Академический Документы
Профессиональный Документы
Культура Документы
Out Licensing
Загружено:
TusharVermaИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Out Licensing
Загружено:
TusharVermaАвторское право:
Доступные форматы
LAST UPDATE – 4 October 2010
The Pharmaceutical Out-licensing Course
By:
David Scott
Over 24 years licensing experience in the healthcare sector - the first ten
years within the pharma industry.
As a freelance licensing and business development consultant since 1996, he
has successfully concluded numerous inward and outward licensing
agreements for clients covering small molecules, biologicals and delivery
technologies.
Author of Scrip’s best-selling Practical Guide to Pharmaceutical Licensing.
By attending this course, you will:
1. Understand the key factors leading to a successful out-licensing deal within the pharmaceutical
and biotech industries.
2. Learn how to profile your product and prepare product information to maximise its attractiveness
to third parties.
3. Understand the key factors leading to valuing your product and how to set up a spreadsheet to
optimize the commercial structure of the deal.
4. Find out how to target potential partners - and the best way to make successful contacts.
5. Learn what to include in a term sheet, as well as in CDAs and MTAs, and which issues to watch out
for during negotiations.
6. Understand the due diligence process and what will be expected from you.
7. Get expert advice on negotiation strategy and on managing a deal post-signature.
What sets this course apart?
The expert: David Scott is well-respected in the pharmaceutical licensing world and is actively in- and out-
licensing products for clients in the pharmaceutical, biotech and technology sectors. He also has a strong
track record in running successful training courses and workshops, so he combines a hands-on approach to
licensing with training skills.
To register, complete the online registration form at www.celforpharma.com
43
LAST UPDATE – 4 October 2010
The content: This course is designed to provide delegates with all the practical skills needed to out-license a
development based product. The course is hard work but fulfilling and covers all the main aspects of
licensing. It comes along with useful templates for future activities, including a spreadsheet to calculate
optimal deal values.
Who should attend?
The Pharmaceutical Out-licensing Course is designed for those likely to be involved in the licensing process:
Senior executives and scientists in companies developing or planning to develop products for out-
licensing.
Junior to mid-level managers, including scientists, commercial and legal managers, who are likely to be
involved in the licensing and due diligence process.
Business development managers, members of legal and IP teams with no formal training in licensing and
staff joining the business development and licensing functions.
Managers looking to broaden their personal career skills with a thorough understanding of the licensing
process.
Course agenda
The course starts on day 1 at 09:30h with a welcome coffee and ends on day 2 at 16:00h.
Day 1
Welcome & General Introduction
o Introduction of the programme and the delegates
o Overview of the out-licensing process
Preparing the Ground
o The importance of an out-licensing strategy
o Questions to be addressed when preparing an out-licensing plan
o Deciding on the best time to do a deal
Deciding What Type of Deal to Seek
o What are the options in terms of deal types?
o An explanation of how joint ventures and co-promotion work
o An introduction to typical commercial deal structures
o The value of performance and offset arrangements
Contractual Issues
o What to include in Confidential Disclosure Agreements (CDAs) and Materials Transfer Agreements (MTAs)
o Termsheets – a detailed layman’s review of all the key clauses, including:
Exclusivity, Sub-licenses, Field and Territory
Milestones, Royalties and Royalty stacking
Termination, Warranties and Jurisdiction
Valuing the Deal
o What are the key factors influencing deal values?
o What is a sensible way of establishing the value of a product?
o Modelling the deal
Exercise: delegates will be given a spreadsheet and an exercise to calculate an optimal deal structure for themselves
GROUP DINNER
To register, complete the online registration form at www.celforpharma.com
44
LAST UPDATE – 4 October 2010
Day 2
Preparing to Out-license
o How to draw up an action plan and what to include
o Setting up the licensing team
o How to market the deal – gain a full understanding of how to prepare the required documentation, including the non-
confidential brochure, confidential prospectus and presentation, due diligence and target termsheets
Finding Potential Partners
o Assembling and refining target lists and the resources used for this
o How to make effective contact with potential partners
o A checklist for effective record-keeping
The Evaluation Process
o What is involved in the evaluation and due diligence process undertaken by both licensors and licensees
o Factors that can influence a successful outcome
Negotiation Pointers
o How to make your negotiation more effective
Managing the Deal
o Building a team – task forces
o Managing your partner
o What to do if everything goes wrong
Preparing a Term Sheet
o Example of an actual termsheet used in a successful deal
o Exercise: delegates will be given the opportunity to draft their own target and fall-back terms for a fictitious but
realistic case. The results will be critically examined by the expert and discussed in plenary.
