprovementsДокумент20 страницEnergies: Advanced and Intensified Seawater Flue Gas Desulfurization Processes: Recent Developments and ImprovementsnuvanОценок пока нет
- 155:501 Advanced Transport Phenomena - Final ProjectДокумент1 страница155:501 Advanced Transport Phenomena - Final ProjectFahim Bin Abdur RahmanОценок пока нет
- QRA On Leakage Failure of Bayesian NetworkДокумент11 страницQRA On Leakage Failure of Bayesian NetworknuvanОценок пока нет
- Dynamic Sim Gas Processing Plant SAhmed SImtiazДокумент42 страницыDynamic Sim Gas Processing Plant SAhmed SImtiaznuvanОценок пока нет
- Spreadsheet Techniques For Engineering Professors The Case of Excel and Engineering EconomicsДокумент13 страницSpreadsheet Techniques For Engineering Professors The Case of Excel and Engineering EconomicsnuvanОценок пока нет
- A Fuzzy Bayesian Belief Network For Safety Assessment of Oil and Gas PipelinesДокумент17 страницA Fuzzy Bayesian Belief Network For Safety Assessment of Oil and Gas PipelinesnuvanОценок пока нет
- Assignment 1 Engineering Failures: PETR 2134:02 Materials and Equipment DesignДокумент8 страницAssignment 1 Engineering Failures: PETR 2134:02 Materials and Equipment DesignnuvanОценок пока нет
- Quantitive Assessment of CorroДокумент21 страницаQuantitive Assessment of CorronuvanОценок пока нет
- Pipe Line Leak DetectionДокумент28 страницPipe Line Leak DetectionARCC2030Оценок пока нет
- Spreadsheet Techniques For Engineering Professors The Case of Excel and Engineering EconomicsДокумент13 страницSpreadsheet Techniques For Engineering Professors The Case of Excel and Engineering EconomicsnuvanОценок пока нет
- Material Requisition For Leak Detection System: Page 1 of 14Документ14 страницMaterial Requisition For Leak Detection System: Page 1 of 14nuvanОценок пока нет
- (14076179 - Transport and Telecommunication Journal) Condition Monitoring of Operating Pipelines With Operational Modal Analysis ApplicationДокумент15 страниц(14076179 - Transport and Telecommunication Journal) Condition Monitoring of Operating Pipelines With Operational Modal Analysis ApplicationnuvanОценок пока нет
- Data Sheet For Leak Detection SystemДокумент4 страницыData Sheet For Leak Detection SystemnuvanОценок пока нет
- Termpaper TonnyДокумент11 страницTermpaper TonnynuvanОценок пока нет
- KROHNE Gerhard Geiger Principles of Leak Detection 2012Документ68 страницKROHNE Gerhard Geiger Principles of Leak Detection 2012Alvaro Luiz SmiderleОценок пока нет
- ASSIGNMENT 1 FINAL in 2003Документ30 страницASSIGNMENT 1 FINAL in 2003nuvanОценок пока нет
- DOE Term Paper Wind TurbineДокумент11 страницDOE Term Paper Wind TurbinenuvanОценок пока нет
- PETR 3134 Codes and Specifications in The Petroleum IndustryДокумент18 страницPETR 3134 Codes and Specifications in The Petroleum IndustrynuvanОценок пока нет
- DOE Term Paper Wind TurbineДокумент11 страницDOE Term Paper Wind TurbinenuvanОценок пока нет
- Termpaper TonnyДокумент11 страницTermpaper TonnynuvanОценок пока нет
- Engi 9625 MUN Assignment 1Документ15 страницEngi 9625 MUN Assignment 1nuvanОценок пока нет
- PDF - 2 - Non - DaeFinal PERSONAL TLD APPLICATION FORMДокумент3 страницыPDF - 2 - Non - DaeFinal PERSONAL TLD APPLICATION FORMvivekmaini0% (1)
- Lung Cancer - WikipediaДокумент25 страницLung Cancer - WikipediaMaria Vanda Ribeiro Moreira MoreiraОценок пока нет
- Radiotherapy and OncologyДокумент7 страницRadiotherapy and OncologyMichael Steve Camelo GuevaraОценок пока нет
- The Biology of CancerДокумент20 страницThe Biology of CancerAlishka Garg59% (22)
- Daily QA™ 3 User's GuideДокумент40 страницDaily QA™ 3 User's Guideruben50% (2)
- 3d MamaДокумент26 страниц3d Mamadavid2493Оценок пока нет
- Breast CancersДокумент9 страницBreast CancersMS AntikaОценок пока нет
- INP Report Final 1Документ10 страницINP Report Final 1walidОценок пока нет
- Internal Fiducial MarkerДокумент9 страницInternal Fiducial MarkerAndreas RonaldОценок пока нет
- Spinal Cord Tumors: By: Divine Incillo Shiela Marie LaraДокумент27 страницSpinal Cord Tumors: By: Divine Incillo Shiela Marie LaraDivine IncilloОценок пока нет
- Thesis RadiodiagnosisДокумент8 страницThesis Radiodiagnosisgcq5c1pv100% (2)
- Ecancermedicalscience 2010 Article 190Документ5 страницEcancermedicalscience 2010 Article 190alejandromfunes1749Оценок пока нет
- Cervix DelineationДокумент22 страницыCervix Delineationfadli puteraОценок пока нет
- Liver CancerДокумент9 страницLiver Cancerapi-240354710Оценок пока нет
- Andrés Felipe Cardona: Eduardo Obando)Документ3 страницыAndrés Felipe Cardona: Eduardo Obando)Zarit Diseños CaliОценок пока нет
- Thiourea Derivatives in Drug Design and Medicinal Chemistry: A Short ReviewДокумент11 страницThiourea Derivatives in Drug Design and Medicinal Chemistry: A Short ReviewYun NikОценок пока нет
- Brain Tumors (Benign and Malignant) - Symptoms, Causes, TreatmentДокумент4 страницыBrain Tumors (Benign and Malignant) - Symptoms, Causes, TreatmentThuvija DarshiniОценок пока нет
- Moderate and Extreme HypofractionationДокумент32 страницыModerate and Extreme HypofractionationsamuelfsjОценок пока нет
- RPT 79Документ84 страницыRPT 79risalatifahОценок пока нет
- Oncology Practice ExamДокумент8 страницOncology Practice ExamgrabshellОценок пока нет
- Sykes Hmo Benefit Plan 2016Документ16 страницSykes Hmo Benefit Plan 2016Secret SecretОценок пока нет
- Internal Radiation: Researchers Studying Breast RadiationДокумент3 страницыInternal Radiation: Researchers Studying Breast RadiationYoza FendrianiОценок пока нет
- Splenomegaly Treatment & Management - Approach Considerations, Activity, Pharmacologic TherapyДокумент7 страницSplenomegaly Treatment & Management - Approach Considerations, Activity, Pharmacologic TherapyAndri Feisal NasutionОценок пока нет
- Hyperbaric Oxygen in Cancer Treatment - CANCERactiveДокумент6 страницHyperbaric Oxygen in Cancer Treatment - CANCERactivejide.atolagbe3737Оценок пока нет
- Radiotherapy: Basic Concepts and Recent AdvancesДокумент17 страницRadiotherapy: Basic Concepts and Recent AdvancesutokaОценок пока нет