Академический Документы
Профессиональный Документы
Культура Документы
Boyles Law
Загружено:
Maria Jessa M. ArenasОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Boyles Law
Загружено:
Maria Jessa M. ArenasАвторское право:
Доступные форматы
Elma Joy M.
Arenas
10-Diamond
Boyle's Law
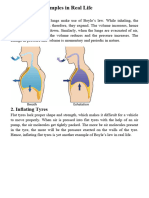
Typically, when we open a bottle of soda, we slowly turn the cap to allow the air to
escape before we completely remove the lid. We do this because we've learned over time that
twisting it open too fast causes it to fizz up and spill all over. This happens because the liquid is
pumped full of carbon dioxide, causing it to bubble up as the CO2 makes its escape.
When a soda bottle is filled, it is also pressurized. Much like the aerosol can mentioned
earlier, when you slowly open the cap, the gas is able to increase its volume and the pressure
decreases.
Normally you can let the gas out of a can or bottle release cleanly, but if the bottle is
shaken up and the gas is mixed into the liquid, then you may have a mess on your hands. This is
because the gas trying to escape is mixed into the fluid, so, when it does escape, it brings the
foamy fluid out with it. Pressure in the bottle goes down, volume of the gas goes up, and you
have yourself a mess to clean up.
Вам также может понравиться
- How To Make The TrueДокумент3 страницыHow To Make The TrueShree Uday88% (8)
- Water Vapor and Humidity PowerpointДокумент12 страницWater Vapor and Humidity Powerpointapi-282246608100% (1)
- Boom! 50 Fantastic Science Experiments to Try at Home with Your Kids (PB)От EverandBoom! 50 Fantastic Science Experiments to Try at Home with Your Kids (PB)Оценок пока нет
- Red LionДокумент9 страницRed Liontravellerfellow0% (1)
- Make A Big Dry Ice Bubble: What You'll NeedДокумент9 страницMake A Big Dry Ice Bubble: What You'll Needฮันนี่ คริสОценок пока нет
- Fire Stone Via KermesДокумент4 страницыFire Stone Via Kermesforest ravensonОценок пока нет
- Red LionДокумент10 страницRed Lionyakeen88100% (5)
- Astm A706-16Документ7 страницAstm A706-16李明环100% (1)
- Method Statement For Pile Chipping and Capping Beam: Vascon Engineers LTDДокумент12 страницMethod Statement For Pile Chipping and Capping Beam: Vascon Engineers LTDshahaji0% (1)
- Technical specs for TCG 2032V16 gas engineДокумент20 страницTechnical specs for TCG 2032V16 gas enginePanda1388100% (4)
- Charles Law Explained Gas Volume TemperatureДокумент2 страницыCharles Law Explained Gas Volume TemperatureHelmaОценок пока нет
- Gary Young Simple Calcium Chloride Magnesium Method For OrmusДокумент5 страницGary Young Simple Calcium Chloride Magnesium Method For OrmusgedfireОценок пока нет
- Sci10 LM U3Документ131 страницаSci10 LM U3Romel B Agno86% (7)
- Fire, Salt, and Water That Does Not Wet The HandsДокумент4 страницыFire, Salt, and Water That Does Not Wet The HandsottoОценок пока нет
- Charles LawДокумент12 страницCharles LawLerr Real RelleОценок пока нет
- Problemario Prácticas TermoДокумент35 страницProblemario Prácticas TermoEsaú RSОценок пока нет
- Boyles LawДокумент1 страницаBoyles LawMaria Jessa M. ArenasОценок пока нет