Learning methodology
David Scott is an experienced “hands-on” licensing manager - his report, “Scrip’s Practical Guide to
Pharmaceutical Licensing” has been called the “quintessential pharmaceutical licensing work”. He matches a
systematic presentation of the involved processes with practical anecdotes drawn from personal experience.
Delegates are encouraged to raise specific issues in the group to take full advantage of his experience and advice.
Each day ends with an exercise that allows delegates to put into practice the techniques. Delegates also receive a
number of pro-formas (including a draft CDA) dealing with the issues discussed during the course.
Meet the expert: David Scott
David Scott is a freelance healthcare consultant and a skilled negotiator who has closed a number of major deals
for inward and outward licensing for pharmaceutical products, delivery systems and technologies. He has also
provided licensing training for a number of multinational pharma companies and training organizations and has
published widely. David has a BSc in Chemistry from Nottingham and post-graduate qualifications in marketing
and market research from Kingston Business School. He is also an accredited Certified Licensing Professional and
a member of the Licensing Executives Society and the Pharmaceutical Licensing Group.
He joined Fisons plc (acquired by RPR in 1995, which itself has been acquired by Sanofi-aventis) in 1973 where he
held a number of roles including marketing, corporate development, finance director of their Spanish subsidiary,
To register, complete the online registration form at www.celforpharma.com
45
LAST UPDATE – 4 October 2010
business development manager and licensing. As Fisons Licensing Manager, he closed a number of major deals
and collaborative ventures involving both drugs and delivery systems.
David has spent the past 13 years as a consultant and has provided strategic advice. He has successfully
concluded both inward and outward licensing agreements on behalf of a range of worldwide clients. His client
base includes top-ranking global companies, European regional companies, biotech companies, technology start-
ups and universities. He currently sits on the board of three UK-based pharmaceutical businesses.
Dates
25-26 November 2010
6-7 June 2011, Copenhagen
8-9 November 2011, Brussels
Venue & Accommodation
25-26 November 2010
8-9 November 2011
These courses take place at the Sheraton Airport Hotel & Conference Center which is situated opposite Brussels
National Airport, literally at 2 minutes’ walking distance from the arrival hall.
Sheraton Brussels Airport Hotel
Brussels National Airport
1930 Zaventem
Belgium
Tel: +32 (0) 2 710 80 00
Fax: +32 (0) 2 710 80 80
We have secured preferential room rates at this four-star hotel for our delegates. Registering three weeks or more
prior to the course will secure your room at a preferential rate. Upon your registration, C.E.L.forpharma will send
you a Hotel Accommodation Sheet to complete and send back. Do not hesitate to contact Marie Stricklesse,
Programme Coordinator, if you need assistance in this matter (marie.stricklesse@celforpharma.com ; tel +32 (0)2
709 22 41).
6-7 June 2011
This course takes place at the Hilton Copenhagen Airport Hotel which is directly connected to Terminal 3 by a
covered walkway. It is located only 12 minutes' away from the city centre and its top attractions. The metro will
take you from the airport to Nørreport Station in central Copenhagen in 15 minutes.
Hilton Copenhagen Airport Hotel
Ellehammersvej 20
2770 Copenhagen
Denmark
Tel: 45-32-501-501
Fax: 45-32-528-528
We have secured preferential room rates at this four-star hotel for our delegates. Registering three weeks or more
prior to the course will secure your room at a preferential rate. Upon your registration, C.E.L.forpharma will send
you a Hotel Accommodation Sheet to complete and send back. Do not hesitate to contact Marie Stricklesse,
Programme Coordinator, if you need assistance in this matter (marie.stricklesse@celforpharma.com; tel
+32(0)27092241).
