Академический Документы
Профессиональный Документы
Культура Документы
ch237 Tut9 2002
Загружено:
Ervin CrespoИсходное описание:
Оригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
ch237 Tut9 2002
Загружено:
Ervin CrespoАвторское право:
Доступные форматы
UNIVERSITY OF MALTA
DEPARTMENT OF CHEMISTRY
CH237 - Chemical Thermodynamics and Kinetics
Tutorial Sheet IX
1. NOTE: For this question, the rate constants should be labelled with the number of the step
in the proposed reaction mechanism, and any reverse steps be labelled similarly but with a
prime. For example, see q. 1a.
(a) Derive the rate law for the decomposition of ozone in the reaction 2O3(g) → 3O2(g) on the E26.1a
basis of the following proposed mechanism:
(1) O3 ! O2 + O (Rate constants: forward k1, reverse k’1)
(2) O + O3 → 2O2 (Rate constant: k2)
(b) (i) On the basis of the following proposed mechanism, account the experimental fact that E26.1b
the rate law for the decomposition 2N2O5(g) → 4NO2(g) + O2(g) is v = k[N2O5]. E26.2a
(1) 2N2O5 ! NO2 + NO3
(2) NO2 + NO3 → NO2 + O2 + NO
(3) NO + N2O5 → 3NO2
(ii) A slightly different mechanism for the decomposition of N2O5 from that in (i) has also
been proposed. It differs only in the last step, which is replaced by
(3) NO + NO3 → 2NO2
Show that this mechanism leads to the same overall rate law.
(c) Consider the following mechanism for the thermal decomposition of R2: E26.2b
(1) R2 → 2R
(2) R + R2 → PB + R’
(3) R’ → PA + R
(4) 2R → PA + PB
where R2, PA and PB are stable hydrocarbons and R and R’ are radicals. Find the
dependence of the rate of decomposition of R2 on the concentration of R2.
(d) The condensation reaction of propanone, (CH3)2CO, in aqueous solution is catalysed by E26.6a
-
bases, B, which react reversibly with propanone to form the carbanion O3H5O . The
carbanion then reacts with a molecule of propanone to give the product. A simplified
version of the mechanism is as follows:
Version 2.0 - 2001/2002 Tutorial Sheet IX Page 1
(1) AH + B → BH+ +A-
(2) A- + BH+ → AH + B
(3) A- + AH → product
where AH stands for propanone and A- denotes its carbanion. Use the steady-state
approximation to find the concentration of the carbanion and derive the rate equation for the
formation of the product.
(e) Consider the acid-catalysed reaction: E26.6b
(1) HA + H+ ! HAH+ (k1 forward, k`1 reverse, both fast)
(2) HAH+ + B ! BH+ + AH (k2, slow)
Deduce the rate law and show that it can be made independent of the specific term [H+].
(f) Consider the following chain mechanism: E26.7a
(1) AH → A ⋅ + H ⋅
(2) A⋅→ B ⋅+C
(3) AH + B → A ⋅+ D
(4) A ⋅+ B⋅→ P
Identify the initiation, propagation, and termination steps, and use the steady-state
approximation to deduce that the decomposition of AH is first-order in AH.
(g) Consider the following chain mechanism: E26.7b
(1) A2 → A ⋅ + A ⋅
(2) A⋅→ B ⋅+ C
(3) A ⋅+ P → B ⋅
(4) A ⋅+ B⋅→ P
Identify any initiation, propagation, retardation, inhibition, and termination steps, and use
the steady-state approximation to deduce the rate law for the consumption of A2.
(h) (i) Show that the pyrolysis of ethanal (acetaldehyde) exhibits the rate law: JNG
∆
CH 3CHO(g) → CH 4 (g) + CO(g)
d CH 4 [ ] [
= k CH 3CHO
3/2
]
(P26.10)
dt
given that the reaction proceeds according the Rice-Herzfeld mechanism, i.e.:
(a) CH 3CHO →⋅CH 3 + ⋅CHO
(b) CH 3CHO + ⋅CH 3 → CH 4 + CH 3CO ⋅
(c) CH 3CO⋅→⋅CH 3 + CO
(d) ⋅ CH 3 + ⋅CH 3 → CH 3CH 3
and hence deduce the rate of disappearance of ethanal.
(ii) Classify steps (a) - (d) according to initiation, propagation, etc.
