Академический Документы
Профессиональный Документы
Культура Документы
Hiperparatiro e Hipoparatiroismo
Загружено:
xander trujilloАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Hiperparatiro e Hipoparatiroismo
Загружено:
xander trujilloАвторское право:
Доступные форматы
MED ICA L PROGR ES S
Medical Progress logic activity.10 The effects of parathyroid hormone on
mineral metabolism are initiated by the binding of
parathyroid hormone to the type 1 parathyroid hor-
H YPERPARATHYROID AND mone receptor in the target tissues.11 Parathyroid hor-
H YPOPARATHYROID D ISORDERS mone thereby regulates large calcium fluxes across
bone, kidneys, and intestines1 (Fig. 1). Another par-
STEPHEN J. MARX, M.D. athyroid hormone receptor (type 2) has been found
in the brain and the intestines. Its main ligand is a pep-
tide different from parathyroid hormone12; the func-
T
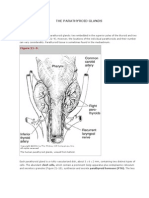
HE four parathyroid glands, through the secre- tions of this receptor are not known.
tion of parathyroid hormone, regulate serum Parathyroid hormone–related peptide is a distant
calcium concentrations and bone metabolism.1 homologue of parathyroid hormone and is not a true
In turn, serum calcium concentrations regulate par- hormone. It is synthesized in cartilage and in many
athyroid hormone secretion; high concentrations in- more tissues than is parathyroid hormone, and its se-
hibit secretion by the parathyroid glands of parathy- cretion is not regulated by serum calcium.13 Its local
roid hormone and low concentrations stimulate it.2 release activates the type 1 parathyroid hormone re-
Low or falling serum calcium concentrations act with- ceptor, and its affinity for this receptor is similar to
in seconds to stimulate parathyroid hormone secre- that of parathyroid hormone (Fig. 1).
tion, initiated by means of a calcium-sensing receptor
on the surface of the parathyroid cells.2 This receptor MEASUREMENT OF PARATHYROID
is a heptahelical molecule, like the receptors for light, HORMONE IN SERUM
odorants, catecholamines, and many peptide hor- Measurements of serum calcium, parathyroid hor-
mones.3 Parathyroid hormone secretion is 50 per- mone, 25-hydroxyvitamin D, and 1,25-dihydroxyvi-
cent of the maximal level at a serum ionized calcium tamin D are used regularly in the diagnosis and treat-
concentration of 4 mg per deciliter (1 mmol per li- ment of hyperparathyroidism and hypoparathyroidism;
ter); this is considered the calcium set point for par- only the measurement of serum parathyroid hormone
athyroid hormone secretion. A slower regulation of is covered here. Serum calcium should usually be
parathyroid hormone secretion occurs over a period measured at the same time as serum parathyroid hor-
of hours as a result of cellular changes in parathyroid mone; since the ionized fraction of serum calcium is
hormone messenger RNA (mRNA). Vitamin D and the biologically active form, it is a more useful index
its metabolites 25-hydroxyvitamin D and 1,25-dihy- of hyperparathyroidism and hypoparathyroidism than
droxyvitamin D, acting through vitamin D receptors, are other indexes of calcium in serum. It is therefore
decrease the level of parathyroid hormone mRNA,4 the preferred form of serum calcium to measure.
and hypocalcemia increases the level of that mRNA.5,6 Current assays for serum parathyroid hormone are
The slowest regulation of parathyroid hormone se- two-site assays designed to detect both amino-termi-
cretion occurs over days or even months and reflects nal and carboxy-terminal epitopes of the peptide.14,15
changes in the growth of the parathyroid glands. The better assays are those that are well standardized,
Metabolites of vitamin D directly inhibit the mass of do not cross-react with parathyroid hormone–relat-
parathyroid cells7; hypocalcemia stimulates the growth ed peptide, and are sufficiently sensitive that normal
of parathyroid cells independently of the contrary values can be distinguished from subnormal values
action of vitamin D metabolites.8,9 Disruptions in (Fig. 2). Parathyroid hormone molecules that are re-
these processes cause hyperparathyroidism or hypo- active in these two-site immunoassays are considered
parathyroidism. “intact,” but some have no bioactivity.14-17 For exam-
ple, a loss of only six amino acids to yield parathyroid
STRUCTURE AND ACTIONS OF
hormone (7–84) eliminates all bioactivity but does
PARATHYROID HORMONE
not affect the immunoreactivity measured in most or
Parathyroid hormone is stored and secreted main- all of these assays.10 In fact, about half of the para-
ly as an 84-amino-acid peptide.1 A synthetic amino- thyroid hormone detected with these assays in the
terminal fragment, parathyroid hormone (1–34), is serum of patients with chronic renal disease is bio-
fully active; modifications at the amino terminal, par- logically inactive.16,17
ticularly at the first two residues, can abolish its bio- Measurements of parathyroid hormone can help
characterize parathyroid tumors. Parathyroid hormone
can be measured in fluid obtained from a lesion by
From the Metabolic Diseases Branch, National Institute of Diabetes and
Digestive and Kidney Diseases, Bethesda, Md. Address reprint requests to
fine-needle aspiration (usually guided ultrasonograph-
Dr. Marx at the National Institute of Diabetes and Digestive and Kidney ically) or in serum from the veins of the neck and
Diseases, Bldg. 10, Rm. 9C-101, National Institutes of Health, 9000 mediastinum, catheterized selectively.18 Serum test re-
Rockville Pike, Bethesda, MD 20892-1802, or at StephenM@intra.niddk.
nih.gov. sults that can be obtained in 10 to 15 minutes allow
©2000, Massachusetts Medical Society. physicians to assess the completeness of the removal
Vol ume 343 Numb e r 25 · 1863
The New England Journal of Medicine
Downloaded from nejm.org on April 23, 2012. For personal use only. No other uses without permission.
Copyright © 2000 Massachusetts Medical Society. All rights reserved.
The Ne w E n g l a nd Jo u r n a l o f Me d ic i ne
Extracellular ionized 6
calcium
Renal6
tubule
Calcium-6
sensing6
Ca2+
receptor
Parathyroid cell PTH6
receptor
Ca2+
PTH Bone
Calcium-6 1,25(OH)2D
Ca2+
sensing6 Endocrine6
receptor PTHrP
mechanism6
6
Duodenal6
lumen
PTHrP Blood and other6
extracellular fluid
PTH receptor Cartilage and PTHrP6
Autocrine–paracrine6 target cells in many 6
mechanism other tissues
Figure 1. The Parathyroid Axis.
The synthesis of parathyroid hormone (PTH) and parathyroid hormone–related peptide (PTHrP) is shown on the left, and their target
sites of action are shown on the right. Both act by means of the same receptor (also termed the type 1 PTH receptor). Negative
feedback of 1,25-dihydroxyvitamin D is not shown. See the text for further descriptions. An excess or deficiency of parathyroid hor-
mone may be treated either at the level of parathyroid hormone release (and the parathyroid hormone receptors) or at selected
sites distal to the parathyroid hormone receptors. Blue arrows indicate extracellular calcium flow.
of hyperfunctioning parathyroid tissue during the The Parathyroid Gland in Primary Hyperparathyroidism
operation.19 Solitary parathyroid adenomas are monoclonal or
oligoclonal tumors.20 Similarly, in multiglandular hy-
PRIMARY HYPERPARATHYROIDISM
perparathyroidism, most of the parathyroid tumors are
Most patients with primary hyperparathyroidism monoclonal or oligoclonal,21 reflecting overgrowth
have high serum parathyroid hormone concentrations. from somatic or germ-line mutations in parathyroid-
Most also have high serum calcium concentrations, tumor precursor cells. The underlying genes that de-
and even more have high serum ionized calcium con- velop mutations in hyperparathyroidism have been
centrations. The most important diagnostic tests for identified only in a minority of tumors. They help to
this disorder are thus measurements of serum para- pinpoint the molecular pathway of oncogenesis and
thyroid hormone and ionized calcium (Fig. 2). thus help to determine possible targets for treatment
1864 · Decem b er 2 1 , 2 0 0 0
The New England Journal of Medicine
Downloaded from nejm.org on April 23, 2012. For personal use only. No other uses without permission.
Copyright © 2000 Massachusetts Medical Society. All rights reserved.
MED IC A L PROGR ES S
10,000
Serum Parathyroid Hormone (pg/ml)
1,000
Uremic6 Primary6
hyperpara-6 hyperparathyroidism
thyroidism
100
Normal
10
Primary6 Tumor6
hyperpara-6 hypercalcemia
thyroidism
1
Lower limit6
of detection
6 7 8 9 10 11 12 13 14 15
Serum Calcium (mg/dl)
Figure 2. Serum Calcium and Parathyroid Hormone Concentrations in Patients with Hypercalcemia and
Hypocalcemia Due to Various Causes.
The diagnosis of a serious mineral disorder is usually clear, as illustrated by the nonoverlapping do-
mains in the figure, but in the early stages of these disorders, the values for either serum calcium or
parathyroid hormone may overlap with the normal ranges. The following diagnoses are not shown:
familial hypocalciuric hypercalcemia (midpoint of the range for serum calcium, 11.5 mg per deciliter,
and for serum parathyroid hormone, 30 pg per milliliter); neonatal severe primary hyperparathyroid-
ism (midpoint for serum calcium, 18 mg per deciliter, and for serum parathyroid hormone, 500 pg per
milliliter); hypercalciuric hypocalcemia (midpoint for serum calcium, 7 mg per deciliter, and for serum
parathyroid hormone, 10 pg per milliliter); tertiary uremic hyperparathyroidism (midpoint for serum
calcium, 11 mg per deciliter, and for serum parathyroid hormone, 2000 pg per milliliter); tertiary hy-
perparathyroidism after renal transplantation that corrected uremia (midpoint for serum calcium, 12
mg per deciliter, and for serum parathyroid hormone, 200 pg per milliliter); and adynamic bone dis-
ease with uremia (midpoint for serum calcium, 9 mg per deciliter, and for serum parathyroid hormone,
50 pg per milliliter). To convert values for serum calcium to millimoles per liter, multiply by 0.25, and
to convert values for serum parathyroid hormone to picomoles per liter, multiply by 0.11.
