Академический Документы
Профессиональный Документы
Культура Документы
SNB Vs AC
Загружено:
willyoueverlovemenkОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
SNB Vs AC
Загружено:
willyoueverlovemenkАвторское право:
Доступные форматы
The new england journal of medicine
original article
A Randomized Comparison of Sentinel-Node
Biopsy with Routine Axillary Dissection
in Breast Cancer
Umberto Veronesi, M.D., Giovanni Paganelli, M.D., Giuseppe Viale, F.R.C.Path.,
Alberto Luini, M.D., Stefano Zurrida, M.D., Viviana Galimberti, M.D.,
Mattia Intra, M.D., Paolo Veronesi, M.D., Chris Robertson, Ph.D.,
Patrick Maisonneuve, Eng., Giuseppe Renne, M.D., Concetta De Cicco, M.D.,
Francesca De Lucia, M.D., and Roberto Gennari, M.D.
abstract
background
From the Divisions of Senology (U.V., A.L., Although numerous studies have shown that the status of the sentinel node is an accu-
S.Z., V.G., M.I., P.V., R.G.), Nuclear Medi- rate predictor of the status of the axillary nodes in breast cancer, the efficacy and safety
cine (G.P., C.D.), Pathology (G.V., G.R.),
Epidemiology (C.R., P.M.), and Anaesthe- of sentinel-node biopsy require validation.
siology (F.D.), European Institute of On-
cology; and the University of Milan School methods
of Medicine (G.V.) — both in Milan, Italy.
Address reprint requests to Dr. Umberto From March 1998 to December 1999, we randomly assigned 516 patients with primary
Veronesi at the Istituto Europeo di Oncolo- breast cancer in whom the tumor was less than or equal to 2 cm in diameter either to
gia, Via G. Ripamonti 435, 20141 Milan, Italy, sentinel-node biopsy and total axillary dissection (the axillary-dissection group) or to
or at umberto.veronesi@ieo.it.
sentinel-node biopsy followed by axillary dissection only if the sentinel node contained
N Engl J Med 2003;349:546-53. metastases (the sentinel-node group).
Copyright © 2003 Massachusetts Medical Society.
results
The number of sentinel nodes found was the same in the two groups. A sentinel node
was positive in 83 of the 257 patients in the axillary-dissection group (32.3 percent),
and in 92 of the 259 patients in the sentinel-node group (35.5 percent). In the axillary-
dissection group, the overall accuracy of the sentinel-node status was 96.9 percent, the
sensitivity 91.2 percent, and the specificity 100 percent. There was less pain and better
arm mobility in the patients who underwent sentinel-node biopsy only than in those who
also underwent axillary dissection. There were 15 events associated with breast cancer in
the axillary-dissection group and 10 such events in the sentinel-node group. Among the
167 patients who did not undergo axillary dissection, there were no cases of overt axillary
metastasis during follow-up.
conclusions
Sentinel-node biopsy is a safe and accurate method of screening the axillary nodes for
metastasis in women with a small breast cancer.
546 n engl j med 349;6 www.nejm.org august 7, 2003
Downloaded from www.nejm.org at TATA MEMORIAL HOSPITAL on June 16, 2009 .
Copyright © 2003 Massachusetts Medical Society. All rights reserved.
sentinel-node biopsy in breast cancer
of 649 consecutive patients were randomly assigned
m odern screening methods make
it possible to diagnose breast cancer at
an early stage, when the axillary lymph
nodes are free of metastases. As a result, routine ax-
illary dissection may constitute excessive treatment.
to one of the two study groups. Of the other 117 pa-
tients, 78 were deemed ineligible, 25 declined to
undergo randomization, and 14 did not undergo
randomization for other reasons. Of the 532 ran-
The technique of the sentinel-node biopsy was de- domized patients, 16 were not able to be evaluated
veloped to provide surgeons with information that (Table 1). Table 2 summarizes the characteristics of
allows axillary dissection to be avoided if the sentinel the remaining 516 patients.
node is negative. Numerous studies have shown that All the patients were informed of the aims of the
the findings in the sentinel node accurately predict study, the potential effects of the procedures, the
the status of the other axillary nodes.1-3 Neverthe- risks associated with surgery, and the meaning of
less, the efficacy and safety of this method require the randomization. All the patients signed a consent
validation. form approved by the institutional ethics committee
We performed a randomized comparison of sen- (an institutional review board) before the operation.
tinel-node biopsy followed by routine axillary dis-
section with sentinel-node biopsy followed by ax- localization of the sentinel node
illary dissection only if, on intraoperative histologic All 516 patients were scheduled to undergo surgery
examination, the sentinel node was found to be early in the morning. In 410 of them (79 percent),
positive.
methods Table 1. Numbers of Patients Eligible for Enrollment, Randomly Assigned
to a Study Group, and Able to Be Evaluated.
