Академический Документы
Профессиональный Документы
Культура Документы
Dengue Report
Загружено:
jed larsen capulong gavino0 оценок0% нашли этот документ полезным (0 голосов)
506 просмотров1 страницаОригинальное название
dengue report.docx
Авторское право
© © All Rights Reserved
Доступные форматы
DOCX, PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате DOCX, PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
506 просмотров1 страницаDengue Report
Загружено:
jed larsen capulong gavinoАвторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате DOCX, PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 1
LABORATORY DEPARTMENT
Concern: NON-REACTIVE results on suspected Dengue Patients
Requested by: Dr. Emy Sangalang
All request were subjected to proper procedure by the MTOD as required and stated by the inserts of SD
Dengue NS1 + Ab Combo and Bio Tracer Dengue IgG/IgM Rapid Card.
Bio Tracer Dengue IgG/IgM Rapid Card Exp Date: 12-2017
1. Add 5ul of specimen (serum, plasma or whole blood) to sample well
2. Add 4 drops of sample diluent to diluent well
3. Read after 15 – 20 minutes
SD DENGUE NS1 + Ab Combo Exp Date: 01-2018
NS1
1. Add 100ul of serum, plasma or whole blood into sample well
Ab Combo
1. Add 10ul of serum, plasma or whole blood into sample well marked “S”
2. Put 4 drops of assay diluent into the round-shaped assay diluent well
READ after 15-20minutes
All of this procedure was done properly and accurately by MTODs.
With regards to the concern of NON-REACTIVE results with marked platelet drops, we can correlate it to the
indications and principle of the procedures:
Bio Tracer Dengue IgG/IgM Rapid Card
Principle: Immunochromatography
In-vitro test for the qualitative determination of dengue virus specific IgG or IgM
***If antibodies against dengue are present enough in the sample, a colored band of dengue IgG/IgM in
the test zone will appear. If there are no antibodies or not sufficient in the sample, the area will remain colourless.
The sample continues to move to the control reaction zone and forms a red or purple colour, indicating the test is
working and result is valid
SD DENGUE NS1 + Ab Combo
Principle: Immunochromatography
In-vitro test designed to detect both Dengue NS1 Dengue virus and differential IgG/IgM
antibodies for detection in acute dengue infection.
Limitation of the Test:
1. A negative result can occur if the quantity of Dengue virus NS1 antigen is present in the
specimen is below the detection limits of the assay, or the antigens that are detected by the
test are not present during the stage of disease in which sample is collected
2. A negative result cannot exclude a recent infection
3. In early infections and some secondary infections, detectable levels of IgM antibodies may be
low. Some patients may not produce detectable levels of IgM antibodies within the first seven
to 10 days after infection. Where symptoms persist, patients should be re-tested 3 – 4 days
after the first specimen.
Factors to consider:
1. Date of examination after disease / antigen exposure
2. Quantity of antigen and antibodies in the specimen
3. Other diseases to consider : Chikungunya
Вам также может понравиться
- Dengue 1Документ1 страницаDengue 1Sure NavyasriОценок пока нет
- WM68Документ2 страницыWM68AbhijayОценок пока нет