(Fig. 3).22-27 As in other tumors, it is likely that two activating mutations of the cyclin D1 gene
or more genes have mutated in parathyroid adenomas, (CCND1).20,24,39 These mutations result in the over-
reflecting a stepwise development of the adenoma.28-31 expression of the protein cyclin D1, but cyclin D1
Many of the known and unknown genes that have overexpression is even more common in parathyroid
mutated in parathyroid tumors are probably tumor- adenomas without cyclin D1 mutations.40
suppressor genes; that is to say, they contribute to the The abnormal parathyroid cells in primary (and
formation of the tumor through a sequential inacti- secondary) hyperparathyroidism have deficient sen-
vation of both copies of the gene.31-34 The multiple sitivity to inhibition by calcium; this may result in part
endocrine neoplasia type 1 gene (MEN1) is a tumor- from a deficiency of calcium-sensing receptors on par-
suppressor gene and the known gene that most of- athyroid cells.41 Deficiency of these receptors is prob-
ten has somatic mutations in both copies in parathy- ably a consequence and not a cause of neoplastic pri-
roid adenomas (in 20 percent of cases).35 Since the mary hyperparathyroidism.
calcium-sensing receptor and the vitamin D receptor
also mediate the inhibition of parathyroid-gland func- Categories and Causes of Sporadic Primary
Hyperparathyroidism
tion, it is noteworthy that no inactivating mutation
of either gene has been identified in parathyroid ad- Solitary parathyroid adenomas account for 85 per-
enomas.35-38 cent of cases of primary hyperparathyroidism; hyper-
A small minority of parathyroid adenomas have function in multiple parathyroid glands (a broad cat-
Vol ume 343 Numb e r 25 · 1865
The New England Journal of Medicine
Downloaded from nejm.org on April 23, 2012. For personal use only. No other uses without permission.
Copyright © 2000 Massachusetts Medical Society. All rights reserved.
The Ne w E n g l a nd Jo u r n a l o f Me d ic i ne
Secretory6
granule
Menin
JunD
PTH
CDK 4 or 6
p53
P E2F
Cytoplasm
DNA
Cyclin3
D1
6 pRb
RET
Nucleus
G?
SHC
RRL
Calcium-sensing6 Ca2+
receptor
RR-GPI
Plasma membrane
Figure 3. Protein That Causes Parathyroid-Gland Hyperfunction When Mutated.
Mutation may occur through inheritance (germ-line mutation) or postnatally (somatic mutation) in abnormal parathyroid tissue. In-
activating mutations characterize tumor-suppressor–encoded proteins (menin [the product of the MEN1 tumor-suppressor gene],
p53, and retinoblastoma protein [pRb]), which are shown in red. Similarly, the calcium-sensing receptor is a growth suppressor
(shown in red). Activating mutations characterize proto-oncoproteins (cyclin D1 and RET) and are shown in yellow. Menin binds to
JunD, a transcription factor, which dimerizes (connects) with another member of the Jun–Fos family of transcription factors; menin
thereby inhibits transcriptional activation by JunD.22 The p53 protein binds to DNA through a specific DNA response element.23
Cyclin D1 is a cell-cycle regulator that activates the catalytic units of cyclin-dependent kinases (CDK) 4 and 6.24 One substrate for
phosphorylation (P) and thus blockade by CDK 4 or 6 is pRb,25 which binds to the transcription factor E2F as well as to several other
transcription factors.25 Black T bars indicate binding to a specific sequence of DNA. Calcium ions probably bind to the calcium-
sensing receptor in the plasma membrane, which transmits information on extracellular calcium to an unidentified guanine-nucle-
otide–binding protein (G?) in the cytoplasm.26 The RET -encoded tyrosine kinase in the plasma membrane (RET [yellow]) is a dimer
that phosphorylates Src-homology collagen (SHC) and other substrates. RET is regulated by an extracellular RET receptor attached
to the membrane by its glycosylphosphatidylinositol anchor (RR-GPI [turquoise]).27 There are at least four extracellular RET recep-
tors, each with different extracellular protein ligands (RRL). The full mechanisms by which any of these mutant proteins contribute
to tumor formation are not known. Arrows show the flow of a molecule to or away from the plasma membrane.
egory that includes hyperplasia, multiple adenomas, annual incidence of primary hyperparathyroidism
and polyclonal hyperfunction) occurs in most of the among postmenopausal women in Olmsted County,
remainder; and a few patients (less than 1 percent) Minnesota, peaked at 112 per 100,000 in 1974; the
have parathyroid carcinoma. About 75 percent of pa- high number of diagnoses in that year is attributable
tients with sporadic primary hyperparathyroidism are to the introduction of screening measurements of se-
women; the average age at diagnosis is 55 years. The rum calcium. The annual incidence then fell markedly
1866 · Decem b er 2 1 , 2 0 0 0
The New England Journal of Medicine
Downloaded from nejm.org on April 23, 2012. For personal use only. No other uses without permission.
Copyright © 2000 Massachusetts Medical Society. All rights reserved.
MED ICA L PROGR ES S
to 8 per 100,000 in 1992, a decline in diagnoses that by an inactivating germ-line mutation of a tumor-
may be due to the prior removal of tumors in pa- suppressor gene (the MEN1 gene) that is inherited
tients whose hypercalcemia was diagnosed when they as an autosomal dominant trait. Acquired or somatic
were younger.42 During a similar period, there was mutation of the second MEN1 copy can give a cell
no decline in the incidence of primary hyperparathy- the growth advantage to become a tumor.
roidism in Sweden.43
Familial Hypocalciuric Hypercalcemia
The factors associated with sporadic primary hy-
perparathyroidism include the external irradiation of Familial hypocalciuric (or benign) hypercalcemia
the neck and therapy with lithium salts.44,45 Lithium is characterized by lifelong hypercalcemia with nor-
stimulates parathyroid cells in vitro.46 Mild hyper- mal urinary calcium excretion. It is inherited as an
parathyroidism occurs in approximately 5 percent of autosomal dominant trait (Table 1).41 It is caused by
patients receiving long-term lithium therapy, and it inactivating germ-line mutations of the calcium-sens-
often persists after the therapy is discontinued.45 ing receptor, which result in an insensitivity of the
parathyroid cells to inhibition by serum calcium.41
Syndromes of Hereditary Primary Hyperparathyroidism The hypercalcemia persists after subtotal parathyroid-
Among the minority of patients with primary hy- ectomy; thus, such surgery is contraindicated. Para-
perparathyroidism caused by hyperfunction of mul- thyroid-cell hyperfunction is polyclonal and non-neo-
tiple parathyroid glands, the disorder is inherited in plastic.50 The normal urinary calcium excretion despite
about 20 percent. Any of these hereditary syndromes, hypercalcemia is an effect of the mutated calcium-
such as multiple endocrine neoplasia type 1, may sensing receptors in the kidneys.41
present as isolated hyperparathyroidism in some fam-
Neonatal Severe Primary Hyperparathyroidism
ilies.38,47,48 Each syndrome raises special issues for di-
agnosis and management (Table 1). Neonatal severe primary hyperparathyroidism is a
rare and potentially lethal disorder (Table 1). Affect-
Multiple Endocrine Neoplasia Type 1
ed neonates have a marked enlargement of all para-
Patients with multiple endocrine neoplasia type 1 thyroid glands, very high serum parathyroid hormone
have various combinations of parathyroid, enteropan- concentrations, and marked hypercalcemia (calcium
creatic, anterior pituitary, and other tumors.49 By the concentration, more than 16 mg per deciliter [4 mmol
age of 40, patients with multiple endocrine neopla- per liter]). It is usually caused by homozygous inac-
sia type 1 have endocrine disorders with the follow- tivating germ-line mutations of the calcium-sensing
ing frequencies: hyperparathyroidism in 85 percent receptor gene.41 The effects of these mutations con-
of patients, Zollinger–Ellison syndrome in 35 percent, firm the importance of the calcium-sensing receptor
prolactinoma in 25 percent, and other tumors less in the regulation of secretion and growth of parathy-
often.49 Multiple endocrine neoplasia type 1 is caused roid cells.
TABLE 1. CATEGORIES OF PRIMARY HYPERPARATHYROIDISM.*
MULTIPLE ENDOCRINE FAMILIAL HYPOCALCIURIC NEONATAL SEVERE PRIMARY
CHARACTERISTIC SPORADIC ADENOMA NEOPLASIA TYPE 1 HYPERCALCEMIA HYPERPARATHYROIDISM
Inheritance Not inherited Autosomal dominant Autosomal dominant Autosomal recessive
Age at onset of 55 yr 25 yr Birth Birth
hypercalcemia
Urinary calcium excretion Normal to high Normal to high Low to normal Low to normal
Serum parathyroid hormone High High Normal Very high
concentration
Parathyroid glands
No. abnormal One Multiple Multiple Multiple
Enlargement 20 times normal size 5 times normal size Minimally enlarged Very enlarged
Clonality Monoclonal or oligoclonal Monoclonal or oligoclonal Polyclonal Polyclonal
Effectiveness of 95% cured 90% cured, but many recur Surgery not indicated Total parathyroidectomy
parathyroidectomy required
Pathophysiology Stepwise acquired muta- Sequential inactivation of both Monoallelic inherited inacti- Biallelic inactivation of the
tions of certain genes, copies (first copy by inherit- vation of the calcium-sens- calcium-sensing receptor
such as MEN1, promote ance) of the MEN1 gene ing receptor gene decreases gene impairs calcium
the emergence of a leads to the growth of one or the sensing of serum calci- sensing in parathyroid
neoplastic clone more neoplastic clones in par- um by parathyroid cells and cells more than does
in parathyroid gland athyroid glands by renal tubules monoallelic inactivation
*All entries are typical for that disorder. Ranges are broad, with overlap (not shown) among categories.
Vol ume 343 Numb e r 25 · 1867
The New England Journal of Medicine
Downloaded from nejm.org on April 23, 2012. For personal use only. No other uses without permission.
Copyright © 2000 Massachusetts Medical Society. All rights reserved.