study design and main end points
The study was approved by the ethics committee of Patients No. of Patients
the European Institute of Oncology. It was a single- Initially considered for enrollment 649
center, randomized trial involving two study groups. Not eligible 78
Patients with primary breast cancer in whom the tu-
Noninvasive breast cancer 12
mor was less than or equal to 2 cm in diameter were
randomly assigned to undergo, after breast-con- Tumor diameter >2 cm 32
serving surgery, either sentinel-node biopsy and to- Multicentric disease 26
tal axillary dissection (the axillary-dissection group) Sentinel node not revealed by scintigraphy 8
or sentinel-node biopsy followed by axillary dissec-
Eligible for enrollment 571
tion only if the sentinel node contained metastatic
breast cancer (the sentinel-node group). The pri- Not randomly assigned to a study group 39
mary end point of the study was the predictive pow- Patient’s decision 25
er of the status of the sentinel node, measured in Sentinel node not evident on preoperative probe-guided 3
terms of the percentage of cases of axillary involve- inspection
ment detected by sentinel-node biopsy in relation Frozen sectioning not feasible 3
to the percentage found by routine axillary dissec-
Other 8
tion. Additional end points were indicators of the
quality of life, the number of axillary-node metas- Randomly assigned to a study group 532
tases appearing during follow-up in patients in the Not able to be evaluated 16
sentinel-node group with a negative sentinel node, Multicentric, bilateral, or extensive multifocal disease 5
and disease-free and overall survival.
Sentinel node not identified at surgery 5
patients Benign lesion on final histologic examination 4
Patients 40 to 75 years of age with invasive breast Metastatic disease 2
carcinoma and no history of another cancer except Able to be evaluated 516
skin cancer were eligible for enrollment. Patients
Axillary-dissection group 257
who had multicentric cancer or who had previously
undergone excisional biopsy were not eligible. Sentinel-node group 259
Between March 1998 and December 1999, 532
n engl j med 349;6 www.nejm.org august 7, 2003 547
Downloaded from www.nejm.org at TATA MEMORIAL HOSPITAL on June 16, 2009 .
Copyright © 2003 Massachusetts Medical Society. All rights reserved.
The new england journal of medicine
radioactive tracer was injected during the evening
Table 2. Characteristics of the Patients in the Two Study Groups.* before surgery; in the other 106 patients (21 percent)
Axillary- Sentinel- radioactive tracer was injected on the day of surgery.
Dissection Node Five to 10 MBq of technetium-99m–labeled particles
Group Group Total of colloidal human albumin (each 50 to 200 nm in
Characteristic (N=257) (N=259) (N=516) P Value
diameter) in 0.2 ml of saline was injected close to
no. of patients (%) the tumor.5 Injection was subdermal if the tumor
Age 0.74 was superficial and peritumoral if it was deep. Ante-
rior and anterior–oblique lymphoscintigraphic pro-
40–45 yr 35 (13.6) 32 (12.4) 67 (13.0)
jections of the breast and axilla were subsequently
46–55 yr 88 (34.2) 99 (38.2) 187 (36.2)
obtained to determine the exact position of the sen-
56–65 yr 92 (35.8) 92 (35.5) 184 (35.7) tinel node.
66–75 yr 42 (16.3) 36 (13.9) 78 (15.1) Four to 20 hours after the injection of tracer,
Diameter of tumor 0.90
sentinel-node biopsy was performed during breast
surgery. A gamma-ray–detecting probe in a sterile
<1.0 cm 65 (25.3) 65 (25.1) 130 (25.2)
glove was used to identify the radioactive sentinel
1.1–1.5 cm 123 (47.9) 120 (46.3) 243 (47.1) node and facilitate its removal.
>1.5 cm 69 (26.8) 74 (28.6) 143 (27.7)
Site of tumor 0.84 surgery
All the patients underwent quadrantectomy or wide
Outer quadrant 187 (72.8) 186 (71.8) 373 (72.3)
resection, immediately followed by sentinel-node
Inner or central quadrant 70 (27.2) 73 (28.2) 143 (27.7) biopsy. In the operating room, patients were random-
Histologic type 0.63 ly assigned to one of the two study groups after it was
Ductal infiltrating 212 (82.5) 209 (80.7) 421 (81.6) verified that a sentinel node could be detected by the
Lobular infiltrating 20 (7.8) 18 (6.9) 38 (7.4)
gamma probe and after the macroscopical size of
the tumor was determined. The node was removed
Other 25 (9.7) 32 (12.4) 57 (11.0)
through the incision used for tumor resection if the
Estrogen-receptor status† 1.00 tumor was in the upper outer quadrant and through
Positive 236 (91.8) 237 (91.9) 473 (91.8) a separate, axillary incision if the tumor was in any
Negative 21 (8.2) 21 (8.1) 42 (8.2) other quadrant. The removed sentinel node was sent
for immediate frozen-section examination.
Rate of proliferation‡ 0.78
In the patients assigned to the axillary-dissection
<20% of nuclei dividing 166 (64.6) 170 (65.9) 336 (65.2) group, sentinel-node biopsy was immediately fol-
≥20% of nuclei dividing 91 (35.4) 88 (34.1) 179 (34.8) lowed by complete axillary dissection.6 In patients in
Tumor grade§ 0.68 the sentinel-node group, the next event depended
on the result of the intraoperative pathological ex-
I 81 (31.9) 82 (31.9) 163 (31.9)
amination of the sentinel node. If the node was neg-
II 119 (46.9) 128 (49.8) 247 (48.3) ative for metastasis, the operation was concluded at
III 54 (21.3) 47 (18.3) 101 (19.8) that point; if the node contained a metastasis, com-
Peritumoral vascular invasion 1.00 plete axillary dissection was performed immediate-
Yes 43 (16.7) 44 (17.0) 87 (16.9)
ly. In both the patients in the sentinel-node group
with a positive node and in all the patients in the ax-
No 214 (83.3) 215 (83.0) 429 (83.1)
illary-dissection group, all the axillary nodes, at all
three Berg levels, were removed. According to Berg,
* Because of rounding, not all percentages total 100.