Version 2.0 - 2001/2002 Tutorial Sheet IX Page 2
(i) Although the stoichiometric equation for the reaction between hydrogen and bromine to JNG
give HBr looks very simple, the rate law is very complicated, i.e.:
d [HBr] k [H 2 ][Br2 ]
H 2 (g) + Br2 (g) → 2HBr =
dt [Br2 ]+ k'[HBr]
The proposed mechanism for this reaction is as follows:
Br2 + M → Br ⋅ + Br ⋅+ M
(M = Br2 or H 2 )
Br ⋅ +H 2 → HBr + H ⋅
H ⋅ +Br2 → HBr + Br ⋅
H ⋅ +HBr → H 2 + Br ⋅
Br ⋅+ Br ⋅+ M → Br2 + M *
where the third body M in the final step removes the energy of recombination.
(i) Classify the above steps as initiation, propagation, etc.
(ii) Prove that if the formation of HBr proceeds though this mechanism, then the rate law is
indeed as stated above.
2. (a) Discuss methods for measuring the rates of chemical reactions. JNG
(b) Write brief notes on: JNG
(i) The variation of reaction rates with changes in temperature;
(ii) The verification of the Arrhenius equation through the collision theory
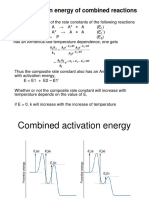
(iii) The relationship between the activation energy of different steps in a series
consecutive reactions and the overall rate constant.
(iv) The variation of the rate constant of a reaction between ions with the ionic
strength of the solution.
Version 2.0 - 2001/2002 Tutorial Sheet IX Page 3
Вам также может понравиться
- Semester-6 3360503 CRE MCQ KRD PDFДокумент9 страницSemester-6 3360503 CRE MCQ KRD PDFDhruv RanaОценок пока нет
- Solution: For A First-Order Reaction, The Following Rate Coefficients Were FoundДокумент16 страницSolution: For A First-Order Reaction, The Following Rate Coefficients Were FoundDeepak SharmaОценок пока нет
- Counter-Current Extraction: An Introduction to the Design and Operation of Counter-Current ExtractorsОт EverandCounter-Current Extraction: An Introduction to the Design and Operation of Counter-Current ExtractorsОценок пока нет
- Chapter 2 - Data InterpretationДокумент24 страницыChapter 2 - Data InterpretationPHƯƠNG ĐẶNG YẾNОценок пока нет
- 10 PDFДокумент23 страницы10 PDFTysir SarhanОценок пока нет
- PR 1-5Документ18 страницPR 1-5Febryan CaesarОценок пока нет
- 03 Equilibria (I)Документ11 страниц03 Equilibria (I)David LevisteОценок пока нет
- 3 31Документ2 страницы3 31Rahmania FatimahОценок пока нет
- Distillation Chapter 2Документ35 страницDistillation Chapter 2fatien zakaria100% (1)
- CRE GATE Question Paper PDFДокумент28 страницCRE GATE Question Paper PDFChandra prakash GuptaОценок пока нет
- Rr320802chemicalreactionengineeringiДокумент8 страницRr320802chemicalreactionengineeringiSanthosh KumarОценок пока нет
- Multiple Reaction System-1Документ35 страницMultiple Reaction System-1ANZWAYNEОценок пока нет
- A. Answer The Following Questions With Proper ExplanationsДокумент1 страницаA. Answer The Following Questions With Proper ExplanationsRohitОценок пока нет
- RaoultДокумент11 страницRaoultNurul AfifahОценок пока нет
- Mass Transfer Operations II Rr320801Документ8 страницMass Transfer Operations II Rr320801Nagwa MansyОценок пока нет
- (P01, C01, C02, C2, C3) : Confidential EH/JUN 2014/CHE584/594Документ11 страниц(P01, C01, C02, C2, C3) : Confidential EH/JUN 2014/CHE584/594Addison JuttieОценок пока нет
- Answer For TutorialДокумент7 страницAnswer For TutorialFatur RohimОценок пока нет
- Thus, This Term Actually Means A in A Constant-Volume System The Measure of Reaction Rate of Component I BecomesДокумент23 страницыThus, This Term Actually Means A in A Constant-Volume System The Measure of Reaction Rate of Component I Becomesalice Annabelle100% (1)
- Experiment No. 4Документ5 страницExperiment No. 4fareeha saeedОценок пока нет
- Lect 2 Response of First Order SystemsДокумент19 страницLect 2 Response of First Order SystemsZaidoon MohsinОценок пока нет