The Ne w E n g l a nd Jo u r n a l o f Me d ic i ne
Multiple Endocrine Neoplasia Type 2a There is some controversy about whether any of these
Multiple endocrine neoplasia type 2a is character- changes decrease life expectancy. A recent population-
ized primarily by medullary thyroid carcinoma and based study found that there was no excess mortality
pheochromocytoma.27 Primary hyperparathyroidism among all patients with hyperparathyroidism, but
can occur by the age of 70 in up to 70 percent of there was excess mortality among the patients in the
patients and is usually mild.51 Multiple endocrine neo- highest quartile for serum calcium concentrations.59
plasia type 2a is caused by an activating mutation of
the RET proto-oncogene and is inherited as an au- Effects on Bone
tosomal dominant trait.27 Parathyroid hormone increases the rate of bone
Hyperparathyroidism–Jaw Tumor Syndrome turnover, and its effects on bone may be catabolic or
anabolic, depending on the age of the patient, the
The hyperparathyroidism–jaw tumor syndrome is
skeletal site, and the pattern of serum concentrations
rare and is characterized by hyperparathyroidism, ce-
of the hormone over time.60,61 In general, persistent-
mento-ossifying fibromas of the jaw, renal cysts,
ly high serum parathyroid hormone concentrations
Wilms’ tumor, and renal hamartomas.47,52,53 By the age
have catabolic effects on bone, whereas intermittent
of 40, about 80 percent of patients with this syn-
mild increases have anabolic effects.
drome have hyperparathyroidism, and about 10 per-
On balance, the effects of mild primary hyperpara-
cent of those have a parathyroid carcinoma. Often,
thyroidism on bone seem to be slightly anabolic.62
at the first presentation of hyperparathyroidism, only
However, the disorder can cause a demineralization
one parathyroid adenoma is present, but multiple ad-
of bone, distributed variably between cortical sites (i.e.,
enomas can occur either simultaneously or at differ-
mainly long bones) and trabecular sites (i.e., mainly
ent times. The disorder is caused by a mutation in
vertebrae).63,64 Approximately one in four patients has
an unknown gene on chromosome 1q2452 and is in-
osteopenia (a z score lower than –2; “z” refers to the
herited as an autosomal dominant trait.
number of standard deviations from an age- and sex-
Manifestations of Primary Hyperparathyroidism matched mean) in cortical or trabecular bone.63,64
The parathyroids are small endocrine glands, and Overall, the risk of bone fractures in patients with
increases in their size or enhancements of their func- mild hyperparathyroidism is similar to that in matched
tion have no effect on neighboring tissues. Instead, normal subjects (one new fracture per decade); still,
the effects of an excessive secretion of parathyroid hor- the presence of hyperparathyroidism significantly in-
mone are manifested chemically as abnormal fluxes of creased the risk of fracture in several bones, particu-
calcium and phosphate in bone, in the kidneys, and larly the vertebrae, in a population-based, controlled
in the gastrointestinal tract (Fig. 1). The main results study.65 Successful parathyroidectomy is followed by
are hypercalcemia, hypercalciuria, and increased rates an increase in bone mass over a period of 6 to 12
of bone turnover. months,55,63,66 with continued increases for up to 10
Primary hyperparathyroidism is usually first sus- years after surgery.55
pected when a patient is found on biochemical screen-
Natural History and Treatment of Primary
ing to have hypercalcemia; less often it is suspected Hyperparathyroidism
because nephrolithiasis or osteopenia is present. The
anticalciuric effect of thiazide drugs can raise serum In most patients, primary hyperparathyroidism pro-
calcium concentrations slightly, thereby uncovering gresses slowly, if at all. Among asymptomatic patients,
occult hyperparathyroidism. With the current restric- only about 25 percent have progressive disease, which
tions on reimbursement for biochemical screening, is usually manifested as a decrease in bone mass dur-
the proportion of newly diagnosed cases of hyper- ing a 10-year period of follow-up.55 Thus, there has
parathyroidism that are asymptomatic should decrease. been some controversy regarding the indications for
Currently, most patients in whom hyperparathy- surgery, the only effective treatment. A consensus con-
roidism is diagnosed at first appear to be asympto- ference of the National Institutes of Health conclud-
matic,54,55 but up to half of them have subtle neuro- ed in 1990 that surgery was not routinely needed in
behavioral symptoms such as fatigue and weakness.56,57 asymptomatic patients 50 years old or older who
In many of these patients, the fact that fatigue or had a serum calcium concentration 1.0 to 1.6 mg per
weakness is a symptom of hyperparathyroidism be- deciliter (0.25 to 0.40 mmol per liter) above the up-
comes clear only after a successful parathyroidectomy, per limit of normal, a level of urinary calcium excre-
when the symptom resolves. About 20 percent of tion of less than 400 mg (100 mmol) per day, a cre-
patients with hyperparathyroidism have nephrolithi- atinine clearance of at least 70 percent of normal, or
asis.55 Primary hyperparathyroidism can cause cardi- a z score higher than –2 for bone mass.67 These are
ac calcifications and left ventricular hypertrophy; the still reasonable guidelines, but surgery may be rec-
latter can occur in the absence of hypertension and ommended for many of these patients because of the
can be partially reversed after parathyroidectomy.58 evidence that it ameliorates neurobehavioral symp-
1868 · Decem b er 2 1 , 2 0 0 0
The New England Journal of Medicine
Downloaded from nejm.org on April 23, 2012. For personal use only. No other uses without permission.
Copyright © 2000 Massachusetts Medical Society. All rights reserved.
MED IC A L PROGR ES S
toms that may be hard to detect.56,57 Surgery is not
only an effective but also a safe treatment for primary
Parotid
hyperparathyroidism, even in patients who are more
than 70 years old.68 Submandibular
Several methods for characterizing overactive par-
athyroid glands are available, including ultrasonog- Thyroid
raphy and imaging with technetium-99m sestamibi
before or during surgery (Fig. 4), but these are not
used routinely.69-71 Rapid assays for measuring serum Mediastinal6
parathyroid hormone during surgery are also available, parathyroid
as discussed above. So-called minimally invasive sur- Heart
gical methods have become possible because imaging
can be used to detect parathyroid adenomas and can
sometimes be coupled with a rapid assay of parathy-
Figure 4. Anterior Planar Image of the Neck and Chest of a Pa-
roid hormone during surgery.72 Such approaches can tient with Primary Hyperparathyroidism Obtained with Techne-
decrease the duration of the surgery, but the rate of tium-99m Sestamibi, Showing a Parathyroid Adenoma in the
success may not match that of standard parathyroid- Mediastinum.
ectomy.73,74 When patients require repeated operation, The patient had undergone an unsuccessful parathyroid explo-
every effort should be made to identify abnormal ration. The image shown was obtained two hours after the ad-
tissue preoperatively, and intraoperative testing is of- ministration of 20 mCi of the radionuclide. The lobes of the thy-
roid and the salivary glands are clearly visible. (Image courtesy
ten included as well.18,19 of Dr. Clara Chen.)
Patients who do not undergo surgery should be
evaluated clinically, and serum calcium, creatinine,
and parathyroid hormone should be measured at
6-to-12-month intervals, and cortical and trabecular
bone density at 12-month intervals. Such patients HYPERCALCEMIA MEDIATED BY
should be advised to avoid dehydration and to keep PARATHYROID HORMONE–RELATED
their calcium intake at or below 1000 mg per day. PEPTIDE
Some patients with more severe primary hyper-
parathyroidism may not undergo surgery because of Hypercalcemia is sometimes caused by serum fac-
contraindications or because they decline the proce- tors, which may be released by nonparathyroid tu-
dure; in others, surgery may have been unsuccessful. mors, whether or not there are skeletal metastases.
For these patients, several treatments directed at the Most of these tumors are malignant and secrete par-
target tissues of parathyroid hormone action are avail- athyroid hormone–related peptide.13 In contrast, hy-
able (Table 2).75 Bisphosphonates such as alendronate persecretion of parathyroid hormone by a nonparathy-
and clodronate inhibit bone resorption; however, they roid tumor is extremely rare.
may be less effective in patients with hyperparathy- UREMIC HYPERPARATHYROIDISM
roidism than in those with hypercalcemia from other
causes.76 Estrogen increases bone density in postmeno- Secondary and Tertiary Hyperparathyroidism
pausal women with hyperparathyroidism but has little Hypocalcemia from any cause stimulates parathy-
effect on serum calcium concentrations.77 A calcium- roid hormone secretion, and chronic hypocalcemia
sensing–receptor agonist acts directly on parathyroid also stimulates the growth of the parathyroid glands.
cells by way of the calcium-sensing receptor (and is This secondary hyperparathyroidism usually resolves
thus calcimimetic) in order to inhibit the secretion with the treatment of the underlying cause of hypo-
of parathyroid hormone78; the further development calcemia. However, in patients with chronic renal fail-
of drugs of this type may provide effective treatments ure, secondary hyperparathyroidism often lasts longer
for primary and secondary hyperparathyroidism.79 Pa- and is more severe than in patients with other hypo-
tients with primary hyperparathyroidism who have calcemic disorders, such as a deficiency or malabsorp-
severe symptomatic hypercalcemia should be treated tion of vitamin D.80 Eventually, either before or, more
with intravenous saline, a bisphosphonate, furosemide, often, after renal transplantation, secondary hyperpara-
and in some cases dialysis.75 thyroidism can develop into a disorder of oversecre-
Most of these treatments for primary hyperpara- tion of parathyroid hormone with hypercalcemia (ter-
thyroidism change the abnormal transfer of calcium tiary hyperparathyroidism).
from the serum to only one target tissue of parathy-
roid hormone action (Table 2 and Fig. 1). Most treat- The Parathyroid Gland in Uremia
ments for hypoparathyroidism also affect the transfer Hypercalcemia in patients with uremia who have
of calcium along only one of these pathways, albeit tertiary hyperparathyroidism might reflect an excess
in the opposite direction. of nearly normal parathyroid cells with a consequent-
Vol ume 343 Numb e r 25 · 1869
The New England Journal of Medicine
Downloaded from nejm.org on April 23, 2012. For personal use only. No other uses without permission.
Copyright © 2000 Massachusetts Medical Society. All rights reserved.
The Ne w E n g l a nd Jo u r n a l o f Me d ic i ne
TABLE 2. TREATMENTS FOR HYPERPARATHYROIDISM AND HYPOPARATHYROIDISM.