To register, complete the online registration form at www.celforpharma.com
46
LAST UPDATE – 4 October 2010
Registration fee
Price*
25-26 November 2010 Course (OUT-01)
▪ Registration before 8 October 2010 €2.450
▪ Registration after 8 October 2010 €2.850
6-7 June 2011 Course (OUT-02)
▪ Registration before 22 April 2011 €2.450
▪ Registration after 22 April 2011 €2.850
8-9 November 2011 Course (OUT-03)
▪ Registration before 23 September 2011 €2.450
▪ Registration after 23 September 2011 €2.850
* (VAT excl.)
To register, complete the online registration form at www.celforpharma.com
47
Вам также может понравиться
- Scientific Due Diligence: A Handbook for Investigators and InvestorsОт EverandScientific Due Diligence: A Handbook for Investigators and InvestorsОценок пока нет
- Biobusines Plan: Ernst & YoungДокумент16 страницBiobusines Plan: Ernst & YoungBeatriz MontaneОценок пока нет
- Financial Valuation Methods For BiotechnologyДокумент3 страницыFinancial Valuation Methods For Biotechnologyavestus100% (1)
- ADMET for Medicinal Chemists: A Practical GuideОт EverandADMET for Medicinal Chemists: A Practical GuideKatya TsaiounОценок пока нет
- Royalty Rates For Technology 4th Edition Sample ReportДокумент21 страницаRoyalty Rates For Technology 4th Edition Sample ReportTodd DaviesОценок пока нет
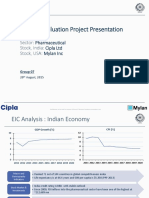
- Business Valuation Project Presentation: Sector: Pharmaceutical Stock, India: Cipla LTD Stock, USA: Mylan IncДокумент32 страницыBusiness Valuation Project Presentation: Sector: Pharmaceutical Stock, India: Cipla LTD Stock, USA: Mylan Incpuneet.glennОценок пока нет
- Using DCF in Biotech ValuationДокумент5 страницUsing DCF in Biotech ValuationOladipupo Mayowa PaulОценок пока нет
- Cracking the Patent Search Code: The Step-By-Step Guidebook for Patent Search & AnalysisОт EverandCracking the Patent Search Code: The Step-By-Step Guidebook for Patent Search & AnalysisОценок пока нет
- Valuing Ip A Short OutlineДокумент11 страницValuing Ip A Short OutlinewordbabeyОценок пока нет
- Royalty Rates and Profitability Across IndustriesДокумент15 страницRoyalty Rates and Profitability Across IndustriesPropertywizzОценок пока нет
- Valuation MethodsДокумент17 страницValuation MethodssaurabhОценок пока нет
- Intellectual Property AnalysisДокумент3 страницыIntellectual Property AnalysisShradha DiwanОценок пока нет
- Biotech Valuation ModelДокумент8 страницBiotech Valuation ModelsachinmatpalОценок пока нет
- Early-Stage Valuation in The Biotechnology IndustryДокумент54 страницыEarly-Stage Valuation in The Biotechnology Industrytransbunko100% (1)
- Financial AnalysisДокумент22 страницыFinancial AnalysisAiswarya MohanОценок пока нет
- Pharma Licensing & Tech Transfer GuideДокумент32 страницыPharma Licensing & Tech Transfer Guidevikram singh nitkОценок пока нет
- Royalty RatesДокумент8 страницRoyalty Ratesblittle100% (2)
- Pharmaceutical Valuation Methodology & Case StudiesДокумент45 страницPharmaceutical Valuation Methodology & Case Studiessonimanish1407Оценок пока нет
- Life SciencesДокумент28 страницLife SciencesMaryanna Istiqomah PratiwiОценок пока нет
- Big Pharma M&AДокумент23 страницыBig Pharma M&ADr Amit Rangnekar97% (39)
- Pharmaceutical LicensingДокумент116 страницPharmaceutical LicensingSudarshan Dujari100% (2)