† Estrogen-receptor status was not determined in one patient in the sentinel- the axillary lymph nodes are divided into three lev-
node group. els according to location: lateral (first level), poste-
‡ The rate of proliferation was measured by determining the percentage of nuclei rior (second level), or medial (third level) to the mi-
in which labeled antigen Ki-67 (a marker of cell division) was expressed. It was
not measured in one patient in the sentinel-node group. nor pectoralis muscle.7
§ Tumors were graded according to the system of Elston and Ellis,4 where I indi- The patients who underwent axillary dissection
cates well differentiated, II moderately differentiated, and III poorly differenti- stayed in the hospital an average of 4.3 days, where-
ated. The grade was not available for three patients in the axillary-dissection
group and two patients in the sentinel-node group. as those who underwent only sentinel-node biopsy
stayed an average of 2.1 days.
548 n engl j med 349;6 www.nejm.org august 7 , 2003
Downloaded from www.nejm.org at TATA MEMORIAL HOSPITAL on June 16, 2009 .
Copyright © 2003 Massachusetts Medical Society. All rights reserved.
sentinel-node biopsy in breast cancer
pathological examination arm mobility (on a scale from 0 [severe restriction]
The sentinel node was bisected along its major axis, to 100 [no restriction]), and the appearance of the
embedded (cut surface up) in optimal-cutting-tem- axillary scar. At the same evaluation the circumfer-
perature compound (CellPath), and frozen in iso- ence of the operated arm was measured and com-
pentane cooled with liquid nitrogen. Lymph nodes pared with that of the contralateral arm.
less than 5 mm in diameter were embedded and
frozen uncut.8 If more than one sentinel lymph node statistical analysis
was obtained from a patient, all the nodes were ex- One aim of the study was to estimate the percentage
amined in this way. of patients in the sentinel-node group whose senti-
For each node that was large enough to be cut, nel node was negative for metastasis but in whom
15 pairs of frozen sections, each 4 µm thick, were cut overt axillary nodal metastases developed within five
at 50-µm intervals in each half of the node, amount- years after surgery. Assuming that 30 percent of 250
ing to about 60 sections per node. If residual tissue recruited patients would have a positive sentinel
was left, additional pairs of sections were cut at node, we expected 175 patients to have a negative
100-µm intervals until the lymph node had been sentinel node. On the basis of data from a previous
sampled completely. One section in each pair of sec- study,9 we expected axillary metastases to develop
tions was stained with hematoxylin and eosin. If the in 5 percent of those with a negative sentinel node.
result was ambiguous, the other section was stained The study had 84 percent power to distinguish be-
for cytokeratins by means of a rapid method (EPOS tween an acceptable percentage (5 percent) and an
Cytokeratin reagent with HRP, Dako) and stained unacceptable percentage (10 percent) of women
for the monoclonal antibody MNF116.8 with nodal metastases at five years; we considered
The lymph nodes removed during conventional 10 percent the probable maximal percentage. A sen-
axillary dissection were examined by standard tech- sitivity analysis of the power of this one-sided test
niques. Nodes greater than 5 mm in diameter were showed that it varied between 77 percent and 91
bisected; those less than or equal to 5 mm in diam- percent for a range of acceptable expected percent-
eter were fixed and embedded uncut. Three to six ages less than 5 percent.
sections were obtained from each lymph node at dif- One-sided tests and confidence intervals were
ferent levels 100 to 500 µm apart and stained with used because we were interested only in seeing
hematoxylin and eosin. whether the percentage of women with overt nodal
metastases in the sentinel-node group was greater
adjuvant treatment than specified values. All P values were two-sided.
All the patients received radiation to the ipsilateral The overall accuracy of the status of the sentinel
breast over a period of eight weeks. A dose of 50 Gy node in the axillary-dissection group was defined as
was delivered through two opposed tangential fields the rate of correct classification of patients, on the
with high-energy photons. A 10-Gy boost was given basis of this status, as having or as not having axil-
to the skin surrounding the surgical scar. Patients lary metastases.
with unfavorable prognostic characteristics were Associations between the status of the axillary
given systemic adjuvant therapy according to the nodes and the characteristics of the primary tumor
standard protocols used at the European Institute were assessed by means of Fisher’s exact test.10
of Oncology. Logistic-regression methods were used to adjust
for the effects of the prognostic factors. Confidence
evaluation of side effects intervals were calculated by the exact binomial
The evaluation of side effects included 100 consec- method. The log-rank test was used to compare
utive patients from the axillary-dissection group and curves representing disease-free survival. All the
100 consecutive patients from the sentinel-node calculations were carried out using S-Plus 2000,
group who did not undergo total axillary dissection version 3.11
because the sentinel node was negative for metas-
tasis. These 200 patients were interviewed by physi- results
cians from the Breast Department 6 months and 24
months after surgery and were asked to complete a identification of the sentinel node
questionnaire concerning the intensity of pain, the Between March 1998 and December 1999, 649 con-
presence or absence of paresthesias, the extent of secutive patients underwent scintigraphy after the
n engl j med 349;6 www.nejm.org august 7, 2003 549
Downloaded from www.nejm.org at TATA MEMORIAL HOSPITAL on June 16, 2009 .