TREATMENTS FOR TREATMENTS FOR
PROCESS AFFECTED BY TREATMENT HYPERPARATHYROIDISM HYPOPARATHYROIDISM
Secretion of parathyroid hormone by parathyroid gland Parathyroidectomy Parathyroid autograft
Calcium-sensing–receptor agonist*
Activation of receptor for parathyroid hormone Blocker of type 1 receptor* Parathyroid hormone (1–34)*
Release of calcium from bone Bisphosphonates
Estrogen
Uptake of calcium from gut Blocker of vitamin D receptor* Vitamin D analogue
Calcium salts
Excretion of calcium in urine Forced natriuresis Thiazide
Exchange with extracorporeal calcium pool Dialysis Intravenous calcium
*This treatment is not currently available.
ly high and nonsuppressible base-line secretion of ease among patients with uremia is similar to the fre-
parathyroid hormone; in fact, however, it most often quency of adynamic bone diseases.86,87
reflects the secretory dysfunction of autonomously Uremic hyperparathyroid bone disease is best treat-
functioning parathyroid cells.21,80,81 Overactive para- ed by raising serum calcium concentrations and there-
thyroid glands that have been removed from patients by decreasing parathyroid hormone secretion. The
with uremia are usually overgrown with monoclonal cause of adynamic bone disease is not known, and
or oligoclonal components.22,82-84 The cause of pro- there is no specific treatment.88,89
gression from early, presumably polyclonal, secondary
Treatment of Hyperparathyroidism in Patients
hyperplasia of the parathyroid to later monoclonal or with Chronic Renal Diseases
oligoclonal tumors is poorly understood.21,80,84 Prob-
ably, some of the genes that are mutated in the par- In patients with chronic renal failure, secondary hy-
athyroid glands of patients with secondary or tertiary perparathyroidism is caused by hypocalcemia, which,
hyperparathyroidism are different from those that are in turn, is caused by hyperphosphatemia and decreased
mutated in primary hyperparathyroidism; in partic- renal production of 1,25-dihydroxyvitamin D. Treat-
ular, MEN1 mutations are less frequent in the para- ment is based on raising serum calcium concentra-
thyroid glands of patients with uremia than in tumors tions by the oral administration of calcium salts; these
of patients with sporadic primary hyperparathyroid- salts also ameliorate hyperphosphatemia by chelating
ism.82,83 phosphate in the intestines. Additional measures for
treating hypocalcemia include raising the calcium
Bone Disease in Patients with Chronic Renal Disease concentration in the dialysis fluid and administering
and Hyperparathyroidism some form of vitamin D. When treatment is initiated
Bone disease in patients with chronic renal disease early, severe secondary hyperparathyroidism can be
is caused by both hyperparathyroidism and other fac- prevented or at least delayed. There is some contro-
tors.85,86 Some patients with chronic renal disease have versy regarding the most appropriate dosage, type, and
hyperparathyroid uremic bone disease, which is char- route of administration of vitamin D or vitamin D
acterized by an activation of osteoblasts and osteo- analogue90,91 and the most appropriate phosphate
clasts with excess bone resorption. Other patients have binder for these patients.92 1,25-Dihydroxyvitamin D3
an adynamic bone disease or osteomalacia. Adynam- (calcitriol) has sometimes been given intravenously
ic bone disease is characterized by low activity of the in pulsed doses in the hope of inhibiting parathyroid
bone cells, no excess accumulation of matrix, and lit- hormone secretion without raising serum calcium con-
tle parathyroid hypersecretion.86,87 Osteomalacia in centrations,90,91 but calcitriol given orally has similar
renal failure is characterized by excess accumulation effects.93
of osteoid and a minimal degree of hyperparathy- Severe secondary hyperparathyroidism is an im-
roidism and has been associated with the accumula- portant indication for parathyroidectomy in patients
tion of aluminum in bone. This disorder has become with chronic renal disease who cannot be treated ad-
less common as a result of the minimization of use equately with the measures described above.94 Para-
of products with high concentrations of aluminum, thyroidectomy is also appropriate for some patients
such as are found in some antacids and dialysis flu- with tertiary hyperparathyroidism.
ids.86,87 The frequency of hyperparathyroid bone dis- After renal transplantation, secondary hyperpara-
1870 · Dec em b er 2 1 , 2 0 0 0
The New England Journal of Medicine
Downloaded from nejm.org on April 23, 2012. For personal use only. No other uses without permission.
Copyright © 2000 Massachusetts Medical Society. All rights reserved.
MED IC A L PROGR ES S
thyroidism usually regresses over a period of 1 to 10 diasis–ectodermal dystrophy syndrome.100 The hypo-
years, but the regression may be incomplete, as re- parathyroidism, like other manifestations of the syn-
flected in persistently high serum parathyroid hor- drome, occurs during childhood; for this reason and
mone concentrations.95 About one third of patients because of such associated abnormalities as hypoadre-
who receive renal transplants have parathyroid hor- nalism and intestinal malabsorption, the hypoparathy-
mone–induced hypercalcemia postoperatively that, roidism may be difficult to treat. The syndrome is in-
depending on its magnitude and duration, can present herited as an autosomal recessive trait and is caused by
a threat to the renal graft and to other functions. mutations in an autoimmune regulator gene (AIRE)
The hypercalcemia usually subsides within months or with a known sequence but an unknown function.101
at most a few years, but 1 to 3 percent of patients re-
Defects in the Parathyroid Hormone Molecule
quire parathyroidectomy an average of three years af-
ter renal transplantation because of persistent hyper- A few cases of familial hypoparathyroidism have
calcemia.96 been described in which the cause was a mutation in
the gene for parathyroid hormone that resulted in the
HYPOPARATHYROIDISM synthesis of a defective parathyroid hormone mole-
Hypoparathyroidism can cause hypocalcemia with cule and undetectable amounts of parathyroid hor-
consequent paresthesias, muscle spasms (i.e., tetany), mone in serum.102
and seizures, especially when it occurs rapidly. In con-
Defective Regulation of Parathyroid Hormone Secretion
trast, chronic hypoparathyroidism generally causes hy-
pocalcemia so gradually that the only symptom may Hypocalcemia and hypercalciuria are the chief fea-
be visual impairment from cataracts caused by years tures of autosomal dominant hypercalciuric hypocal-
of hypoparathyroidism. cemia, which is caused by activating mutations of the
parathyroid and renal calcium-sensing receptor. These
Diagnosis and Causes mutations cause excessive calcium-induced inhibition
Like hyperparathyroidism, hypoparathyroidism is of parathyroid hormone secretion. The hypocalcemia
diagnosed on the basis of measurements of serum is usually mild and asymptomatic. When it is mild,
calcium and parathyroid hormone (Fig. 2).14,15 The it should be treated cautiously, if at all, because rais-
causes of hypoparathyroidism are diverse, represent- ing serum calcium concentrations further increases
ing disruptions of one or more of the steps in the urinary calcium excretion and may cause nephrocal-
development and maintenance of parathyroid hor- cinosis.41,103
mone secretion. TREATMENT OF HYPOPARATHYROIDISM
Damage to the Parathyroid Glands from Surgery Calcium and Vitamin D Analogues
Injury to or removal of the parathyroid glands dur- The main treatments available for patients with
ing neck surgery is the most common cause of acute acute or chronic hypoparathyroidism are calcium salts,
or chronic hypoparathyroidism. vitamin D or vitamin D analogues, and drugs that
increase renal tubular reabsorption of calcium (i.e., thi-
Developmental Defects in the Parathyroid Glands
azides) (Table 2). The parathyroid hormone–depend-
Agenesis of the parathyroid glands occurs in in- ent renal production of 1,25-dihydroxyvitamin D is
fants with the DiGeorge syndrome (and the closely deficient in all hypoparathyroid states. Therefore, ther-
related velocardiofacial syndrome). The manifestations apy with a vitamin D analogue is used to ensure that
of these syndromes include incomplete development there is a steady serum concentration of an active vi-
in the branchial arches, resulting in varying degrees tamin D analogue. If parathyroid hormone is absent
of parathyroid and thymic hypoplasia, conotruncal or nonfunctional, its hypocalciuric action cannot oc-
cardiac defects, facial malformations, and learning dis- cur; therefore, raising the serum calcium concentra-
ability. Both syndromes are associated with rearrange- tion may cause hypercalciuria, nephrolithiasis, and
ments and microdeletions affecting an unknown gene renal damage.
or genes on the short arm of chromosome 22.97 Any Patients in whom hypocalcemia develops suddenly
resultant defect should be treated, depending on its — for example, after neck surgery — are best treated
severity.98 Isolated agenesis of the parathyroid glands with intravenous calcium and with oral or intravenous
in one family has been attributed to a recessive de- calcitriol. Those with chronic hypocalcemia should
letion in the gene on chromosome 6 that normally be treated with oral calcium and either calcitriol or
encodes a transcription factor.99 vitamin D. Patients in whom the efficacy of treatment
may vary, such as those with autoimmune polyglan-
Autoimmune Hypoparathyroidism
dular syndrome type 1, are best treated with vitamin D
Hypoparathyroidism is a prominent component analogues that have a short half-life. Calcitriol raises
of autoimmune polyglandular syndrome type 1, also serum calcium concentrations within one or two
known as autoimmune polyendocrinopathy–candi- days after treatment begins, and its action dissipates
Vol ume 343 Numb e r 25 · 1871
The New England Journal of Medicine
Downloaded from nejm.org on April 23, 2012. For personal use only. No other uses without permission.
Copyright © 2000 Massachusetts Medical Society. All rights reserved.
The Ne w E n g l a nd Jo u r n a l o f Me d ic i ne
equally rapidly; the action of vitamin D begins and ited as an autosomal recessive trait. The growth plates
dissipates over a period of two to four weeks. show accelerated calcification and a near-absence of
proliferating chondrocytes.
Parathyroid-Tissue Transplantation or Parathyroid
Hormone Defects of the Stimulatory Guanine-Nucleotide–Binding
Protein
Transplantation of parathyroid tissue is appealing
but rarely possible. A parathyroid allograft would re- Parathyroid hormone signaling in cells is mediated
quire immunosuppression and so would be more dan- by the type 1 parathyroid hormone receptor, which
gerous than the disease it was meant to treat. Parathy- then acts on a stimulatory guanine-nucleotide–bind-
roid autografts are sometimes placed in the forearm ing (Gs) protein, which is composed of three sub-
and can consist of either fresh parathyroid tissue or units (a, b, and g). The Gsa subunit (encoded by the
parathyroid tissue removed earlier and cryopreserved. GNAS1 gene) mediates cyclic AMP stimulation by
The indication for a parathyroid autograft is a high parathyroid hormone and by several other peptide
likelihood of postoperative hypoparathyroidism. As hormones, including thyrotropin.26
many as half of these grafts fail, and among those
Pseudohypoparathyroidism Type 1a
that survive and function, the potential for late auto-
graft-mediated recurrences of hyperparathyroidism Pseudohypoparathyroidism type 1a is characterized
is substantial, since the parathyroid tissue used for by short stature and other skeletal abnormalities, which
the graft was abnormal.84,94 are known collectively as Albright’s hereditary osteo-
Patients with hypoparathyroidism have been treated dystrophy, as well as hypocalcemia and high serum
successfully with synthetic human parathyroid hor- concentrations of parathyroid hormone. It is caused
mone (1–34) given subcutaneously once daily.104 The by inactivating mutations in the a subunit of Gs 26 and
increase in urinary calcium excretion in these patients is inherited as an autosomal dominant trait. Many pa-
was smaller than that which occurs in patients treat- tients with pseudohypoparathyroidism type 1a have
ed with calcium and calcitriol or vitamin D. Howev- resistance not only to parathyroid hormone but also
er, synthetic human parathyroid hormone (1–34) is to thyrotropin.
not currently available.