- Biotech Valuation ModelДокумент8 страницBiotech Valuation ModelMichel KropfОценок пока нет
- Johnson & Johnson Equity Research ReportДокумент13 страницJohnson & Johnson Equity Research ReportPraveen R V100% (3)
- FPДокумент29 страницFPKevin ParkerОценок пока нет
- Utilization of IP and Need For ValuationДокумент15 страницUtilization of IP and Need For ValuationBrain LeagueОценок пока нет
- Biotech Valuation Model 2Документ21 страницаBiotech Valuation Model 2w_fibОценок пока нет
- Pharmaceutical ValuationДокумент75 страницPharmaceutical ValuationJohn DoeОценок пока нет
- Valuation Report - PfizerДокумент7 страницValuation Report - Pfizermcdonaghmatthew0% (1)
- Biotech Valuation ModelДокумент8 страницBiotech Valuation Modelw_fibОценок пока нет
- Case Study 1 - Fall 2020Документ2 страницыCase Study 1 - Fall 2020Gianna DeMarcoОценок пока нет
- Saudi Pharma Market Shifts as Global Giants and Generics RiseДокумент20 страницSaudi Pharma Market Shifts as Global Giants and Generics RiseSandip Rana BallavОценок пока нет
- IP Valuation FinalДокумент46 страницIP Valuation FinalNancy EkkaОценок пока нет
- Valuation GoodwilllДокумент22 страницыValuation GoodwilllShubashPoojariОценок пока нет
- Industry & Company ProfileДокумент141 страницаIndustry & Company ProfileMike Peña100% (1)
- WACC CalculationДокумент17 страницWACC Calculationrm1912Оценок пока нет
- Maulik Shah Master Thesis DocumentДокумент107 страницMaulik Shah Master Thesis DocumentchintanОценок пока нет
- Intangible ValuationДокумент29 страницIntangible ValuationCamelia VechiuОценок пока нет
- Improve Patent Search EfficiencyДокумент17 страницImprove Patent Search Efficiencypatent_info100% (1)
- Ey Study Opportunities in The Consolidating Cdmo IndustryДокумент16 страницEy Study Opportunities in The Consolidating Cdmo IndustryRakesh KumarОценок пока нет
- IPO UnderpricingДокумент9 страницIPO UnderpricingHendra Gun DulОценок пока нет
- S&P Pharma Industry Overview - 11252010Документ49 страницS&P Pharma Industry Overview - 11252010earajesh100% (1)
- Pharma Sector AnalysisДокумент11 страницPharma Sector AnalysisKritika TОценок пока нет
- AdvanceAutoPartsConference Call Presentation101613Документ18 страницAdvanceAutoPartsConference Call Presentation101613aedcbf123Оценок пока нет
- A6967DE8-A1F0-446A-B423-6FB2815A2E4D (2)Документ74 страницыA6967DE8-A1F0-446A-B423-6FB2815A2E4D (2)zainОценок пока нет
- Stage One (Where Are We Now?) : A. Environment AuditДокумент59 страницStage One (Where Are We Now?) : A. Environment AuditmzakifОценок пока нет
- Mini Case AFSДокумент3 страницыMini Case AFSOwais YaseenОценок пока нет
- Appraoches To Valuation 1Документ67 страницAppraoches To Valuation 1tj00007Оценок пока нет
- S&PIndustryReport PharmaceuticalsДокумент58 страницS&PIndustryReport PharmaceuticalsJessa RosalesОценок пока нет
- Royalty Rates For Trademarks and Copyrights Sample PagesДокумент4 страницыRoyalty Rates For Trademarks and Copyrights Sample PagesValentina TrentОценок пока нет
- Pharma For BeginnersДокумент327 страницPharma For Beginnersgunc100% (1)
- HCL Tech Q3 FY22 Investor ReleaseДокумент28 страницHCL Tech Q3 FY22 Investor Releaseashokdb2kОценок пока нет
- A Conceptual Study On Brand Valuation: A. Seetharaman Zainal Azlan Bin Mohd Nadzir S. GunalanДокумент14 страницA Conceptual Study On Brand Valuation: A. Seetharaman Zainal Azlan Bin Mohd Nadzir S. GunalanMara MatesОценок пока нет