Copyright © 2003 Massachusetts Medical Society. All rights reserved.
The new england journal of medicine
injection of radiolabeled albumin as a prelude to Seventy other patients also proved ineligible. Of the
sentinel-node biopsy. In eight patients (1.2 percent), 571 eligible patients, 39 (6.8 percent) were not ran-
scintigraphy revealed no uptake of radioactivity to domly assigned to a study group, either because they
the axilla; these patients were considered ineligible. chose not to participate (25 patients) or because
other factors precluded their participation (14 pa-
tients). Of the 532 patients who were randomly as-
Table 3. Side Effects in the Two Study Groups. signed to a study group, 16 (3.0 percent) could not
Axillary-Dissection Sentinel-Node
be evaluated, for various reasons (Table 1).
Group Group A total of 429 sentinel nodes were removed and
Side Effect (N=100) (N=100) examined from the 257 patients in the axillary-dis-
no. of patients
section group and 424 from the 259 patients in the
sentinel-node group. A mean of 24 nonsentinel ax-
6 mo 24 mo 6 mo 24 mo
illary nodes were removed in both groups.
Axillary pain*
No 9 61 84 92 detection of positive axillary nodes
Yes, sporadic 72 34 14 7
The primary end point of the study was the capacity
of the sentinel-node biopsy to predict the presence
Yes, continuous 19 5 2 1
or absence of axillary nodes positive for metastasis.
Numbness or paresthesias on Of the 257 patients in the axillary-dissection group,
operated side†
83 had a positive sentinel node (32.3 percent; 95
No 15 32 98 99 percent confidence interval, 26.6 to 38.4) and 174
Yes 85 68 2 1 had a negative sentinel node (67.7 percent). Of the
Arm mobility‡
259 patients in the sentinel-node group, 92 had a
positive sentinel node (35.5 percent; 95 percent con-
80–100% 73 79 100 100
fidence interval, 29.7 to 41.7), and 167 had a nega-
60–79% 22 18 0 0 tive sentinel node (64.5 percent; 95 percent confi-
40–59% 5 2 0 0 dence interval, 58.3 to 70.3).
20–39% 0 1 0 0
In the axillary-dissection group 8 of 174 pa-
tients with negative sentinel nodes (4.6 percent; 95
<20% 0 0 0 0
percent confidence interval, 2.0 to 8.9) were subse-
Aesthetic appearance of axillary scar§ quently found to have metastatic disease in the ax-
Good 91 85 98 100 illary nodes; in 2 of these patients, there was micro-
Bad 9 15 2 0
metastatic disease in only one axillary node. The
overall accuracy of the sentinel-node status in the
Arm swelling (difference in circumference)¶
axillary-dissection group was therefore 96.9 percent
No difference 31 25 89 93 (in that the results were accurate in 249 of 257 pa-
<1 cm 44 38 11 6 tients), the sensitivity was 91.2 percent (83 of 91
1–2 cm 17 25 0 1
patients with positive nodes were identified), and
the specificity was 100 percent. The false negative
>2 cm 8 12 0 0
rate of 8.8 percent (8 of 91 patients with positive
nodes were not identified) (95 percent confidence
* Postoperative axillary pain was evaluated as continuous (lasting >50 percent
of the day), sporadic, or absent. interval, 3.9 to 16.6) and the negative predictive val-
† Numbness and paresthesias were assessed by comparing skin sensitivity on ue of 95.4 percent (95 percent confidence interval,
the inner and outer upper arms, axillae, and chest wall on the operated side 91.1 to 98.0) are consistent with data from previ-
with that on the untreated side. Sensitivity was recorded as either the presence
or absence of numbness. ous studies.1-3,9
‡ Arm mobility was judged by asking the patient to assign the restriction in mo-
tion in the operated arm a value on a scale of 0 percent (severe restriction) to micrometastasis
100 percent (no restriction).
§ The appearance of the axillary scar was judged by asking the patient simply to In 60 of the 175 patients with a positive sentinel
say whether the result was good or bad. node, only micrometastases (foci of metastatic cells
¶ Arm swelling and edema were assessed by comparing the circumference ≤2 mm in diameter) were found. In the axillary-dis-
(in centimeters) of the treated arm 15 cm above the lateral epicondyle with
that of the untreated arm. section group, 29 patients had a micrometastatic
sentinel node; in 24 of these patients, all the other
550 n engl j med 349;6 www.nejm.org august 7 , 2003
Downloaded from www.nejm.org at TATA MEMORIAL HOSPITAL on June 16, 2009 .
Copyright © 2003 Massachusetts Medical Society. All rights reserved.
sentinel-node biopsy in breast cancer
axillary nodes were negative, and in 5, only one oth-
er node was positive. In the sentinel-node group, Table 4. Unfavorable Events and Deaths in the Two Study Groups.
31 patients had a micrometastatic sentinel node; in Axillary-Dissection Sentinel-Node
26 of these patients, all the other axillary nodes were Group Group
negative, and in 5, only one other node was positive. Event (N=257) (N=259)
no. of events
side effects and unfavorable events
Events other than death
Side effects are listed in Table 3. The patients who
Axillary metastasis 0 0
underwent only sentinel-node biopsy had less pain
and numbness and better arm mobility than those Supraclavicular metastasis 2 0
who underwent axillary-node dissection as well. Recurrence in ipsilateral breast 1 1
They also had less arm swelling than those who un- Cancer in contralateral breast 2 3
derwent immediate axillary-node dissection.