Pseudo-pseudohypoparathyroidism
GENETIC DISORDERS OF PARATHYROID Pseudo-pseudohypoparathyroidism occurs in fam-
HORMONE ACTION ilies with pseudohypoparathyroidism type 1a. It con-
Hereditary defects in parathyroid hormone action sists of a combination of inactivating mutations of
are rare but informative. Each confirms the role of an GNAS1 and Albright’s osteodystrophy without the
important signaling molecule. To some extent, these resistance to multiple hormones that characterizes
states mimic disorders of parathyroid hormone excess pseudohypoparathyroidism. The hormone resistance
or deficiency. is suppressed when the mutated GNAS1 gene is in-
herited from the father (i.e., paternal imprinting, or
Defects of the Type 1 Parathyroid Hormone Receptor suppression, of the mutant copy occurs in selected
Two defects with opposite effects on the type 1 tissues).107,108
parathyroid hormone receptor have a surprisingly sim-
Pseudohypoparathyroidism Type 1b
ilar effect on bone growth.11,13
Pseudohypoparathyroidism type 1b is characterized
Jansen’s Chondrodystrophy by isolated resistance to parathyroid hormone with-
Jansen’s chondrodystrophy is characterized by short out the accompanying Albright’s osteodystrophy. It is
limbs, mild hypercalcemia, and low serum parathyroid associated with defective methylation within GNAS1,
hormone concentrations. It is caused by activating mu- which is most likely caused by a mutation in or near
tations of the type 1 parathyroid hormone receptor105 GNAS1.109
and is inherited as an autosomal dominant trait. It is Hypocalcemia in patients with pseudohypoparathy-
associated with increased proliferation and delayed roidism type 1a or 1b should be treated in the same
maturation of chondrocytes, which may weaken the way as it is in patients with true hypoparathyroidism.
growth plates, thereby causing the short limbs.
CONCLUSIONS
Blomstrand’s Chondrodystrophy
Despite a confusing disease nomenclature that is
Blomstrand’s chondrodystrophy is characterized by a remnant of past eras, substantial insight has been
growth impairment, primarily in the form of short gained into many disorders of the parathyroid axis.
limbs. It has been lethal prenatally, and therefore the With the advent of reliable and specific assays for par-
regulation of serum calcium has not been evaluated athyroid hormone, the diagnosis of parathyroid dys-
in vivo. It is caused by inactivating mutations of the function has become much easier. Treatments are gen-
type 1 parathyroid hormone receptor106 and is inher- erally satisfactory and are logically related to the defects
1872 · Dec em b er 2 1 , 2 0 0 0
The New England Journal of Medicine
Downloaded from nejm.org on April 23, 2012. For personal use only. No other uses without permission.
Copyright © 2000 Massachusetts Medical Society. All rights reserved.
MED IC A L PROGR ES S
in the parathyroid gland or to their expression in the AP1 transcription factor JunD and represses JunD-activated transcription.
Cell 1999;96:143-52.
target organs. Controversies persist, however, particu- 23. Malkin D. Li-Fraumeni syndrome. In: Scriver CR, Beaudet A, Sly WS,
larly about the treatment of primary hyperparathy- Valle D, Vogelstein B, eds. The metabolic and molecular bases of inherited
roidism. disease. 8th ed. Vol. X. New York: McGraw-Hill (in press).
24. Pestell RG, Albanese C, Reutens AT, Segall JE, Lee RJ, Arnold A. The
cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of
REFERENCES proliferation and differentiation. Endocr Rev 1999;20:501-34.
25. Masciullo V, Khalili K, Giordano A. The Rb family of cell cycle regu-
1. Jüppner H, Gardella TJ, Brown EM, Kronenberg HM, Potts JT Jr. Par- latory factors: clinical implications. Int J Oncol 2000;17:897-902.
athyroid hormone and parathyroid hormone-related peptide in the regula- 26. Spiegel AM. Mutations in G proteins and G protein-coupled receptors
tion of calcium homeostasis and bone development. In: DeGroot LJ, ed. in endocrine disease. J Clin Endocrinol Metab 1996;81:2434-42.
Endocrinology. 4th ed. Philadelphia: W.B. Saunders (in press). 27. Hoff AO, Cote GJ, Gagel RF. Multiple endocrine neoplasias. Annu
2. Brown EM, Vassilev PM, Quinn S, Hebert SC. G-protein-coupled, ex- Rev Physiol 2000;62:377-411.
tracellular Ca2+-sensing receptor: a versatile regulator of diverse cellular 28. Tahara H, Smith AP, Gaz RD, Cryns VL, Arnold A. Genomic local-
functions. Vitam Horm 1999;55:1-71. ization of novel candidate tumor suppressor gene loci in human parathy-
3. Marchese A, George SR, Kolakowski LF, Lynch KR, O’Dowd BF. Nov- roid adenomas. Cancer Res 1996;56:599-605.
el GPCRs and their endogenous ligands: expanding the boundaries of 29. Farnebo F, Kytola S, Teh BT, et al. Alternative genetic pathways in par-
physiology and pharmacology. Trends Pharmacol Sci 1999;20:370-5. athyroid tumorigenesis. J Clin Endocrinol Metab 1999;84:3775-80.
[Erratum, Trends Pharmacol Sci 1999;20:447.] 30. Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer.
4. Demay MB, Kiernan MS, DeLuca HF, Kronenberg HM. Sequences in Cell 1996;87:159-70.
the human parathyroid hormone gene that bind the 1,25-dihydroxyvitamin 31. Knudson AG. Hereditary cancer: two hits revisited. J Cancer Res Clin
D3 receptor and mediate transcriptional repression in response to 1,25- Oncol 1996;122:135-40.
dihydroxyvitamin D3. Proc Natl Acad Sci U S A 1992;89:8097-101. 32. Cryns VL, Thor A, Xu H-J, et al. Loss of the retinoblastoma tumor-
5. Moallem E, Kilav R, Silver J, Naveh-Many T. RNA-protein binding and suppressor gene in parathyroid carcinoma. N Engl J Med 1994;330:757-
post-transcriptional regulation of parathyroid hormone gene expression by 61.
calcium and phosphate. J Biol Chem 1998;273:5253-9. 33. Cryns VL, Rubio MP, Thor AD, Louis DN, Arnold A. p53 Abnor-
6. Chung U, Igarashi T, Nishishita T, et al. The interaction between Ku malities in human parathyroid carcinoma. J Clin Endocrinol Metab 1994;
antigen and REF1 protein mediates negative gene regulation by extracel- 78:1320-4.
lular calcium. J Biol Chem 1996;271:8593-8. 34. Agarwal SK, Schrock E, Kester MB, et al. Comparative genome hy-
7. Naveh-Many T, Rahamimov R, Livni N, Silver J. Parathyroid cell pro- bridization analysis of human parathyroid tumors. Cancer Genet Cytogen-
liferation in normal and chronic renal failure rats: the effects of calcium, et 1998;106:30-6.
phosphate, and vitamin D. J Clin Invest 1995;96:1786-93. 35. Heppner C, Kester MB, Agarwal SK, et al. Somatic mutation of the
8. Li YC, Amling M, Pirro AE, et al. Normalization of mineral ion ho- MEN1 gene in parathyroid tumours. Nat Genet 1997;16:375-8.
meostasis by dietary means prevents hyperparathyroidism, rickets, and os- 36. Hosokawa Y, Pollak MR, Brown EM, Arnold A. Mutational analysis
teomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocri- of the extracellular Ca(2+)-sensing receptor gene in human parathyroid
nology 1998;139:4391-6. tumors. J Clin Endocrinol Metab 1995;80:3107-10.
9. Malloy PJ, Pike JW, Feldman D. The vitamin D receptor and the syn- 37. Brown SB, Brierley TT, Palanisamy N, et al. Vitamin D receptor as a
drome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr Rev candidate tumor-suppressor gene in severe hyperparathyroidism of uremia.
1999;20:156-88. J Clin Endocrinol Metab 2000;85:868-72.
10. Chorev M, Alexander JM, Rosenblatt M. Interactions of parathyroid 38. Carling T, Szabo E, Bai M, et al. Familial hypercalcemia and hypercal-
hormone and parathyroid homone–related protein with their receptors. In: ciuria caused by a novel mutation in the cytoplasmic tail of the calcium re-
Bilezikian JP, Levine MA, Marcus R, eds. The parathyroids: basic and clin- ceptor. J Clin Endocrinol Metab 2000;85:2042-7.
ical concepts. 2nd ed. San Diego, Calif.: Academic Press (in press). 39. Motokura T, Bloom T, Kim HG, et al. A novel cyclin encoded by a
11. Juppner H. Receptors for parathyroid hormone and parathyroid hor- bcl1-linked candidate oncogene. Nature 1991;350:512-5.
mone-related peptide: exploration of their biological importance. Bone 40. Hsi ED, Zukerberg LF, Yang WI, Arnold A. Cyclin D1/PRAD1 ex-
1999;25:87-90. pression in parathyroid adenomas: an immunohistochemical study. J Clin
12. Usdin TB, Hoare SRJ, Wang T, Mezey E, Kowalak JA. TIP39: a new Endocrinol Metab 1996;81:1736-9.
neuropeptide and PTH2-receptor agonist from hypothalamus. Nat Neuro- 41. Brown EM, Pollak M, Hebert SC. The extracellular calcium-sensing
sci 1999;2:941-3. receptor: its role in health and disease. Annu Rev Med 1998;49:15-29.
13. Strewler GJ. The physiology of parathyroid hormone–related protein. 42. Wermers RA, Khosla S, Atkinson EJ, Hodgson SF, O’Fallon WM,
N Engl J Med 2000;342:177-85. Melton LJ III. The rise and fall of primary hyperparathyroidism: a popu-
14. Kao PC, van Heerden JA, Grant CS, Klee GG, Khosla S. Clinical per- lation-based study in Rochester, Minnesota, 1965-1992. Ann Intern Med
formance of parathyroid hormone immunometric assays. Mayo Clin Proc 1997;126:433-40.
1992;67:637-45. 43. Lundgren E, Rastad J, Thurfjell E, Akerstrom G, Ljunghall S. Popu-
15. Michelangeli VP, Heyma P, Colman PG, Ebeling PR. Evaluation of a lation-based screening for primary hyperparathyroidism with serum calci-
new, rapid and automated immunochemiluminometric assay for the meas- um and parathyroid hormone values in menopausal women. Surgery 1997;
urement of serum intact parathyroid hormone. Ann Clin Biochem 1997; 121:287-94.