- Serology Report: Test Units Normal RangeДокумент1 страницаSerology Report: Test Units Normal RangeKrishna Shrestha100% (2)
- Sonu ReportДокумент8 страницSonu ReportVeeraj SinghОценок пока нет
- Saurav RajДокумент8 страницSaurav RajSaurav RajОценок пока нет
- Mor DengueДокумент3 страницыMor Dengueveenit2512Оценок пока нет
- Specimen: Nasopharyngeal Swab: Dr. Girish Gaur Lab Director and Senior Consultant Molecular DiagnosticsДокумент1 страницаSpecimen: Nasopharyngeal Swab: Dr. Girish Gaur Lab Director and Senior Consultant Molecular DiagnosticsBhavy BansalОценок пока нет
- DENGUE FEVER Test Report Format Example Sample Template Drlogy Lab ReportДокумент1 страницаDENGUE FEVER Test Report Format Example Sample Template Drlogy Lab ReportBhojraj SinghОценок пока нет
- Interpretation: L30 - Para-Cc Shop No 3, Shubh Complex, Para, Old para Thana, para LucknowДокумент5 страницInterpretation: L30 - Para-Cc Shop No 3, Shubh Complex, Para, Old para Thana, para LucknowYogesh BaranwalОценок пока нет
- R3Документ1 страницаR3Asif ButtОценок пока нет
- DR Waseem Hammoudeh - Jordan and Hepatitis B Virus - Medics Index MemberДокумент1 страницаDR Waseem Hammoudeh - Jordan and Hepatitis B Virus - Medics Index MemberMedicsindex Telepin SlidecaseОценок пока нет
- MrsSNIGDHA 43Y FemaleДокумент3 страницыMrsSNIGDHA 43Y FemalePathkind LabОценок пока нет
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Te Name Result Unit Bio. Ref. Range MethodДокумент2 страницыDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Te Name Result Unit Bio. Ref. Range MethodAnirban MondalОценок пока нет
- L96 - Patna Lab II R K Estate (Opp. Igims Gate), Rajabazar PATNA-14 # 7632995990 & 0612-2295550 PatnaДокумент3 страницыL96 - Patna Lab II R K Estate (Opp. Igims Gate), Rajabazar PATNA-14 # 7632995990 & 0612-2295550 PatnaAnkit AnandОценок пока нет
- Molecular Analysis For Qualitative Detection of Sars-Cov-2.: Negative Negative Negative PassДокумент4 страницыMolecular Analysis For Qualitative Detection of Sars-Cov-2.: Negative Negative Negative PassmeezОценок пока нет
- Department of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range MethodДокумент2 страницыDepartment of Molecular Biology. Covid 19 RTPCR With Home Collection Test Name Result Unit Bio. Ref. Range Methodmaneesh babuОценок пока нет
- Dengue ReportДокумент1 страницаDengue ReportAS POSTОценок пока нет
- Patient 15-c20721 Bcr-Abl Quali ReportДокумент4 страницыPatient 15-c20721 Bcr-Abl Quali ReportNishant Kumar GuptaОценок пока нет
- DSBPOPV26746Документ2 страницыDSBPOPV26746Abha MahapatraОценок пока нет
- Stool Test Report PDFДокумент2 страницыStool Test Report PDFAbhishek DubeyОценок пока нет
- L21 - FPSC Medical Road Aligarh Shop No-18, Near JNMC, Zakria Market, Aligarh-Mob - 7417522734Документ1 страницаL21 - FPSC Medical Road Aligarh Shop No-18, Near JNMC, Zakria Market, Aligarh-Mob - 7417522734FaizanAli100% (1)
- Shera Wood en PDFДокумент16 страницShera Wood en PDFFACTS- WORLDОценок пока нет
- Untitled DesignДокумент6 страницUntitled Designwork.krishnam9988Оценок пока нет
- Medical CertificateДокумент5 страницMedical CertificatePrasanna VenkatОценок пока нет
- Dengue Test RPT 2 EditДокумент3 страницыDengue Test RPT 2 EditMuhammad KhudriОценок пока нет