Distant metastasis 10 6
The 516 patients were followed for a median of
46 months. As of the most recent follow-up, there Other primary tumor 6 3
have been 34 events (21 in the axillary-dissection Total 21 13
group and 13 in the sentinel-node group; P=0.13 Death due to breast cancer 2 1
by the log-rank test) (Table 4). Of the 25 events as-
Death from other causes 4 1
sociated with breast cancer, 15 occurred in the axil-
lary-dissection group (recurrence in the ipsilateral
breast in 1, a tumor in the contralateral breast in 2,
an axillary metastasis in 2, and distant metastasis third, and skip metastases are infrequent.7,12,13
in 10), and 10 occurred in the sentinel-node group These findings encouraged investigations in breast
(recurrence in the ipsilateral breast in 1, a tumor in cancer of sentinel-node biopsy, a procedure that is
the contralateral breast in 3, and distant metastasis effective in melanoma.14 Initial results1,2,9,15 and
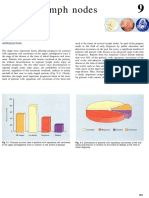
in 6) (P=0.26 by the log-rank test) (Fig. 1). In addi- many subsequent studies indicated that sentinel-
tion, other primary tumors developed in six patients node biopsy is a reliable axillary staging technique,
in the axillary-dissection group and in three in the although a limited number of false negative re-
sentinel-node group (Table 4). As of the most recent sults has been a feature of all the reports published
follow-up, axillary metastasis has not been detected to date.
by physical or ultrasonographic examination in any In the current study, randomization began in
of the patients. March 1998. As a single-center study, it had the ad-
Thus far, the rate of events associated with breast vantages that the procedures used for sentinel-node
cancer is 16.4 per 1000 per year (95 percent confi- identification, the surgical techniques, and the
dence interval, 9.2 to 26.9) in the axillary-dissection standards of pathological examination were uni-
group and 10.1 per 1000 per year (95 percent con- form. Recruitment was completed quickly (in De-
fidence interval, 4.9 to 18.5) in the sentinel-node cember 1999), after 532 patients had been randomly
group. Eight patients have died, six of them in the assigned to one of the two study groups. It is note-
axillary-dissection group (two from metastatic worthy that only 25 of the 649 patients initially con-
breast cancer) and two of them in the sentinel-node sidered for enrollment (3.9 percent) chose not to
group (one from metastatic breast cancer) (P=0.15 participate in the randomization, some requesting
by the log-rank test). There was no statistically sig- complete axillary dissection and others requesting
nificant difference between the two groups in the sentinel-node biopsy procedure.16
rate of overall survival (Fig. 2). We used a radioactive tracer (technetium-99m)
and not blue dye to detect the sentinel node. Because
discussion our rate of sentinel-node identification was close to
99 percent, blue dye was not required as an adjuvant
The aim of this study was to determine the ability of localization tool.
sentinel-node biopsy to reduce the need for com- An important result of this study is that sentinel-
plete axillary dissection in women with breast can- node biopsy is effective in identifying cases in which
cer. In most cases, lymphatic spread within the axilla one or more axillary nodes are positive for metasta-
is orderly, proceeding from the first Berg level to the sis. Of the 259 patients in the sentinel-node group,
n engl j med 349;6 www.nejm.org august 7, 2003 551
Downloaded from www.nejm.org at TATA MEMORIAL HOSPITAL on June 16, 2009 .
Copyright © 2003 Massachusetts Medical Society. All rights reserved.
The new england journal of medicine
10
100 Sentinel-node group
Cumulative Incidence of Events (%)
Axillary-dissection group
8 P=0.26
Proportion of Patients Surviving (%)
90 P=0.15
6
80
4 Axillary-dissection group
70
2
Sentinel-node group 60
0
0 6 12 18 24 30 36 42 48 54 60 50
Months
0
0 6 12 18 24 30 36 42 48 54 60
Figure 1. Cumulative Incidence of Events Associated
Months
with Breast Cancer in the Two Study Groups.
Figure 2. Overall Survival in the Two Study Groups.
92 (35.5 percent) had a positive sentinel node and tween “routine” findings on frozen section and sub-
underwent total axillary dissection — a proportion sequent findings on permanent section.8 Such
similar to that in the axillary-dissection group, in discordant results would mean that patients with
which 91 of 257 patients (35.4 percent) were found negative findings on intraoperative examination
to have axillary metastases. However, only 83 of of frozen sections of the sentinel node and positive
those 91 patients had had a positive sentinel node; findings on the final pathological examination will
hence, the accuracy of sentinel-node biopsy in the have to undergo axillary dissection some days after
axillary-dissection group was 96.9 percent. The false the breast operation, a process imposing consider-
negative rate (8.8 percent) is similar to that observed able psychological trauma on the patients. For this
in our previous series.1,3,9,17 reason, we used a new method of intraoperative fro-
Assuming that the proportion of patients in the zen-section examination, involving complete sec-
sentinel-node group who had a negative sentinel tioning of the entire lymph node and examination
node and undetected axillary involvement is similar of a large number of sections (30 to 60).8 The intra-
to that in the axillary-dissection group, we can esti- operative diagnosis is therefore definitive, and no
mate that 8 (95 percent confidence interval, 3 to 15) tissue is left for permanent sectioning.