34:97-103. 44. Schneider AB, Gierlowski TC, Shore-Freedman E, Stovall M, Ron E,
16. Lepage R, Roy L, Brossard JH, et al. A non-(1-84) circulating para- Lubin J. Dose-response relationships for radiation-induced hyperparathy-
thyroid hormone (PTH) fragment interferes significantly with intact PTH roidism. J Clin Endocrinol Metab 1995;80:254-7.
commercial assay measurements in uremic samples. Clin Chem 1998;44: 45. Bendz H, Sjodin I, Toss G, Berglund K. Hyperparathyroidism and
805-9. long-term lithium therapy — a cross-sectional study and the effect of lith-
17. John MR, Goodman WG, Gao P, Cantor TL, Salusky IB, Juppner H. ium withdrawal. J Intern Med 1996;240:357-65.
A novel immunoradiometric assay detects full-length human PTH but not 46. Brown EM. Lithium induces abnormal calcium-regulated PTH release
amino-terminally truncated fragments: implications for PTH measure- in dispersed bovine parathyroid cells. J Clin Endocrinol Metab 1981;52:
ments in renal failure. J Clin Endocrinol Metab 1999;84:4287-90. 1046-8.
18. Jaskowiak N, Norton JA, Alexander HR, et al. A prospective trial eval- 47. Teh BT, Farnebo F, Twigg S, et al. Familial isolated hyperparathyroid-
uating a standard approach to reoperation for missed parathyroid adenoma. ism maps to the hyperparathyroidism-jaw tumor locus in 1q21-q32 in a
Ann Surg 1996;224:308-20. subset of families. J Clin Endocrinol Metab 1998;83:2114-20.
19. Irvin GL III, Molinari AS, Figueroa C, Carneiro DM. Improved suc- 48. Kassem M, Kruse TA, Wong FK, Larsson C, Teh BT. Familial isolated
cess rate in reoperative parathyroidectomy with intraoperative PTH assay. hyperparathyroidism as a variant of multiple endocrine neoplasia type 1 in
Ann Surg 1999;229:874-8. a large Danish pedigree. J Clin Endocrinol Metab 2000;85:165-7.
20. Arnold A. Molecular basis of primary hyperparathyroidism. In: Bile- 49. Marx SJ, Spiegel AM, Skarulis MC, Doppman JL, Collins FS, Liotta
zikian JP, Levine MA, Marcus R, eds. The parathyroids: basic and clinical LA. Multiple endocrine neoplasia type 1: clinical and genetic topics. Ann
concepts. 2nd ed. San Diego, Calif.: Academic Press (in press). Intern Med 1998;129:484-94.
21. Arnold A, Brown MF, Urena P, Gaz RD, Sarfati E, Drueke TB. Mono- 50. Marx SJ. Contrasting paradigms for hereditary hyperfunction of endo-
clonality of parathyroid tumors in chronic renal failure and in primary par- crine cells. J Clin Endocrinol Metab 1999;84:3001-9.
athyroid hyperplasia. J Clin Invest 1995;95:2047-53. 51. Schuffenecker I, Virally-Monod M, Brohet R, et al. Risk and pene-
22. Agarwal SK, Guru SC, Heppner C, et al. Menin interacts with the trance of primary hyperparathyroidism in multiple endocrine neoplasia
Vol ume 343 Numb e r 25 · 1873
The New England Journal of Medicine
Downloaded from nejm.org on April 23, 2012. For personal use only. No other uses without permission.
Copyright © 2000 Massachusetts Medical Society. All rights reserved.
The Ne w E n g l a nd Jo u r n a l o f Me d ic i ne
type 2A families with mutations at codon 634 of the RET proto-oncogene. mone replacement therapy on bone mineral density in postmenopausal
J Clin Endocrinol Metab 1998;83:487-91. women with mild primary hyperparathyroidism: a randomized, controlled
52. Szabo J, Heath B, Hill VM, et al. Hereditary hyperparathyroidism-jaw trial. Ann Intern Med 1996;125:360-8.
tumor syndrome: the endocrine tumor gene HRPT2 maps to chromosome 78. Silverberg SJ, Bone HG III, Marriott TB, et al. Short-term inhibition
1q21-q31. Am J Hum Genet 1995;56:944-50. of parathyroid hormone secretion by a calcium-receptor agonist in patients
53. Williamson C, Cavaco BM, Jauch A, et al. Mapping the gene causing with primary hyperparathyroidism. N Engl J Med 1997;337:1506-10.
hereditary primary hyperparathyroidism in a Portuguese kindred to chro- 79. Antonsen JE, Sherrard DJ, Andress DL. A calcimimetic agent acutely
mosome 1q22-q31. J Bone Miner Res 1999;14:230-9. [Erratum, J Bone suppresses parathyroid hormone levels in patients with chronic renal fail-
Miner Res 1999;14:1472.] ure: rapid communication. Kidney Int 1998;53:223-7.
54. Bilezikian JP, Silverberg SJ, Gartenberg F, et al. Clinical presentation 80. Parfitt AM. The hyperparathyroidism of chronic renal failure: a disor-
of primary hyperparathyroidism. In: Bilezikian JP, Levine MA, Marcus R, der of growth. Kidney Int 1997;52:3-9.
eds. The parathyroids: basic and clinical concepts. 2nd ed. San Diego, Cal- 81. Malberti F, Farina M, Imbasciati E. The PTH-calcium curve and the
if.: Academic Press (in press). set point of calcium in primary and secondary hyperparathyroidism. Neph-
55. Silverberg SJ, Shane E, Jacobs TP, Siris E, Bilezikian JP. A 10-year pro- rol Dial Transplant 1999;14:2398-406.
spective study of primary hyperparathyroidism with or without parathyroid 82. Shan L, Nakamura Y, Murakami M, et al. Clonal emergence in uremic
surgery. N Engl J Med 1999;341:1249-55. [Erratum, N Engl J Med 2000; parathyroid hyperplasia is not related to MEN1 gene abnormality. Jpn J
342:144.] Cancer Res 1999;90:965-9.
56. Chan AK, Duh QY, Katz MH, Siperstein AE, Clark OH. Clinical 83. Imanishi Y, Tahara H, Salusky I, et al. MEN1 gene mutations in re-
manifestations of primary hyperparathyroidism before and after parathy- fractory hyperparathyroidism of uremia. J Bone Miner Res 1999;14:Suppl
roidectomy: a case-control study. Ann Surg 1995;222:402-14. 1:S446. abstract.
57. Burney RE, Jones KR, Christy B, Thompson NW. Health status im- 84. Horandner HH, Neyer U, Gruber U, et al. Pathomorphology of par-
provement after surgical correction of primary hyperparathyroidism in pa- athyroid autografts. Nieren Hochdruckkrankheiten 1997;26:319-27.
tients with high and low preoperative calcium levels. Surgery 1999;125: 85. Solal M-EC, Sebert J-L, Boudailliez B, et al. Comparison of intact,
608-14. midregion, and carboxy terminal assays of parathyroid hormone for the di-
58. Steffenelli T, Abela C, Frank H, et al. Cardiac abnormalities in patients agnosis of bone disease in hemodialyzed patients. J Clin Endocrinol Metab
with primary hyperparathyroidism: implications for follow-up. J Clin En- 1991;73:516-24.
docrinol Metab 1997;82:106-12. 86. Sherrard DJ, Hercz G, Pei Y, et al. The spectrum of bone disease in
59. Wermers RA, Khosla S, Atkinson EJ, et al. Survival after diagnosis of end-stage renal failure — an evolving disorder. Kidney Int 1993;43:436-
hyperparathyroidism: a population-based study. Am J Med 1998;104:115- 42.
22. 87. Mucsi I, Hercz G. Relative hypoparathyroidism and adynamic bone
60. Ishizuya T, Yokose S, Hori M, et al. Parathyroid hormone exerts dis- disease. Am J Med Sci 1999;317:405-9.
parate effects on osteoblastic differentiation depending on exposure time 88. Wang M, Hercz G, Sherrard DJ, Maloney NA, Segre GV, Pei Y. Re-
in rat osteoblastic cells. J Clin Invest 1997;99:2961-70. lationship between intact 1-84 parathyroid hormone and bone histomor-
61. Schiller PC, D’Ippolito G, Roos BA, Howard GA. Anabolic or cata- phometric parameters in dialysis patients without aluminum toxicity. Am J
bolic responses of MC3T3-E1 osteoblastic cells to parathyroid hormone Kidney Dis 1995;26:836-44.
depend on time and duration of treatment. J Bone Miner Res 1999;14: 89. Hampl H, Steinmuller T, Frohling P, et al. Long-term results of total
1504-12. parathyroidectomy without autotransplantation in patients with and with-
62. Dempster DW, Parisien M, Silverberg SJ, et al. On the mechanism of out renal failure. Miner Electrolyte Metab 1999;25:161-70.
cancellous bone preservation in postmenopausal women with mild primary 90. Hasnain M, Hauncher C, Pegoraro AA, Arruda JAL, Dunea G. Sup-
hyperparathyroidism. J Clin Endocrinol Metab 1999;84:1562-6. pression of hyperparathyroidism by calcitriol therapy. ASAIO J 1999;45:
63. Abdelhadi M, Nordenstrom J. Bone mineral recovery after parathy- 424-7.
roidectomy in patients with primary and renal hyperparathyroidism. J Clin 91. Bacchini G, Fabrizi F, Pontoriero G, Marcelli D, Filippo SD, Locatelli
Endocrinol Metab 1998;83:3845-51. F. ‘Pulse oral’ versus intravenous calcitriol therapy in chronic hemodialysis
64. Silverberg SJ, Locker FG, Bilezikian JP. Vertebral osteopenia: a new patients: a prospective and randomized study. Nephron 1997;77:267-72.
indication for surgery in primary hyperparathyroidism. J Clin Endocrinol [Erratum, Nephron 1998;79:509.]
Metab 1996;81:4007-12. 92. Loghman-Adham M. Phosphate binders for control of phosphate re-
65. Khosla S, Melton LJ III, Wermers RA, Crowson CS, O’Fallon WM, tention in chronic renal failure. Pediatr Nephrol 1999;13:701-8.