- Avexis Investor PresentationДокумент20 страницAvexis Investor PresentationmedtechyОценок пока нет
- Pharma & Biotech: Development & ValuationДокумент32 страницыPharma & Biotech: Development & ValuationParas Bhola100% (1)
- EMIS Insights - India Pharma and Healthcare Sector Report 2021 - 2025Документ87 страницEMIS Insights - India Pharma and Healthcare Sector Report 2021 - 2025arreeyaarОценок пока нет
- Global Biotechnology IBISДокумент52 страницыGlobal Biotechnology IBISJun LiuОценок пока нет
- Finance - Cost of Capital TheoryДокумент30 страницFinance - Cost of Capital TheoryShafkat RezaОценок пока нет
- Partnership Accounting.Документ15 страницPartnership Accounting.セニタОценок пока нет
- AUSTRALIAN WOOLLEN MILLS PTY LTD V COMMONWEA PDFДокумент17 страницAUSTRALIAN WOOLLEN MILLS PTY LTD V COMMONWEA PDFjohndsmith22Оценок пока нет
- Tax Comp ExampleДокумент115 страницTax Comp ExampleJayjay FarconОценок пока нет
- All 760953Документ13 страницAll 760953David CheishviliОценок пока нет
- Common Bank Interview QuestionsДокумент23 страницыCommon Bank Interview Questionssultan erboОценок пока нет
- Ac 518 Hand-Outs Government Accounting and Auditing TNCR: The National Government of The PhilippinesДокумент53 страницыAc 518 Hand-Outs Government Accounting and Auditing TNCR: The National Government of The PhilippinesHarley Gumapon100% (1)
- Financial Management September 2013 Mark Plan ICAEW PDFДокумент9 страницFinancial Management September 2013 Mark Plan ICAEW PDFMuhammad Ziaul HaqueОценок пока нет
- The Peoples State Bank of Ellinwood, Kansas, and Marian Isern v. Marlette Coach Company, 336 F.2d 3, 10th Cir. (1964)Документ4 страницыThe Peoples State Bank of Ellinwood, Kansas, and Marian Isern v. Marlette Coach Company, 336 F.2d 3, 10th Cir. (1964)Scribd Government DocsОценок пока нет
- Professional Legal Services and Attorneys in ArmeniaДокумент17 страницProfessional Legal Services and Attorneys in ArmeniaAMLawFirmОценок пока нет
- Budgetary Control - L G ElectonicsДокумент86 страницBudgetary Control - L G ElectonicssaiyuvatechОценок пока нет
- Review 105 - Day 17 P1: How Much of The Proceeds From The Issuance of Convertible Bonds Should Be Allocated To Equity?Документ10 страницReview 105 - Day 17 P1: How Much of The Proceeds From The Issuance of Convertible Bonds Should Be Allocated To Equity?Pola PolzОценок пока нет
- SDDP 12 21 Fec ReportДокумент84 страницыSDDP 12 21 Fec ReportPat PowersОценок пока нет
- Cost Accounting Assignment 2Документ9 страницCost Accounting Assignment 2RCGОценок пока нет
- SBD HPPWD Final2016Документ108 страницSBD HPPWD Final2016KULDEEP KAPOORОценок пока нет
- Welcome To First ReviewДокумент17 страницWelcome To First Reviewkarthy143Оценок пока нет
- Bank of PunjabДокумент72 страницыBank of PunjabZia Shoukat100% (1)
- Term Paper On The Topic Monetary Policy of Nepal 2080 - 81 Aadhar Babu KhatiwadaДокумент15 страницTerm Paper On The Topic Monetary Policy of Nepal 2080 - 81 Aadhar Babu KhatiwadaPrashant GautamОценок пока нет
- Synopsis: Study On Merger and Acquisition in Automobile SectorДокумент3 страницыSynopsis: Study On Merger and Acquisition in Automobile SectorSubhash KumarОценок пока нет