- L51 - Home Collection KRL (Kolkata Reference Lab) Premises No - 031-0199 Plot No - CB 31/1 Street 199 Action Area 1C, KOLKATAДокумент3 страницыL51 - Home Collection KRL (Kolkata Reference Lab) Premises No - 031-0199 Plot No - CB 31/1 Street 199 Action Area 1C, KOLKATADebraj Das0% (1)
- TestReport 2100101650Документ1 страницаTestReport 2100101650Kashi RajpootОценок пока нет
- Research Gaps in Viral HepatitisДокумент6 страницResearch Gaps in Viral HepatitisMuhammad Anwer QambraniОценок пока нет
- Collected Male Dr. Svasti Report Status: Final: Name Lab No. P 159709569 Mr. Dushyant HoodaДокумент2 страницыCollected Male Dr. Svasti Report Status: Final: Name Lab No. P 159709569 Mr. Dushyant Hoodadushyant33% (3)
- Path Lab ReportДокумент3 страницыPath Lab Reportsushant kumar100% (2)
- Report 8b3a3101Документ11 страницReport 8b3a3101Pooja AgarwalОценок пока нет
- TYPHIDOT IgG - IgM - ICTДокумент1 страницаTYPHIDOT IgG - IgM - ICTAlveera Zafar100% (1)
- Dengue: Subjective FindingsДокумент5 страницDengue: Subjective FindingsRaghu VenkatОценок пока нет
- PdfText PDFДокумент10 страницPdfText PDFshakila banuОценок пока нет
- A03 - Mr. Pradeep Kumar Tripathi - FPSC George Town 18/1A, A.N. Jha Marg Georgetown, Allahabad, UpДокумент2 страницыA03 - Mr. Pradeep Kumar Tripathi - FPSC George Town 18/1A, A.N. Jha Marg Georgetown, Allahabad, UpAbhisesh Dev Narayan MishraОценок пока нет
- SL Report-278574812Документ2 страницыSL Report-278574812SiddharthОценок пока нет
- Dengue Test RPT 1 EditДокумент2 страницыDengue Test RPT 1 EditMuhammad KhudriОценок пока нет
- 1-Dengue Antigen NS1, IgG & IgM - PO1576121305-961 PDFДокумент16 страниц1-Dengue Antigen NS1, IgG & IgM - PO1576121305-961 PDFArijit GoraiОценок пока нет
- Dangue ReportДокумент1 страницаDangue ReportPranavpuri GoswamiОценок пока нет
- Department of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodДокумент2 страницыDepartment of Molecular Biology. Covid 19 Test Name Result Unit Bio. Ref. Range MethodDevi Sri PrasadОценок пока нет
- Fatima Jinnah Chest and General Hospital: COVID-19 Test ReportДокумент1 страницаFatima Jinnah Chest and General Hospital: COVID-19 Test ReportMuhammad IlyasОценок пока нет
- Doctor PrescriptionДокумент2 страницыDoctor PrescriptionKhurram ShahzadОценок пока нет
- Mktkiunughe4re544imlv1fuДокумент3 страницыMktkiunughe4re544imlv1fuअभिषेक सिंह पटेल100% (1)
- Microalbumin Creatinine Ratio - Spot Urine: SR - No Investigation Observed Value Reference Range UnitДокумент1 страницаMicroalbumin Creatinine Ratio - Spot Urine: SR - No Investigation Observed Value Reference Range Unitdebabrata_dutta678Оценок пока нет
- Diagnostic Report: Patient Name: Ashwani Singh 0088UD006521 ASHWM280719800Документ2 страницыDiagnostic Report: Patient Name: Ashwani Singh 0088UD006521 ASHWM280719800Ankit AgarwalОценок пока нет
- Sars-Cov-2 by RT PCR (Qualitative) : Icmr Reg .No. - SanpalagДокумент1 страницаSars-Cov-2 by RT PCR (Qualitative) : Icmr Reg .No. - SanpalagHaimanti NathОценок пока нет
- Lab Report: 2696700 LAB/20N/45867 16/sep/2020 MR - Manish Vaish 13464912 StatusДокумент2 страницыLab Report: 2696700 LAB/20N/45867 16/sep/2020 MR - Manish Vaish 13464912 StatusmanishОценок пока нет
- Laboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Документ5 страницLaboratory Test Report: Test Name Result Sars-Cov-2 (RDRP Gene)Imran AhmedОценок пока нет
- Test Report: MR - Raghavan Venkatraman (39/M)Документ2 страницыTest Report: MR - Raghavan Venkatraman (39/M)Raghavan VenkatramanОценок пока нет