- Cloud in A Bottle ExperimentДокумент2 страницыCloud in A Bottle ExperimentKristal Jade ConstantinoОценок пока нет
- Applications of Gas LawsДокумент3 страницыApplications of Gas LawsCharm GorospeОценок пока нет
- Applications of Boyle's LaawДокумент2 страницыApplications of Boyle's Laawcherrymaeregalario2001Оценок пока нет
- Soda BottleДокумент3 страницыSoda BottleBenedick CruzОценок пока нет
- De LEON, Cyrus Q. BSIE 1A (Cloud in A Bottle)Документ2 страницыDe LEON, Cyrus Q. BSIE 1A (Cloud in A Bottle)Cyrus De LeonОценок пока нет
- ExamplesДокумент12 страницExamplesMoldir SerikОценок пока нет
- I. Title of Experiment Cloud in A BottleДокумент3 страницыI. Title of Experiment Cloud in A Bottlechappy_leigh118Оценок пока нет
- Why Do Gas Bubbles Get Larger As They Rise?: Why Does The Bubble Grow?Документ4 страницыWhy Do Gas Bubbles Get Larger As They Rise?: Why Does The Bubble Grow?Princes Katherine VergaraОценок пока нет
- Boyles LawДокумент6 страницBoyles LawMara TillesОценок пока нет
- Boyle's Law ExplainedДокумент2 страницыBoyle's Law ExplainedjaОценок пока нет
- Boyle's Law: Breathing, Spray Paint & Soda BottlesДокумент1 страницаBoyle's Law: Breathing, Spray Paint & Soda BottlesRevilloОценок пока нет
- Boyle's LawДокумент24 страницыBoyle's LawRalph Jasil Esparagoza EscanillasОценок пока нет
- Cloud in A BottleДокумент3 страницыCloud in A BottleAditya GaurОценок пока нет
- Make Your Own Cloud in A Bottle: EquipmentДокумент2 страницыMake Your Own Cloud in A Bottle: Equipmentlahsivlahsiv684Оценок пока нет
- The Can CrusherДокумент2 страницыThe Can CrusherMildred FerrerОценок пока нет
- Andi Aryanto Bangsawan - BC21. 2 - 2. Praktikum Sains Untuk AnakДокумент8 страницAndi Aryanto Bangsawan - BC21. 2 - 2. Praktikum Sains Untuk AnakJ-eidolonix andi aryantoОценок пока нет
- States and Properties of Matter ExplainedДокумент5 страницStates and Properties of Matter ExplainedDon MalasagaОценок пока нет
- Roselyn 1Документ5 страницRoselyn 1Lecsos clyde Pang-agОценок пока нет
- Make A Big Dry Ice BubbleДокумент3 страницыMake A Big Dry Ice BubbleHelberth RodriguezОценок пока нет
- Bee Swarm Simulator AutofarmДокумент2 страницыBee Swarm Simulator Autofarmcananke4cxОценок пока нет
- 4Q W1 WORKSHEET With ANSWER KEYSДокумент2 страницы4Q W1 WORKSHEET With ANSWER KEYSjia aganaОценок пока нет
- Pressure law experimentsДокумент6 страницPressure law experimentszahidahoseinОценок пока нет
- The Air Pressure ExperimentДокумент11 страницThe Air Pressure ExperimentMeor Muhammad SyafiqОценок пока нет
- Balloon Blow-Up Science ExperimentДокумент2 страницыBalloon Blow-Up Science ExperimentcodigotruenoОценок пока нет
- Class5 Evs Lesson 15 - Blow Hotb Blow ColdДокумент7 страницClass5 Evs Lesson 15 - Blow Hotb Blow ColdVikram Tukaram Gandhale PRTОценок пока нет
- Saturated Vapour Pressure and Boiling PointДокумент2 страницыSaturated Vapour Pressure and Boiling Pointpackiandavid1982Оценок пока нет
- How Boyle's Law Applies to Spray Paint and SodaДокумент2 страницыHow Boyle's Law Applies to Spray Paint and SodaohjiiОценок пока нет
- Eks 1Документ6 страницEks 1Medline TahaОценок пока нет
- Properties of Air ExplainedДокумент6 страницProperties of Air ExplainedDaoud KhanОценок пока нет