- H.W 7Документ9 страницH.W 7Mirvat ShamseddineОценок пока нет
- Assessment: Test 2: Chemical Engineering ThermodynamicsДокумент3 страницыAssessment: Test 2: Chemical Engineering Thermodynamicscarleston thurgoodОценок пока нет
- Ps2 in PDCДокумент3 страницыPs2 in PDClily august0% (1)
- Contoh Soal CreДокумент11 страницContoh Soal CreMuhammad Irfan SalahuddinОценок пока нет
- Chemical Engineering KineticsДокумент45 страницChemical Engineering KineticsMelissa Marie DimaculanganОценок пока нет
- General Case For Diffusion of Gases A & B Plus Bulk MovementДокумент10 страницGeneral Case For Diffusion of Gases A & B Plus Bulk MovementMayar H. HaggagОценок пока нет
- Gate 2006 PDFДокумент21 страницаGate 2006 PDFVammsy Manikanta SaiОценок пока нет
- Assignment#3 Report PDFДокумент9 страницAssignment#3 Report PDFseraj ibramemОценок пока нет
- Fundamentals of Heat and Mass Transfer 6th Edition-901-1000-51-100Документ50 страницFundamentals of Heat and Mass Transfer 6th Edition-901-1000-51-100abibas olaОценок пока нет
- CHE244 Project GuidelinesДокумент5 страницCHE244 Project GuidelinesEiman UzmiОценок пока нет
- Fogler Mosanto Sauget IllinoisДокумент7 страницFogler Mosanto Sauget IllinoisjosealvaroОценок пока нет
- Cylinder T (K) P (Bar) V (M)Документ3 страницыCylinder T (K) P (Bar) V (M)Harshit MittalОценок пока нет
- Exercises For Lecture x2Документ8 страницExercises For Lecture x2Tara EdwardsОценок пока нет
- Transport Phenomenon NotesДокумент7 страницTransport Phenomenon Notesvishakha goelОценок пока нет
- Chapter 5 AdsorptionДокумент46 страницChapter 5 AdsorptionSyahmiОценок пока нет
- Ex0 Questions SolutionsДокумент7 страницEx0 Questions SolutionsBiniyam haileОценок пока нет
- Chapter 3 Rates Law and StoichiometryДокумент60 страницChapter 3 Rates Law and StoichiometryMalek Marry AnneОценок пока нет
- CRE 1-3 Unit (2016-2017) PDFДокумент56 страницCRE 1-3 Unit (2016-2017) PDFgouthamОценок пока нет
- Set8ans 10Документ5 страницSet8ans 10Agustina Evania DewiОценок пока нет
- 08 Multiple ReactionsДокумент17 страниц08 Multiple ReactionsFikrie MuhdОценок пока нет
- Professional Reference Shelf: Sothermal Eactor EsignДокумент5 страницProfessional Reference Shelf: Sothermal Eactor EsignSourodip GhoshdastidarОценок пока нет
- Mass Transfer Question PaperДокумент2 страницыMass Transfer Question PaperSachin Krishna Moger 1si17ch026100% (1)
- Je000301o PDFДокумент20 страницJe000301o PDFRLОценок пока нет
- CRE - Solid Catalyzed ReactionДокумент47 страницCRE - Solid Catalyzed Reactionandono kusuma jatiОценок пока нет
- Ch12P1 VLE Models by Margules Van Laar and Wilson EquationsДокумент2 страницыCh12P1 VLE Models by Margules Van Laar and Wilson Equationshana faqihОценок пока нет
- Ideal Reactors Part 2 Solved ProblemsДокумент15 страницIdeal Reactors Part 2 Solved ProblemsWaldi SagalaОценок пока нет
- Chapter 4 Reactor DesignДокумент48 страницChapter 4 Reactor DesignAhmadОценок пока нет
- AT12 MabaoДокумент17 страницAT12 MabaoMichael Alex MabaoОценок пока нет
- Chapter 4 Heat EffectsДокумент6 страницChapter 4 Heat Effectsariana religiosoОценок пока нет
- Sample Test Exam One CH201Документ7 страницSample Test Exam One CH201Ashly PhilipОценок пока нет
- Pressure DropДокумент42 страницыPressure DropSói Con100% (1)
- Chemical Engineering Thermodynamics Final ExaminationДокумент9 страницChemical Engineering Thermodynamics Final ExaminationkevidreadОценок пока нет
- Co (NH3) 6Документ1 страницаCo (NH3) 6Ayotunde OnasanyaОценок пока нет
- Chemical Engineering Mass Transfer NotesДокумент19 страницChemical Engineering Mass Transfer NotesLebohang Czar NkuОценок пока нет
- 1-1 Unit (2016-2017)Документ113 страниц1-1 Unit (2016-2017)goutham100% (1)