of the 167 patients in the sentinel-node group who In 60 of the 175 patients with a positive sentinel
had a negative sentinel node may have had occult ax- node, only micrometastases (foci ≤2 mm in diam-
illary involvement. As of the last follow-up (at the eter) were found in the sentinel nodes, and in only
end of March 2003), no overt axillary metastases 10 of these 60 patients (17 percent) was another ax-
had appeared in these 167 patients after a median illary node found to be involved. This poses the
follow-up of 46 months and a total of 634 patient- question of whether to perform axillary dissection
years at risk. when the sentinel node is micrometastatic or wheth-
The possibility that occult metastasis will never er attentive follow-up is sufficient.
become clinically evident if the axilla is left intact The fact that a small percentage (4.6 percent) of
has been raised many times.18 In a series of 221 pa- patients with a negative sentinel node had occult
tients with breast cancer in whom the primary tu- metastases in other axillary nodes suggests that
mor was small (<1.2 cm in diameter) and who un- there is a risk of “understaging” in such patients.
derwent conservative breast surgery without axillary However, our intraoperative method for examining
dissection, overt axillary metastases developed in the sentinel node is more sensitive than routine
only two women (unpublished data). analysis of permanent sections and may actually
A serious drawback to the use of sentinel-node lead to “overstaging” and hence to possible over-
biopsy arises if there is frequent discordance be- treatment.
552 n engl j med 349;6 www.nejm.org august 7 , 2003
Downloaded from www.nejm.org at TATA MEMORIAL HOSPITAL on June 16, 2009 .
Copyright © 2003 Massachusetts Medical Society. All rights reserved.
sentinel-node biopsy in breast cancer
Postoperative side effects were much less fre- vides evidence justifying the use of sentinel-node bi-
quent in the patients who underwent sentinel-node opsy as part of the assessment of the stage of breast
biopsy only than in those who underwent complete cancer. Use of this method can obviate the need for
axillary dissection. Disease-free survival was slight- total axillary dissection in patients with a negative
ly better in the sentinel-node group than in the axil- sentinel node, thereby reducing postoperative mor-
lary-dissection group. Because the number of pa- bidity and hospitalization costs.
tients was small, however, firm conclusions cannot Supported by the Italian Association for Cancer Research (AIRC).
be drawn. Nevertheless, this randomized trial pro-
references
1. Giuliano AE, Kirgan DM, Guenther JM, 7. Berg JW. The significance of axillary node Technical details of intraoperative lymphat-
Morton DL. Lymphatic mapping and sentinel levels in the study of breast carcinoma. Can- ic mapping for early stage melanoma. Arch
lymphadenectomy for breast cancer. Ann cer 1955;8:776-8. Surg 1992;127:392-9.
Surg 1994;220:391-401. 8. Viale G, Bosari S, Mazzarol G, et al. In- 15. Albertini JJ, Lyman GH, Cox C, et al.
2. Krag D, Weaver D, Ashikaga T, et al. The traoperative examination of axillary sentinel Lymphatic mapping and sentinel node bi-
sentinel node in breast cancer — a multi- lymph nodes in breast carcinoma patients. opsy in the patient with breast cancer. JAMA
center validation study. N Engl J Med 1998; Cancer 1999;85:2433-8. 1996;276:1818-22.
339:941-6. 9. Veronesi U, Paganelli G, Viale G, et al. 16. Veronesi U, Galimberti V, Zurrida S, et
3. Veronesi U, Paganelli G, Galimberti V, et Sentinel lymph node biopsy and axillary dis- al. Sentinel lymph node biopsy as an indica-
al. Sentinel-node biopsy to avoid axillary dis- section in breast cancer: results in a large se- tor for axillary dissection in early breast can-
section in breast cancer with clinically nega- ries. J Natl Cancer Inst 1999;91:368-73. cer. Eur J Cancer 2001;37:454-8.
tive lymph-nodes. Lancet 1997;349:1864-7. 10. Gould AL. Sample sizes for event rate 17. McMasters KM, Giuliano AE, Ross MI,
4. Elston CW, Ellis IO. Pathological prog- equivalence trials using prior information. et al. Sentinel-lymph-node biopsy for breast
nostic factors in breast cancer. I. The value Stat Med 1993;12:2009-23. cancer — not yet the standard of care.
of histological grade in breast cancer: expe- 11. S Plus 2000. S Plus professional release N Engl J Med 1998;339:990-5.
rience from a large study with long-term fol- 3. Seattle: Mathsoft, 2000. 18. Mansi JL, Gogas H, Bliss JM, Gazet JC,
low-up. Histopathology 1991;19:403-10. 12. Veronesi U, Luini A, Galimberti V, Mar- Berger U, Coombes RC. Outcome of pri-
5. De Cicco C, Cremonesi M, Luini A, et al. chini S, Sacchini V, Rilke F. Extent of axillary mary-breast-cancer patients with microme-
Lymphoscintigraphy and radioguided biop- involvement in 1446 cases of breast cancer. tastases: a long-term follow-up study. Lan-
sy of the sentinel axillary node in breast can- Eur J Surg Oncol 1990;16:127-33. cet 1999;354:197-202.
cer. J Nucl Med 1998;39:2080-4. 13. Zurrida S, Morabito A, Galimberti V, et al. Copyright © 2003 Massachusetts Medical Society.