Riggs BL. Primary hyperparathyroidism and the risk of fracture: a popula- 93. Indridason OS, Quarles LD. Comparison of treatments for mild sec-
tion-based study. J Bone Miner Res 1999;14:1700-7. ondary hyperparathyroidism in hemodialysis patients. Kidney Int 2000;57:
66. Christiansen P, Steiniche T, Brixen K, et al. Primary hyperparathyroid- 282-92.
ism: effect of parathyroidectomy on regional bone mineral density in Dan- 94. Tominaga Y. Surgical management of secondary hyperparathyroidism
ish patients: a three-year follow-up study. Bone 1999;25:589-95. in uremia. Am J Med Sci 1999;317:390-7.
67. NIH conference: diagnosis and management of asymptomatic primary 95. Torres A, Rodriguez AP, Concepcion MT, et al. Parathyroid function
hyperparathyroidism: Consensus Development Conference statement. Ann in long-term renal transplant patients: importance of pre-transplant PTH
Intern Med 1991;114:593-7. concentrations. Nephrol Dial Transplant 1998;13:Suppl 3:94-7.
68. Chen H, Parkerson S, Udelsman R. Parathyroidectomy in the elderly: 96. Kerby JD, Rue LW, Blair H, Hudson S, Sellers MT, Diethelm AG. Op-
do the benefits outweigh the risks? World J Surg 1998;22:531-6. erative treatment of tertiary hyperparathyroidism: a single-center experi-
69. Pattou F, Torres G, Mondragon-Sanchez A, et al. Correlation of par- ence. Ann Surg 1998;227:878-86.
athyroid scanning and anatomy in 261 unselected patients with sporadic 97. Novelli A, Sabani M, Caiola A, et al. Diagnosis of DiGeorge and Wil-
primary hyperparathyroidism. Surgery 1999;126:1123-31. liams syndromes using FISH analysis of peripheral blood smears. Mol Cell
70. Wei JP, Burke GJ. Cost utility of routine imaging with Tc-99m-sesta- Probes 1999;13:303-7.
mibi in primary hyperparathyroidism before initial surgery. Am Surg 1997; 98. Markert ML, Boeck A, Hale LP, et al. Transplantation of thymus tissue
63:1097-100. in complete DiGeorge syndrome. N Engl J Med 1999;341:1180-9.
71. Shawker TH, Avila N, Premkumar A, Bradford MH, Doppman JL. 99. Ding C, Buckingham B, Levine MA. Neonatal hypoparathyroidism at-
Ultrasound evaluation of primary hyperparathyroidism. Ultrasound Q tributable to homozygous partial deletion of the human glial cell missing
2000;16:73-87. gene-B. In: Program and abstracts of the 82nd Annual Meeting of the En-
72. Norman J, Chheda H, Farrell C. Minimally invasive parathyroidectomy docrine Society, Toronto, June 21–24, 2000. Bethesda, Md.: Endocrine
for primary hyperparathyroidism: decreasing operative time and potential Society Press, 2000:409. abstract.
complications while improving cosmetic results. Am Surg 1998;64:391-5. 100. Ahonen P, Myllärniemi S, Sipilä I, Perheentupa J. Clinical variation
73. Ryan JA Jr, Lee F. Effectiveness and safety of 100 consecutive parathy- of autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy
roidectomies. Am J Surg 1997;173:441-4. (APECED) in a series of 68 patients. N Engl J Med 1990;322:1829-36.
74. Low RA, Katz AD. Parathyroidectomy via bilateral cervical explora- 101. Bjorses P, Halonen M, Palvimo JJ, et al. Mutations in the AIRE gene:
tion: a retrospective review of 866 cases. Head Neck 1998;20:583-7. effects on subcellular location and transactivation function of the autoim-
75. Stock JL, Marcus R. Medical management of primary hyperparathy- mune polyendocrinopathy-candidiasis-ectodermal dystrophy protein. Am J
roidism. In: Bilezikian JP, Levine MA, Marcus R, eds. The parathyroids: Hum Genet 2000;66:378-92.
basic and clinical concepts. 2nd ed. San Diego, Calif.: Academic Press (in 102. Sunthornthepvarakul T, Churesigaew S, Ngowngarmratana S. A novel
press). mutation of the signal peptide of the preproparathyroid hormone gene as-
76. Brown DL, Robbins R. Developments in the therapeutic applications sociated with autosomal recessive familial isolated hypoparathyroidism.
of bisphosphonates. J Clin Pharmacol 1999;39:651-60. J Clin Endocrinol Metab 1999;84:3792-6.
77. Grey AB, Stapleton JP, Evans MC, Tatnell MA, Reid IR. Effect of hor- 103. Pearce SHS, Williamson C, Kifor O, et al. A familial syndrome of hy-
1874 · Dec em b er 2 1 , 2 0 0 0
The New England Journal of Medicine
Downloaded from nejm.org on April 23, 2012. For personal use only. No other uses without permission.
Copyright © 2000 Massachusetts Medical Society. All rights reserved.
MED IC A L PROGR ES S
pocalcemia with hypercalciuria due to mutations in the calcium-sensing re- peptide receptor causing Blomstrand chondrodysplasia. J Clin Endocrinol
ceptor. N Engl J Med 1996;335:1115-22. Metab 1998;83:3365-8.
104. Winer KK, Yanovski JA, Cutler GB Jr. Synthetic human parathyroid 107. Davies SJ, Hughes HE. Imprinting in Albright’s hereditary osteodys-
hormone 1-34 vs calcitriol and calcium in the treatment of hypoparathy- trophy. J Med Genet 1993;30:101-3.
roidism. JAMA 1996;276:631-6. 108. Yu SH, Yu DW, Lee E, et al. Variable and tissue-specific hormone
105. Schipani E, Langman CB, Parfitt AM, et al. Constitutively activated resistance in heterotrimeric Gs protein alpha subunit (Gsalpha) knockout
receptors for parathyroid hormone and parathyroid hormone–related pep- mice is due to tissue-specific imprinting of the Gsalpha gene. Proc Natl
tide in Jansen’s metaphyseal chondrodysplasia. N Engl J Med 1996;335: Acad Sci U S A 1998;95:8715-20.
708-14. 109. Liu J, Litman D, Rosenberg MJ, Yu S, Biesecker LG, Weinstein LS.
106. Zhang P, Jobert AS, Couvineau A, Silve C. A homozygous inactivat- A GNAS1 imprinting defect in pseudohypoparathyroidism type IB. J Clin
ing mutation in the parathyroid hormone/parathyroid hormone-related Invest 2000;106:1167-74.
Vol ume 343 Numb e r 25 · 1875
The New England Journal of Medicine
Downloaded from nejm.org on April 23, 2012. For personal use only. No other uses without permission.
Copyright © 2000 Massachusetts Medical Society. All rights reserved.
Вам также может понравиться
- Hiper Hipoparatir NejmДокумент13 страницHiper Hipoparatir NejmPriscila Tobar AlcántarОценок пока нет
- Parathyroid Gland LessonДокумент3 страницыParathyroid Gland LessonCherry Ann ColechaОценок пока нет
- The Porphyria Advances in Diagnosis and TreatmeДокумент9 страницThe Porphyria Advances in Diagnosis and TreatmegemaОценок пока нет
- Autacoid - SerotoninДокумент4 страницыAutacoid - SerotoninJohn Ellard M. SaturnoОценок пока нет
- Hormone Replacement in Men PDFДокумент1 страницаHormone Replacement in Men PDFPanser CueОценок пока нет
- Autacoid - SerotoninДокумент4 страницыAutacoid - SerotoninJohn Ellard M. SaturnoОценок пока нет
- Pubmed HecidinaДокумент9 страницPubmed HecidinaeddcitoОценок пока нет
- PIIS0022316622026001Документ9 страницPIIS0022316622026001asagdaevОценок пока нет
- Physiology, Parathyroid Hormone (PTH) : Statpearls (Internet)Документ10 страницPhysiology, Parathyroid Hormone (PTH) : Statpearls (Internet)chafeb febiОценок пока нет
- The Parathyroid GlandsДокумент7 страницThe Parathyroid GlandsRuth AlooОценок пока нет
- Partial Loss-of-Function Mutations in The Steroidogenic Acute Regulatory Clinical, Genetic, and Functional Characterization of Four Patients CarryingДокумент9 страницPartial Loss-of-Function Mutations in The Steroidogenic Acute Regulatory Clinical, Genetic, and Functional Characterization of Four Patients CarryingGokhan SahinОценок пока нет
- Hormones Classification and FunctionsДокумент15 страницHormones Classification and FunctionsMIbrahimОценок пока нет
- Kuliah Keempat (Thyroid and Parathyroid Glands)Документ38 страницKuliah Keempat (Thyroid and Parathyroid Glands)kiki rawitriОценок пока нет
- Endocrine Physiology OverviewДокумент166 страницEndocrine Physiology OverviewdrpnnreddyОценок пока нет
- B. Hormones, General Principles by D SiwaleДокумент73 страницыB. Hormones, General Principles by D SiwaleDonald SiwaleОценок пока нет
- Molecular Mechanism of Phenylhydrazine Induced Haematotoxicity: A ReviewДокумент5 страницMolecular Mechanism of Phenylhydrazine Induced Haematotoxicity: A ReviewJean FlorencondiaОценок пока нет
- 5 Parathyroid Hormone5Документ58 страниц5 Parathyroid Hormone5Rawbeena RamtelОценок пока нет
- PHP2018Документ25 страницPHP2018radu nicolaeОценок пока нет
- tmp9CE3 TMPДокумент10 страницtmp9CE3 TMPFrontiersОценок пока нет
- Molecular Mechanism of Phenylhydrazine Induced HaematotoxicityДокумент5 страницMolecular Mechanism of Phenylhydrazine Induced HaematotoxicityVilim DretarОценок пока нет
- Heme Metabolism: Professor Dr.V.Meera M D DGOДокумент44 страницыHeme Metabolism: Professor Dr.V.Meera M D DGOshiv gautamОценок пока нет
- Semax As A Universal Drug For Therapy and ResearchДокумент12 страницSemax As A Universal Drug For Therapy and ResearchAlvian VianОценок пока нет
- Endocrine Diversity, Hormone Actions, Intracellular Traffic and Sorting of ProteinsДокумент105 страницEndocrine Diversity, Hormone Actions, Intracellular Traffic and Sorting of ProteinsswishwsОценок пока нет