- RAPDRP Part-B implementation and impact in MPPKVVCL IndoreДокумент20 страницRAPDRP Part-B implementation and impact in MPPKVVCL IndoreArvind YadavОценок пока нет
- Viva Ruchi MamДокумент9 страницViva Ruchi Mam21358 NDIMОценок пока нет
- Sky vs BSB payoff matrix analysisДокумент3 страницыSky vs BSB payoff matrix analysisd-fbuser-46026705100% (1)
- Livelihood Project For The Poor in OmanДокумент6 страницLivelihood Project For The Poor in OmanInternational Journal of Business Marketing and ManagementОценок пока нет
- Supplier Payments SampleДокумент23 страницыSupplier Payments SampleAndhika Artha PrayudhaОценок пока нет
- All About BPIДокумент3 страницыAll About BPIJodelyn Tano JumadasОценок пока нет
- Name: Kimberly Anne P. Caballes Year and CourseДокумент12 страницName: Kimberly Anne P. Caballes Year and CourseKimberly Anne CaballesОценок пока нет
- Wiley Chapter 11 Depreciation Impairments and DepletionДокумент43 страницыWiley Chapter 11 Depreciation Impairments and Depletion靳雪娇Оценок пока нет
- The Economist EU 06.24.2023Документ76 страницThe Economist EU 06.24.2023sahilmkkrОценок пока нет
- Financial Intermediation Roles and RisksДокумент62 страницыFinancial Intermediation Roles and RisksShenna Mariano100% (1)
- W19196-PDF-EnG ChimpChange - How To Raise Capital To GrowДокумент17 страницW19196-PDF-EnG ChimpChange - How To Raise Capital To GrowbobbyОценок пока нет
- When to Jump: If the Job You Have Isn't the Life You WantОт EverandWhen to Jump: If the Job You Have Isn't the Life You WantРейтинг: 4.5 из 5 звезд4.5/5 (16)
- The 7 Habits of Highly Effective People: The Infographics EditionОт EverandThe 7 Habits of Highly Effective People: The Infographics EditionРейтинг: 4 из 5 звезд4/5 (2475)
- The Ultimate Sales Letter, 4th Edition: Attract New Customers, Boost Your SalesОт EverandThe Ultimate Sales Letter, 4th Edition: Attract New Customers, Boost Your SalesРейтинг: 4.5 из 5 звезд4.5/5 (98)
- Work Stronger: Habits for More Energy, Less Stress, and Higher Performance at WorkОт EverandWork Stronger: Habits for More Energy, Less Stress, and Higher Performance at WorkРейтинг: 4.5 из 5 звезд4.5/5 (12)
- Designing Your Life by Bill Burnett, Dave Evans - Book Summary: How to Build a Well-Lived, Joyful LifeОт EverandDesigning Your Life by Bill Burnett, Dave Evans - Book Summary: How to Build a Well-Lived, Joyful LifeРейтинг: 4.5 из 5 звезд4.5/5 (61)
- The Confidence Code: The Science and Art of Self-Assurance--What Women Should KnowОт EverandThe Confidence Code: The Science and Art of Self-Assurance--What Women Should KnowРейтинг: 4.5 из 5 звезд4.5/5 (175)
- The Proximity Principle: The Proven Strategy That Will Lead to the Career You LoveОт EverandThe Proximity Principle: The Proven Strategy That Will Lead to the Career You LoveРейтинг: 4.5 из 5 звезд4.5/5 (93)
- Company Of One: Why Staying Small Is the Next Big Thing for BusinessОт EverandCompany Of One: Why Staying Small Is the Next Big Thing for BusinessРейтинг: 3.5 из 5 звезд3.5/5 (14)
- Steal the Show: From Speeches to Job Interviews to Deal-Closing Pitches, How to Guarantee a Standing Ovation for All the Performances in Your LifeОт EverandSteal the Show: From Speeches to Job Interviews to Deal-Closing Pitches, How to Guarantee a Standing Ovation for All the Performances in Your LifeРейтинг: 4.5 из 5 звезд4.5/5 (39)