- Tutorial For Chapter 23Документ9 страницTutorial For Chapter 23Thurgah VshinyОценок пока нет
- Unit 4 Chemical KineticsДокумент8 страницUnit 4 Chemical KineticsRahgul M.S.Оценок пока нет
- Kinetics & Photochemistry Tutorial ProblemsДокумент4 страницыKinetics & Photochemistry Tutorial ProblemsAmbuj Yadav 4-Year B.Tech. Chemical EngineeringОценок пока нет
- Transparent Conductive Oxides: Solems Solems Solems Solems Solems Solems Solems SolemsДокумент2 страницыTransparent Conductive Oxides: Solems Solems Solems Solems Solems Solems Solems SolemsErvin CrespoОценок пока нет
- Lusher 2007Документ4 страницыLusher 2007Ervin CrespoОценок пока нет
- Lec13 2012Документ16 страницLec13 2012Ervin CrespoОценок пока нет
- Ja 08052Документ8 страницJa 08052Ervin CrespoОценок пока нет
- Farmhouse Style Plans - Farm & CountryДокумент6 страницFarmhouse Style Plans - Farm & Countryhanif azriОценок пока нет
- JMO 2023 (7, 8) Question PaperДокумент2 страницыJMO 2023 (7, 8) Question PaperSuryanshu BhardwajОценок пока нет
- مشخصات فنی بیل بکهو فیات کوبلکو b200Документ12 страницمشخصات فنی بیل بکهو فیات کوبلکو b200Maryam0% (1)
- Equipment in The NICUДокумент7 страницEquipment in The NICUGheDine PeracionОценок пока нет
- Goal 6 Unesco Water SanatationДокумент5 страницGoal 6 Unesco Water Sanatationapi-644347009Оценок пока нет
- 65 ActsДокумент178 страниц65 ActsComprachosОценок пока нет
- Hydraulics Course FileДокумент81 страницаHydraulics Course FileSwarna LathaОценок пока нет
- OLFACTIVE TRAINING 101 by SozioДокумент36 страницOLFACTIVE TRAINING 101 by SoziojaviercdeaeОценок пока нет
- Accessories 162-USДокумент20 страницAccessories 162-USعايد التعزيОценок пока нет
- XYZprint User Manual en V1 1003Документ25 страницXYZprint User Manual en V1 1003reza rizaldiОценок пока нет
- Unit 3 InfiltrationДокумент5 страницUnit 3 InfiltrationHRIDYA MGОценок пока нет
- Introduction To Kalman FilterДокумент4 страницыIntroduction To Kalman FilterArghya MukherjeeОценок пока нет
- Mechanism Design: A SeriesДокумент3 страницыMechanism Design: A Seriesamirmasood kholojiniОценок пока нет
- NDT Matrix 12-99-90-1710 - Rev.2 PDFДокумент2 страницыNDT Matrix 12-99-90-1710 - Rev.2 PDFEPC NCCОценок пока нет
- Veg Dum Biryani - Hyderabadi Veg Biryani Recipe - Hyderabadi Biryani - Hebbar's KitchenДокумент2 страницыVeg Dum Biryani - Hyderabadi Veg Biryani Recipe - Hyderabadi Biryani - Hebbar's KitchenmusicalcarpetОценок пока нет
- 3592 Operator GuideДокумент103 страницы3592 Operator GuideNaim GhattasОценок пока нет
- SweetenersДокумент23 страницыSweetenersNur AfifahОценок пока нет
- Qa/Qc Mechanical Monthly Progress Report For June 2015: Area/System Description Status RemarksДокумент1 страницаQa/Qc Mechanical Monthly Progress Report For June 2015: Area/System Description Status RemarksRen SalazarОценок пока нет
- E Numbers Are Number Codes ForДокумент3 страницыE Numbers Are Number Codes ForaradhyaОценок пока нет
- Diabetes in Pregnancy: Supervisor: DR Rathimalar By: DR Ashwini Arumugam & DR Laily MokhtarДокумент21 страницаDiabetes in Pregnancy: Supervisor: DR Rathimalar By: DR Ashwini Arumugam & DR Laily MokhtarHarleyquinn96 DrОценок пока нет
- Course On Quantum ComputingДокумент235 страницCourse On Quantum ComputingAram ShojaeiОценок пока нет
- Nitofloor NДокумент3 страницыNitofloor Nkiranmisale7Оценок пока нет
- API Casing Collapse CalcsДокумент8 страницAPI Casing Collapse CalcsJay SadОценок пока нет
- 114 The Letter S: M 'TafontДокумент9 страниц114 The Letter S: M 'TafontHarry TLОценок пока нет
- Prakab Export 20.8.2018 UkДокумент260 страницPrakab Export 20.8.2018 UkREN JTNОценок пока нет
- Pantalla Anterior Bienvenido: Cr080vbesДокумент3 страницыPantalla Anterior Bienvenido: Cr080vbesJuan Pablo Virreyra TriguerosОценок пока нет