6. Zurrida S, Costa A, Luini A, Galimberti V, Importance of the level of axillary involve-
Sacchini V, Intra M. The Veronesi quadran- ment in relation to traditional variables in
tectomy: an established procedure for the the prognosis of breast cancer. Int J Oncol
conservative treatment of early breast can- 1999;15:475-80.
cer. Int J Surg Investig 2001;2:423-31. 14. Morton DL, Wen DR, Wong JH, et al.
early job alert service available at the new nejm career center
Register to receive weekly e-mail messages with the latest job openings that match your
specialty, as well as preferred geographic region, practice setting, call schedule, and
more. Visit the new NEJM Career Center at www.nejmjobs.org for more information.
n engl j med 349;6 www.nejm.org august 7, 2003 553
Downloaded from www.nejm.org at TATA MEMORIAL HOSPITAL on June 16, 2009 .
Copyright © 2003 Massachusetts Medical Society. All rights reserved.
Вам также может понравиться
- Obstructive Sleep Aponea SurgeriesДокумент638 страницObstructive Sleep Aponea Surgerieswillyoueverlovemenk100% (2)
- Head and Neck Soft Tissue Tumor Diagnosis and TreatmentДокумент37 страницHead and Neck Soft Tissue Tumor Diagnosis and TreatmentwillyoueverlovemenkОценок пока нет
- Nbde ReleasedДокумент4 страницыNbde ReleasedwillyoueverlovemenkОценок пока нет
- Changes between the 6th and 7th editions of the TNM Classification of Malignant TumoursДокумент46 страницChanges between the 6th and 7th editions of the TNM Classification of Malignant TumoursKen HidakaОценок пока нет
- Neurogenic Tumors and Paragangliomas: BenignДокумент36 страницNeurogenic Tumors and Paragangliomas: BenignwillyoueverlovemenkОценок пока нет
- 5B - Standard Forehead FlapДокумент18 страниц5B - Standard Forehead Flapwillyoueverlovemenk100% (1)
- 5J - Tongue FlapДокумент11 страниц5J - Tongue FlapwillyoueverlovemenkОценок пока нет
- Pediatric Sinusitis and Sinus SurgeryДокумент293 страницыPediatric Sinusitis and Sinus SurgerywillyoueverlovemenkОценок пока нет
- Voice Disorders and Their Management, Third Edition: Margaret Freeman Margaret Fawcus, EditorsДокумент1 страницаVoice Disorders and Their Management, Third Edition: Margaret Freeman Margaret Fawcus, EditorswillyoueverlovemenkОценок пока нет
- 5A - Median Forhead FlapДокумент12 страниц5A - Median Forhead FlapwillyoueverlovemenkОценок пока нет
- 5G - Sternocleidomastoid FlapДокумент11 страниц5G - Sternocleidomastoid FlapwillyoueverlovemenkОценок пока нет
- Salivary Glands: Fig. 11.1 Histologic Structure of The Normal Salivary GlandДокумент35 страницSalivary Glands: Fig. 11.1 Histologic Structure of The Normal Salivary GlandwillyoueverlovemenkОценок пока нет
- 5I - Temporalis Muscle FlapДокумент12 страниц5I - Temporalis Muscle Flapwillyoueverlovemenk100% (1)
- 5D - Orbicularis Oris FlapДокумент20 страниц5D - Orbicularis Oris FlapwillyoueverlovemenkОценок пока нет
- 02 - The Reconstruction TriangleДокумент28 страниц02 - The Reconstruction TrianglewillyoueverlovemenkОценок пока нет
- General Principles: BasicДокумент3 страницыGeneral Principles: BasicwillyoueverlovemenkОценок пока нет
- Oral Cavity and Oropharynx CancersДокумент61 страницаOral Cavity and Oropharynx CancerswillyoueverlovemenkОценок пока нет
- Chap 14Документ40 страницChap 14willyoueverlovemenkОценок пока нет
- The Skull BaseДокумент56 страницThe Skull BasewillyoueverlovemenkОценок пока нет
- Oral Cavity and Oropharynx CancersДокумент61 страницаOral Cavity and Oropharynx CancerswillyoueverlovemenkОценок пока нет
- Thyroid and Parathyroid Glands AnatomyДокумент43 страницыThyroid and Parathyroid Glands AnatomywillyoueverlovemenkОценок пока нет
- Chap 8Документ86 страницChap 8willyoueverlovemenkОценок пока нет
- Cervical Lymph NodesДокумент41 страницаCervical Lymph NodeswillyoueverlovemenkОценок пока нет
- Head and Neck Soft Tissue Tumor Diagnosis and TreatmentДокумент37 страницHead and Neck Soft Tissue Tumor Diagnosis and TreatmentwillyoueverlovemenkОценок пока нет
- Salivary Glands: Fig. 11.1 Histologic Structure of The Normal Salivary GlandДокумент35 страницSalivary Glands: Fig. 11.1 Histologic Structure of The Normal Salivary GlandwillyoueverlovemenkОценок пока нет
- Neurogenic Tumors and Paragangliomas: BenignДокумент36 страницNeurogenic Tumors and Paragangliomas: BenignwillyoueverlovemenkОценок пока нет
- The Lips: Fig. 5.1 Site and Gender Distribution: Squamous Cell Carcinoma of TheДокумент24 страницыThe Lips: Fig. 5.1 Site and Gender Distribution: Squamous Cell Carcinoma of ThewillyoueverlovemenkОценок пока нет