- How the World Sees You: Discover Your Highest Value Through the Science of FascinationОт EverandHow the World Sees You: Discover Your Highest Value Through the Science of FascinationРейтинг: 4 из 5 звезд4/5 (7)
- The 30 Day MBA: Your Fast Track Guide to Business SuccessОт EverandThe 30 Day MBA: Your Fast Track Guide to Business SuccessРейтинг: 4.5 из 5 звезд4.5/5 (19)
- From Paycheck to Purpose: The Clear Path to Doing Work You LoveОт EverandFrom Paycheck to Purpose: The Clear Path to Doing Work You LoveРейтинг: 4.5 из 5 звезд4.5/5 (39)
- Real Artists Don't Starve: Timeless Strategies for Thriving in the New Creative AgeОт EverandReal Artists Don't Starve: Timeless Strategies for Thriving in the New Creative AgeРейтинг: 4.5 из 5 звезд4.5/5 (197)
- The First 90 Days: Proven Strategies for Getting Up to Speed Faster and SmarterОт EverandThe First 90 Days: Proven Strategies for Getting Up to Speed Faster and SmarterРейтинг: 4.5 из 5 звезд4.5/5 (122)
- The Search for Self-Respect: Psycho-CyberneticsОт EverandThe Search for Self-Respect: Psycho-CyberneticsРейтинг: 4.5 из 5 звезд4.5/5 (10)
- Start.: Punch Fear in the Face, Escape Average, and Do Work That MattersОт EverandStart.: Punch Fear in the Face, Escape Average, and Do Work That MattersРейтинг: 4.5 из 5 звезд4.5/5 (56)
- The 12 Week Year: Get More Done in 12 Weeks than Others Do in 12 MonthsОт EverandThe 12 Week Year: Get More Done in 12 Weeks than Others Do in 12 MonthsРейтинг: 4.5 из 5 звезд4.5/5 (90)
- The Dictionary of Body Language: A Field Guide to Human BehaviorОт EverandThe Dictionary of Body Language: A Field Guide to Human BehaviorРейтинг: 4.5 из 5 звезд4.5/5 (95)
- Ultralearning: Master Hard Skills, Outsmart the Competition, and Accelerate Your CareerОт EverandUltralearning: Master Hard Skills, Outsmart the Competition, and Accelerate Your CareerРейтинг: 4.5 из 5 звезд4.5/5 (359)
- The Definitive Executive Assistant and Managerial Handbook: A Professional Guide to Leadership for all PAs, Senior Secretaries, Office Managers and Executive AssistantsОт EverandThe Definitive Executive Assistant and Managerial Handbook: A Professional Guide to Leadership for all PAs, Senior Secretaries, Office Managers and Executive AssistantsРейтинг: 3 из 5 звезд3/5 (2)
- Radiographic Testing: Theory, Formulas, Terminology, and Interviews Q&AОт EverandRadiographic Testing: Theory, Formulas, Terminology, and Interviews Q&AОценок пока нет
- How to Be Everything: A Guide for Those Who (Still) Don't Know What They Want to Be When They Grow UpОт EverandHow to Be Everything: A Guide for Those Who (Still) Don't Know What They Want to Be When They Grow UpРейтинг: 4 из 5 звезд4/5 (74)
- Love + Work: How to Find What You Love, Love What You Do, and Do It for the Rest of Your LifeОт EverandLove + Work: How to Find What You Love, Love What You Do, and Do It for the Rest of Your LifeРейтинг: 4.5 из 5 звезд4.5/5 (26)
- The Healthy Virtual Assistant: How to Become a Virtual Assistant for the Health and Wellness IndustryОт EverandThe Healthy Virtual Assistant: How to Become a Virtual Assistant for the Health and Wellness IndustryРейтинг: 4 из 5 звезд4/5 (2)
- Designing Your Life - Summarized for Busy People: How to Build a Well-Lived, Joyful LifeОт EverandDesigning Your Life - Summarized for Busy People: How to Build a Well-Lived, Joyful LifeРейтинг: 4 из 5 звезд4/5 (4)