- R 400651Документ4 страницыR 400651KanchanОценок пока нет
- MrNAVEENKUMAR 20Y MaleДокумент1 страницаMrNAVEENKUMAR 20Y MaleHimmatt PatelОценок пока нет
- Out Patient Department: UHID:105456532Документ1 страницаOut Patient Department: UHID:105456532Raj's Kitchen0% (1)
- FM Report Download PDFДокумент1 страницаFM Report Download PDFNitish PonneboyinaОценок пока нет
- JWALAДокумент2 страницыJWALAVaid Navdeep Singh100% (1)
- Test Report: PAVITHRA (25/F)Документ3 страницыTest Report: PAVITHRA (25/F)Pavi PavichuОценок пока нет
- Report-MK36202 RAVI KUMAR 04nov2018 122520Документ3 страницыReport-MK36202 RAVI KUMAR 04nov2018 122520munagala.20076336Оценок пока нет
- Mahesh Lab Reports 20.2.21Документ14 страницMahesh Lab Reports 20.2.21raw rajОценок пока нет
- Test Result PDFДокумент2 страницыTest Result PDFSouradeep Sen33% (3)
- Dengue Igm / Igg Rapid Test: Intended UseДокумент4 страницыDengue Igm / Igg Rapid Test: Intended UseYvette TiongsonОценок пока нет
- Ficha Tecnica Dengue Duo SDДокумент2 страницыFicha Tecnica Dengue Duo SDDanitxa Leyva100% (1)
- 2022 Pharmacology s2t2 HeartfailureДокумент6 страниц2022 Pharmacology s2t2 Heartfailurejed larsen capulong gavinoОценок пока нет
- Pedia Newborn PhysioДокумент20 страницPedia Newborn Physiojed larsen capulong gavinoОценок пока нет
- Male Pelvic Viscera: NaeemiДокумент36 страницMale Pelvic Viscera: Naeemijed larsen capulong gavinoОценок пока нет
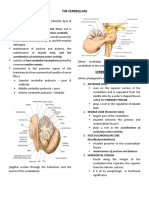
- The Cerebellum: Coordination of Voluntary Motor ActivityДокумент4 страницыThe Cerebellum: Coordination of Voluntary Motor Activityjed larsen capulong gavinoОценок пока нет
- Be It Enacted by The Senate and House of Representatives of The Philippines in Congress AssembledДокумент4 страницыBe It Enacted by The Senate and House of Representatives of The Philippines in Congress Assembledjed larsen capulong gavinoОценок пока нет
- Conditions Affecting The Back and The Upper ExtremityДокумент19 страницConditions Affecting The Back and The Upper Extremityjed larsen capulong gavinoОценок пока нет
- D. Pseudounipolar D. All of The FollowingДокумент5 страницD. Pseudounipolar D. All of The Followingjed larsen capulong gavinoОценок пока нет
- Histology Test Bank PrelimsДокумент40 страницHistology Test Bank Prelimsjed larsen capulong gavino100% (8)
- Study Guide 23 - DIGESTIVE SYSTEMДокумент33 страницыStudy Guide 23 - DIGESTIVE SYSTEMjed larsen capulong gavinoОценок пока нет
- General Health of Dentists. Literature ReviewДокумент11 страницGeneral Health of Dentists. Literature ReviewAdina SerbanОценок пока нет
- A26 - Walk in Paschim Vihar Ii: NoteДокумент2 страницыA26 - Walk in Paschim Vihar Ii: NoteLakshay MahajanОценок пока нет
- Immunization in Children: Mary Beth F. Tanco, MD, FPPS Active Consultant Institute of Pediatrics and Child HealthДокумент92 страницыImmunization in Children: Mary Beth F. Tanco, MD, FPPS Active Consultant Institute of Pediatrics and Child HealthPolychase Magaoay100% (1)
- Journal Benjolan Di LeherДокумент9 страницJournal Benjolan Di LeherStase IPD SoedarsoОценок пока нет
- HD Guideline Cleaning Disinfecting HD MachinesДокумент14 страницHD Guideline Cleaning Disinfecting HD MachinesMendes NovatoОценок пока нет