- The Scalp and Skin of The FaceДокумент31 страницаThe Scalp and Skin of The FacewillyoueverlovemenkОценок пока нет
- Nasal Cavity and Paranasal Sinuses: Fig. 3.1 The Anatomic Location of The Paranasal SinusesДокумент35 страницNasal Cavity and Paranasal Sinuses: Fig. 3.1 The Anatomic Location of The Paranasal SinuseswillyoueverlovemenkОценок пока нет
- Chap2 PDFДокумент20 страницChap2 PDFwillyoueverlovemenkОценок пока нет
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (890)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (399)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2219)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (344)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (265)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (119)
- A Clinically Relevant Gene Signature in Triple Negative and Basal-Like Breast CancerДокумент16 страницA Clinically Relevant Gene Signature in Triple Negative and Basal-Like Breast CancerAlexandra TeodorescuОценок пока нет
- The PainДокумент19 страницThe PainKevin Torres PonteОценок пока нет
- Esophageal Cancer: Diagnosis and Management: Ai Zheng Aizheng Chinese Journal of Cancer October 2010Документ13 страницEsophageal Cancer: Diagnosis and Management: Ai Zheng Aizheng Chinese Journal of Cancer October 2010Al VlaovicОценок пока нет
- 1868-Article Text-6804-1-10-20180620Документ3 страницы1868-Article Text-6804-1-10-20180620Wina ViqaОценок пока нет
- Overview of Medicine - Its Importance and ImpactДокумент9 страницOverview of Medicine - Its Importance and ImpactDuffosОценок пока нет
- OncoДокумент3 страницыOncoTni JolieОценок пока нет
- JURNAL MomayyeziДокумент14 страницJURNAL MomayyeziSeptianОценок пока нет
- Breast Cancer: Presented By: Ola NemriДокумент46 страницBreast Cancer: Presented By: Ola NemriHaitham Ahmed100% (1)
- Breast Cancer TCM 2Документ5 страницBreast Cancer TCM 2Ivonne Flores FernándezОценок пока нет
- Abdominal Lymph Nodes for Common CancersДокумент8 страницAbdominal Lymph Nodes for Common Cancersborst0% (1)
- Upaya Kesehatan Masyarakat Esensial Pencegahan - Pengendalian PenyakitДокумент56 страницUpaya Kesehatan Masyarakat Esensial Pencegahan - Pengendalian PenyakitGiselleОценок пока нет
- GE 15 Chemicals in The EnvironmentДокумент2 страницыGE 15 Chemicals in The EnvironmentAlyssa Paula AltayaОценок пока нет
- Hrvoje Puskaric Danijela Tadic Marija Zahar DjordjevicДокумент10 страницHrvoje Puskaric Danijela Tadic Marija Zahar DjordjevicHom Jyoti AdhikariОценок пока нет
- Kanker Ovarium 2 PDFДокумент6 страницKanker Ovarium 2 PDFhestiОценок пока нет
- Vascular LesionДокумент61 страницаVascular LesionmaskurniawanОценок пока нет
- Estero GenДокумент18 страницEstero Genfitri anggrianiОценок пока нет
- Grammar - Vocab - 6Документ6 страницGrammar - Vocab - 6Phuong Thao NguyenОценок пока нет
- Donald L. Renfrew, MD: Spine PainДокумент16 страницDonald L. Renfrew, MD: Spine PainalmiraerickaiОценок пока нет
- Ramachandran Medical Application FormДокумент3 страницыRamachandran Medical Application FormRuban JebaduraiОценок пока нет
- Anti-Angiogenic Potential of Theobroma Cacao L. (Cacao) Seed Extract On Anas Platyrhynchos (Mallard Duck) EmbryoДокумент41 страницаAnti-Angiogenic Potential of Theobroma Cacao L. (Cacao) Seed Extract On Anas Platyrhynchos (Mallard Duck) EmbryoJulienne Baldonado50% (2)
- Chemical Carcinogens: Understanding Cancer-Causing AgentsДокумент27 страницChemical Carcinogens: Understanding Cancer-Causing Agentsgplese0Оценок пока нет
- Worlds First Licences Gene-Therapy Drug Produced Using NBS Celligen ReactorДокумент3 страницыWorlds First Licences Gene-Therapy Drug Produced Using NBS Celligen ReactormicromanpОценок пока нет
- Pure Patho PDFДокумент9 страницPure Patho PDFlovlyОценок пока нет
- Cancerimmunotherapies: Philip J. BergmanДокумент22 страницыCancerimmunotherapies: Philip J. BergmanRiefkyansyah PutraОценок пока нет
- Case StudyДокумент9 страницCase Studyapi-380684912Оценок пока нет
- Effect of Prehabilitation in Gastrooesophageal AdenocarcinomaДокумент7 страницEffect of Prehabilitation in Gastrooesophageal AdenocarcinomaFlavia VitalОценок пока нет
- Top 15 Therapeutic 2008 PDFДокумент95 страницTop 15 Therapeutic 2008 PDFHerry HendrayadiОценок пока нет
- (Onco) Oncologic EmergenciesДокумент71 страница(Onco) Oncologic EmergencieshatsuneОценок пока нет
- Free Report-Smoking CessationДокумент5 страницFree Report-Smoking CessationdapextilburyОценок пока нет
- Shilajit's potential as a natural panacea for cancerДокумент9 страницShilajit's potential as a natural panacea for cancerRakeshKumarОценок пока нет