- ExperimentДокумент4 страницыExperimentShahfizah EzyanОценок пока нет
- Balloon Lung ExperimentДокумент4 страницыBalloon Lung ExperimentdyanenfalhadapОценок пока нет
- Magic of Science Experiments Under 40 CharactersДокумент6 страницMagic of Science Experiments Under 40 Charactersvic micОценок пока нет
- Dry IceДокумент6 страницDry IceBrei Parayno Laurio0% (1)
- ExperimentsДокумент8 страницExperimentsapi-549074161Оценок пока нет
- CCCCCCC C CCC: C C C C C CДокумент11 страницCCCCCCC C CCC: C C C C C CcoachmylesОценок пока нет
- Air Around Us: Activity 1 Activity 2Документ8 страницAir Around Us: Activity 1 Activity 2Heidi RayОценок пока нет
- Balloon Blow-Up Science Experiment: Items NeededДокумент3 страницыBalloon Blow-Up Science Experiment: Items NeededChitrak4Оценок пока нет
- Experiment #1: Baby Diaper MagicДокумент2 страницыExperiment #1: Baby Diaper MagicRhodora LozanoОценок пока нет
- Boiling Point of LiquidДокумент3 страницыBoiling Point of LiquidDebasish BhattacharjeeОценок пока нет
- (ALBN) FizziologyДокумент4 страницы(ALBN) FizziologyClaudemir HackerОценок пока нет
- Boo Bubbles - Dry Ice Science FlyerДокумент4 страницыBoo Bubbles - Dry Ice Science FlyermmmmmmmmmmmmmmmОценок пока нет
- Lava Lamps ExplainedДокумент2 страницыLava Lamps ExplainedAleeyah WilliamsОценок пока нет
- DISCUSSION Exp 5Документ2 страницыDISCUSSION Exp 5Nurfariha SafarОценок пока нет
- Preschool Science Week 5Документ2 страницыPreschool Science Week 5michelleangel14Оценок пока нет
- 9 Science Imp ch1 2 PDFДокумент5 страниц9 Science Imp ch1 2 PDFParamita KaranОценок пока нет
- Prevent Oiling Out During CrystallizationДокумент1 страницаPrevent Oiling Out During Crystallizationsamdelacruz1030Оценок пока нет
- Cask CleaningДокумент4 страницыCask Cleaningde_klusОценок пока нет
- Lesson Plan in BiologyДокумент12 страницLesson Plan in BiologyakhilaОценок пока нет
- Lessonplan 100512115922 Phpapp02Документ20 страницLessonplan 100512115922 Phpapp02Aiceeh Medrano CortezОценок пока нет
- Lesson Plan in BiologyДокумент12 страницLesson Plan in BiologyakhilaОценок пока нет
- 11228437Документ76 страниц11228437Maria Jessa M. ArenasОценок пока нет
- Elma Joy Madrid Arenas 10Документ3 страницыElma Joy Madrid Arenas 10Maria Jessa M. ArenasОценок пока нет
- Elma Joy Madrid Arenas 10Документ3 страницыElma Joy Madrid Arenas 10Maria Jessa M. ArenasОценок пока нет
- Rodrigo D Valdez Jr resumeДокумент1 страницаRodrigo D Valdez Jr resumeMaria Jessa M. ArenasОценок пока нет
- Arellano ST., Dagupan 2400: ApplicantДокумент3 страницыArellano ST., Dagupan 2400: ApplicantMaria Jessa M. ArenasОценок пока нет
- Handbook of Maintenance Management and Engineering: January 2009Документ9 страницHandbook of Maintenance Management and Engineering: January 2009Deni DamanhuriОценок пока нет
- 3º Eso. Turn in Work.1Документ2 страницы3º Eso. Turn in Work.1djpelocho07Оценок пока нет
- (2-3) Experimental Investigation of Precast Concrete Based Dry Mechanical Column-Column Joints For Precast Concrete FramesДокумент15 страниц(2-3) Experimental Investigation of Precast Concrete Based Dry Mechanical Column-Column Joints For Precast Concrete FramesPhạm Tiến ĐạtОценок пока нет