- International Nosology Heritable Disorders of Connective Tissue, Berlin, 1986Документ14 страницInternational Nosology Heritable Disorders of Connective Tissue, Berlin, 1986Willy AndersonОценок пока нет
- Lumbar PunctureДокумент3 страницыLumbar PunctureKarsten RohlfsОценок пока нет
- Fernandez ED101 Module 2 PDFДокумент28 страницFernandez ED101 Module 2 PDFChristine ElnasОценок пока нет
- Foods That Promote Liver Health - Pritikin Weight Loss Resort PDFДокумент6 страницFoods That Promote Liver Health - Pritikin Weight Loss Resort PDFPallabi RoyОценок пока нет
- Visitors Personal Declaration Form - COVID 19Документ1 страницаVisitors Personal Declaration Form - COVID 19Leoni FrancОценок пока нет
- Hernia: Inguinal - Surgical Anatomy, Presentation, Treatment, ComplicationsДокумент37 страницHernia: Inguinal - Surgical Anatomy, Presentation, Treatment, ComplicationsIvan Olo SarumpaetОценок пока нет
- Streptomycin PenicillinДокумент76 страницStreptomycin PenicillinMuhammad ZeeshanОценок пока нет
- Chellah Final Research (2) EditedДокумент56 страницChellah Final Research (2) EditedCredible Technology ComplexОценок пока нет
- Toij 2020 03 25 PDFДокумент18 страницToij 2020 03 25 PDFHimanshuОценок пока нет
- The Domain BacteriaДокумент51 страницаThe Domain BacterialeyluuuuuhОценок пока нет
- Ypgh 113 1695081Документ12 страницYpgh 113 1695081Pawan MishraОценок пока нет
- Community HESI Study GuideДокумент26 страницCommunity HESI Study Guidemscostello985394% (32)
- What Is Atrial FibrillationДокумент3 страницыWhat Is Atrial FibrillationMoni ShafiqОценок пока нет
- ICON 2016 Febrile Neutropenia GuidelinesДокумент34 страницыICON 2016 Febrile Neutropenia GuidelinesTor Ja100% (1)
- LRP VGFDPI 2021 004 - ProtocolДокумент25 страницLRP VGFDPI 2021 004 - ProtocolDileep CRCОценок пока нет
- Clinical Pharmacology Book 2018 1Документ304 страницыClinical Pharmacology Book 2018 1Salman khanОценок пока нет
- A Century After Yehidar-Beshita (The Spanish U in Ethiopia) : Are We Prepared For The Next Pandemic?Документ5 страницA Century After Yehidar-Beshita (The Spanish U in Ethiopia) : Are We Prepared For The Next Pandemic?Yalew MekonnenОценок пока нет
- Ear Pain Flow Chart: Homeopathic Remedy GuideДокумент4 страницыEar Pain Flow Chart: Homeopathic Remedy GuideAtit Sheth100% (1)
- Nclex Test ReviewДокумент222 страницыNclex Test Reviewrajapp89% (76)
- Hirschsprung Disease Case Study ScenarioДокумент2 страницыHirschsprung Disease Case Study ScenarioKrizzia Angela BacotocОценок пока нет
- Early Breast Cancer - From Screening To Multidisciplinary Management - 3E (PDF) (UnitedVRG)Документ586 страницEarly Breast Cancer - From Screening To Multidisciplinary Management - 3E (PDF) (UnitedVRG)Luciana Bruno MaiaОценок пока нет
- Mitochondrial Disorders A Review of Anesthetic ConsiderationsДокумент10 страницMitochondrial Disorders A Review of Anesthetic ConsiderationsSanaОценок пока нет
- Group C PresentationДокумент45 страницGroup C PresentationFELICIA KASIMUОценок пока нет
- Complete Notes For Class 12 (Yoga and Lifestyle)Документ20 страницComplete Notes For Class 12 (Yoga and Lifestyle)Om Kumar Singh67% (3)
- Censoring Issues in Survival Analysis: Kwan-Moon Leung, Robert M. Elashoff, and Abdelmonem A. AfifiДокумент22 страницыCensoring Issues in Survival Analysis: Kwan-Moon Leung, Robert M. Elashoff, and Abdelmonem A. AfifiLili MolinaОценок пока нет