- Inverse Curve Trip Time Calculation: Enter Values in White CellДокумент3 страницыInverse Curve Trip Time Calculation: Enter Values in White CellVijay FxОценок пока нет
- ReagentsДокумент12 страницReagentsKimscey Yvan DZ SulitОценок пока нет
- FP Lecture Midterm Exam Sec - Sem.2020Документ4 страницыFP Lecture Midterm Exam Sec - Sem.2020SAEEDA ALMUQAHWIОценок пока нет
- Foldable HelmetДокумент16 страницFoldable Helmetharsha kotewarОценок пока нет
- ICH Quality Guidelines: An Implementation GuideОт EverandICH Quality Guidelines: An Implementation GuideAndrew TeasdaleОценок пока нет
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsОт EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsРейтинг: 5 из 5 звезд5/5 (3)
- Quantum Physics: A Beginners Guide to How Quantum Physics Affects Everything around UsОт EverandQuantum Physics: A Beginners Guide to How Quantum Physics Affects Everything around UsРейтинг: 4.5 из 5 звезд4.5/5 (3)
- Periodic Tales: A Cultural History of the Elements, from Arsenic to ZincОт EverandPeriodic Tales: A Cultural History of the Elements, from Arsenic to ZincРейтинг: 3.5 из 5 звезд3.5/5 (137)
- Handbook of Formulating Dermal Applications: A Definitive Practical GuideОт EverandHandbook of Formulating Dermal Applications: A Definitive Practical GuideОценок пока нет
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactОт EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactРейтинг: 5 из 5 звезд5/5 (1)
- Limitless Mind: Learn, Lead, and Live Without BarriersОт EverandLimitless Mind: Learn, Lead, and Live Without BarriersРейтинг: 4 из 5 звезд4/5 (6)
- The Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactОт EverandThe Nature of Drugs Vol. 1: History, Pharmacology, and Social ImpactРейтинг: 5 из 5 звезд5/5 (5)
- Chemistry: a QuickStudy Laminated Reference GuideОт EverandChemistry: a QuickStudy Laminated Reference GuideРейтинг: 5 из 5 звезд5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeОт EverandChemistry for Breakfast: The Amazing Science of Everyday LifeРейтинг: 4.5 из 5 звезд4.5/5 (14)
- It's Elemental: The Hidden Chemistry in EverythingОт EverandIt's Elemental: The Hidden Chemistry in EverythingРейтинг: 4 из 5 звезд4/5 (10)
- AP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeОт EverandAP® Chemistry Crash Course, For the 2020 Exam, Book + Online: Get a Higher Score in Less TimeРейтинг: 5 из 5 звезд5/5 (1)
- AP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeОт EverandAP Chemistry Flashcards, Fourth Edition: Up-to-Date Review and PracticeОценок пока нет
- Mental Math: How to Develop a Mind for Numbers, Rapid Calculations and Creative Math Tricks (Including Special Speed Math for SAT, GMAT and GRE Students)От EverandMental Math: How to Develop a Mind for Numbers, Rapid Calculations and Creative Math Tricks (Including Special Speed Math for SAT, GMAT and GRE Students)Оценок пока нет
- The Production of Volatile Oils and Perfumery Plants in the United StatesОт EverandThe Production of Volatile Oils and Perfumery Plants in the United StatesОценок пока нет
- Monkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeОт EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction, and the Science of Everyday LifeРейтинг: 4 из 5 звезд4/5 (1)
- Build a Mathematical Mind - Even If You Think You Can't Have One: Become a Pattern Detective. Boost Your Critical and Logical Thinking Skills.От EverandBuild a Mathematical Mind - Even If You Think You Can't Have One: Become a Pattern Detective. Boost Your Critical and Logical Thinking Skills.Рейтинг: 5 из 5 звезд5/5 (1)
- Guidelines for Defining Process Safety Competency RequirementsОт EverandGuidelines for Defining Process Safety Competency RequirementsРейтинг: 3 из 5 звезд3/5 (1)