- 03-Lecture-Semiconductor DevicesДокумент7 страниц03-Lecture-Semiconductor DevicesAdnan Hyder SoomroОценок пока нет
- Solutions Manual Internal Combustion Engines: Applied Thermosciences ch08Документ17 страницSolutions Manual Internal Combustion Engines: Applied Thermosciences ch08swastik jenaОценок пока нет
- AMK Tower LampДокумент8 страницAMK Tower LampAdi ZG1Оценок пока нет
- ಕ ಫ ಾಂಶ ಜೂ / ಜು ೖ - ೨೦೧೯. Vtu Provisional Results Of Ug / Pg June / July - 2019 ExaminationДокумент2 страницыಕ ಫ ಾಂಶ ಜೂ / ಜು ೖ - ೨೦೧೯. Vtu Provisional Results Of Ug / Pg June / July - 2019 Examinationvandv printsОценок пока нет
- 80 % Silver-20 % Graphite Sliding Contact Material: Standard Specification ForДокумент2 страницы80 % Silver-20 % Graphite Sliding Contact Material: Standard Specification ForROHITОценок пока нет
- Physics - II (2nd Sem)Документ1 страницаPhysics - II (2nd Sem)Sunder KumarОценок пока нет
- 6th Grade ModifiedДокумент83 страницы6th Grade ModifiedsravanthiОценок пока нет
- Biomechanics of Hip Joint: A Review: Bhaskar Kumar MadetiДокумент19 страницBiomechanics of Hip Joint: A Review: Bhaskar Kumar MadetiIrik PalaciosОценок пока нет
- Measurement of Horizontal and Vertical DistancesДокумент13 страницMeasurement of Horizontal and Vertical DistancesFrannie BorrasОценок пока нет
- 8.06x Problem Set 1Документ5 страниц8.06x Problem Set 1ken adamsОценок пока нет
- HAZDIG A New Software Package For Assessing TheДокумент15 страницHAZDIG A New Software Package For Assessing TheArun HarpalaniОценок пока нет
- 986K Wheel Loader Electrical System (Cab) 486-0964 Chassis and Engine (486-0965)Документ6 страниц986K Wheel Loader Electrical System (Cab) 486-0964 Chassis and Engine (486-0965)abduallah muhammadОценок пока нет
- CYTOP BrochureДокумент11 страницCYTOP BrochureMau RoОценок пока нет
- VRF Prc007 enДокумент35 страницVRF Prc007 enAbdelatiefОценок пока нет
- Types of Wind TunnelsДокумент3 страницыTypes of Wind TunnelsAfaq0% (1)
- Question Bank: Prist UniversityДокумент12 страницQuestion Bank: Prist Universityphillipe baillyОценок пока нет
- Camuplage AntennaДокумент83 страницыCamuplage Antennahamzah zaelaniОценок пока нет
- 11-KINFLEX-MANGUERAS AISLANTES - Conductividad Térmica TUBERIA DE COBREДокумент6 страниц11-KINFLEX-MANGUERAS AISLANTES - Conductividad Térmica TUBERIA DE COBRELeonard Mendoza ChuctayaОценок пока нет
- AE 384 Automatic Control Systems IДокумент25 страницAE 384 Automatic Control Systems ILenduhaОценок пока нет
- ECS002 - Calculation For The Orifice Plate On The AC Lube Oil SystemДокумент9 страницECS002 - Calculation For The Orifice Plate On The AC Lube Oil SystemPaul CarlyОценок пока нет
- Spotlight - Phase-2 - (2022-23) - Week-1 - Paper-2 - Compile (2020-P-2) - (Only Que.)Документ12 страницSpotlight - Phase-2 - (2022-23) - Week-1 - Paper-2 - Compile (2020-P-2) - (Only Que.)Suryansh DummyОценок пока нет
- Horizontal and Vertical Motions of ProjectileДокумент10 страницHorizontal and Vertical Motions of Projectilejaida villanuevaОценок пока нет
- Generador de Efecto SearlДокумент52 страницыGenerador de Efecto SearlAlfonso XhavierОценок пока нет