- How To Diagnose Equine Pituitary Pars Intermedia DysfunctionДокумент3 страницыHow To Diagnose Equine Pituitary Pars Intermedia DysfunctionDorothea Dudli von DewitzОценок пока нет
- tmp87B1 TMPДокумент16 страницtmp87B1 TMPFrontiersОценок пока нет
- 08 Biomedik - Sistem Endokrin &hormonДокумент43 страницы08 Biomedik - Sistem Endokrin &hormonqueenraaa16Оценок пока нет
- LEC 01 - Principles of EndocrinologyДокумент44 страницыLEC 01 - Principles of EndocrinologyIoana Cozma100% (1)
- Drug Metabolism - Chapter 8Документ44 страницыDrug Metabolism - Chapter 8Shaun李好Оценок пока нет
- Endocrinologia EquinaДокумент7 страницEndocrinologia EquinaNiñoTorresОценок пока нет
- Role of Thyroid Hormone in Regulation of PDFДокумент8 страницRole of Thyroid Hormone in Regulation of PDFJose Leonel Fajardo RapaloОценок пока нет
- Comparative Endocrinology Course Covers Hormones Across SpeciesДокумент44 страницыComparative Endocrinology Course Covers Hormones Across Speciessaja MuhammadОценок пока нет
- Hormonal Management of Male InfertilityДокумент22 страницыHormonal Management of Male Infertilitydranibalurologoencancun.mxОценок пока нет
- 2.signal Transduction-2Документ26 страниц2.signal Transduction-2Ragdha MehdawiОценок пока нет
- Agricultural University of Georgia Durmishidze Institute of Biochemistry and BiotechnologyДокумент25 страницAgricultural University of Georgia Durmishidze Institute of Biochemistry and BiotechnologyZainab Jamal SiddiquiОценок пока нет
- Erythrocyte Metabolism and Enzyme Defects: by Guest On 28 May 2018Документ5 страницErythrocyte Metabolism and Enzyme Defects: by Guest On 28 May 2018rismayantifaujiahОценок пока нет
- ERD Biochemistry Lecture 1Документ15 страницERD Biochemistry Lecture 1محمد عليОценок пока нет
- Resveratrol Increases SHBG ProductionДокумент14 страницResveratrol Increases SHBG ProductionGaurav AroraОценок пока нет
- Khatri 2020Документ10 страницKhatri 2020Sukma's Wahyulii TiyasОценок пока нет
- Clinical Chemistry 3: EndocrinologyДокумент21 страницаClinical Chemistry 3: EndocrinologyRomie Solacito100% (3)
- Endrocrinology - PPTX Version 1Документ40 страницEndrocrinology - PPTX Version 1Abishek BhadraОценок пока нет
- Aging of The Endocrine System: Ieva B. AkbarДокумент41 страницаAging of The Endocrine System: Ieva B. AkbarvansrodОценок пока нет
- Regulasi Dan Mekanisme EndokrinДокумент121 страницаRegulasi Dan Mekanisme Endokrinluthfiyya syafiqaОценок пока нет
- The Endocrine SystemДокумент41 страницаThe Endocrine SystemОксана КрасильниковаОценок пока нет
- Tyroid 10Документ4 страницыTyroid 10Anonymous UTUWFODCEYОценок пока нет
- Cyp2c9 2Документ6 страницCyp2c9 2Mesut KirazОценок пока нет
- DocumentДокумент45 страницDocumentPrajwal PatilОценок пока нет
- PKD BiochemistryДокумент7 страницPKD BiochemistryHyun Jae WonОценок пока нет
- Tyroid 10Документ6 страницTyroid 10Anonymous UTUWFODCEYОценок пока нет
- Introduction To HormonesДокумент41 страницаIntroduction To Hormonesنورالهدى حسام عليОценок пока нет
- Liquid Chromatography-Tandem Mass SpectrometryДокумент8 страницLiquid Chromatography-Tandem Mass SpectrometryMónica Adriana Rodríguez CadenaОценок пока нет
- Key Concepts of EndocrinologyДокумент131 страницаKey Concepts of Endocrinologykomal pattabiОценок пока нет
- 4b5eb49a-82a6-4a92-b5a8-d90e68507d7aДокумент25 страниц4b5eb49a-82a6-4a92-b5a8-d90e68507d7amoepiОценок пока нет
- Clinical Pharmacokinetics of Selective Serotonin Reuptake InhibitorsДокумент18 страницClinical Pharmacokinetics of Selective Serotonin Reuptake InhibitorsJucas EscobarОценок пока нет
- Elecsys PTH (1-84) : Cobas e 801 English System InformationДокумент5 страницElecsys PTH (1-84) : Cobas e 801 English System Informationsyafiq_82Оценок пока нет
- Pharmacology of AutacoidsДокумент13 страницPharmacology of AutacoidsInocenteОценок пока нет
- Characteristics and Treatment of Hypertension in PheochromocytomaДокумент17 страницCharacteristics and Treatment of Hypertension in PheochromocytomaJunior TorresОценок пока нет
- Gnrh1: VerificationДокумент6 страницGnrh1: VerificationAmi Kada ImranОценок пока нет
- 5 Reasons Why PH Is Critical For Cell HealthДокумент17 страниц5 Reasons Why PH Is Critical For Cell HealthJLJ_BCNОценок пока нет
- Alopecia Unapproved Treatmentes or IndicationsДокумент10 страницAlopecia Unapproved Treatmentes or IndicationsdanizituОценок пока нет
- ABC of Clinical HematologyДокумент82 страницыABC of Clinical Hematologydokice100% (1)
- Mechanisms of Disease: Review ArticlesДокумент9 страницMechanisms of Disease: Review Articlesxander trujilloОценок пока нет
- Falciforme y Pulmon 2008Документ12 страницFalciforme y Pulmon 2008xander trujilloОценок пока нет
- Distúrbios Ácido Básicos Revisão NEJM 2014-1Документ12 страницDistúrbios Ácido Básicos Revisão NEJM 2014-1sabrinamgrОценок пока нет
- Resistant or Difficult-To-Control HypertensionДокумент8 страницResistant or Difficult-To-Control Hypertensionxander trujilloОценок пока нет
- Eferesis 2008Документ17 страницEferesis 2008xander trujilloОценок пока нет
- WR2 PDFДокумент9 страницWR2 PDFJoni MokodoОценок пока нет
- ADA Guidelines 2012Документ53 страницыADA Guidelines 2012Jones PessoaОценок пока нет
- Clinical Practice: Subclinical HyperthyroidismДокумент5 страницClinical Practice: Subclinical Hyperthyroidismxander trujilloОценок пока нет
- Guia Del EspiritistaДокумент8 страницGuia Del Espiritistaxander trujilloОценок пока нет
- Anmia MicrociticaДокумент8 страницAnmia MicrociticafabitoxxpepeОценок пока нет
- TX LipidosДокумент14 страницTX Lipidosxander trujilloОценок пока нет
- Adrenal Urgencias Urgencias 2014Документ20 страницAdrenal Urgencias Urgencias 2014xander trujilloОценок пока нет
- ADA Guidelines 2012Документ53 страницыADA Guidelines 2012Jones PessoaОценок пока нет
- Adrenal DX y TX EmergenciasДокумент20 страницAdrenal DX y TX Emergenciasxander trujilloОценок пока нет
- Accf Aha Pulmonary HypertensionДокумент49 страницAccf Aha Pulmonary Hypertensionxander trujilloОценок пока нет
- Thyroiditis: Review ArticleДокумент10 страницThyroiditis: Review Articlexander trujilloОценок пока нет
- Osteoporosis Esc Re NingДокумент8 страницOsteoporosis Esc Re Ningxander trujilloОценок пока нет
- Trilogy Suite Op. 5 (Instrumental) : ' B B B B 'Документ21 страницаTrilogy Suite Op. 5 (Instrumental) : ' B B B B 'xander trujillo100% (1)
- Hypothyroidism: Causes, Killers, and Life-Saving TreatmentsДокумент15 страницHypothyroidism: Causes, Killers, and Life-Saving Treatmentsxander trujilloОценок пока нет
- Diabetes Hiperglicemias UrgenciasДокумент16 страницDiabetes Hiperglicemias Urgenciasxander trujilloОценок пока нет
- Antiplatelet DrugsДокумент33 страницыAntiplatelet Drugsxander trujilloОценок пока нет
- Howlett Paper 09182013Документ6 страницHowlett Paper 09182013FutantxxyzОценок пока нет
- Chapter 13 - Diseases of White Blood Cells, Lymph Nodes, Spleen, and ThymusДокумент10 страницChapter 13 - Diseases of White Blood Cells, Lymph Nodes, Spleen, and ThymusAgnieszka WisniewskaОценок пока нет
- Profound Tissue Specifity in Proliferation Control Underlies Cancer Drivers and Aneuploidy PatternsДокумент40 страницProfound Tissue Specifity in Proliferation Control Underlies Cancer Drivers and Aneuploidy PatternsDAYANA ERAZOОценок пока нет
- The Impact of Tumor Biology On Cancer Treatment and Multidisciplinary Strategies - M. Molls, Et Al., (Springer, 2009) WWДокумент363 страницыThe Impact of Tumor Biology On Cancer Treatment and Multidisciplinary Strategies - M. Molls, Et Al., (Springer, 2009) WWiuliОценок пока нет
- Current Diagnosis and Therapy For Head and Neck MalignanciesДокумент249 страницCurrent Diagnosis and Therapy For Head and Neck MalignanciesMara TomaОценок пока нет
- Head and Neck Laryngeal Tumors An OverviewДокумент7 страницHead and Neck Laryngeal Tumors An OverviewpupuayuwandiraОценок пока нет
- A Practical Approach To Diagnosis of B-Cell Lymphomas With Diffuse LДокумент8 страницA Practical Approach To Diagnosis of B-Cell Lymphomas With Diffuse LdkbritobОценок пока нет
- Multiple MyelomaДокумент287 страницMultiple MyelomaMarco Antonio Grajales BuitragoОценок пока нет
- 2021 Article 827Документ39 страниц2021 Article 827Ahmad TaufikОценок пока нет
- R265 FullДокумент13 страницR265 FullMuch MahmudiОценок пока нет
- Gastric CancerДокумент20 страницGastric CancerMaya SariОценок пока нет
- ReishiMax Clinical StudyДокумент7 страницReishiMax Clinical StudynsagelocspaОценок пока нет
- Reviews of Physiology, Biochemistry and Pharmacology 176Документ134 страницыReviews of Physiology, Biochemistry and Pharmacology 176Charles santos da costa100% (1)
- Sleisenger and Fordtran's Gastrointestinal and Liver Disease Part 1 (PDFDrive)Документ1 029 страницSleisenger and Fordtran's Gastrointestinal and Liver Disease Part 1 (PDFDrive)Emi Valcov100% (1)
- Cancer Preventive and Therapeutic Effects of EGCG The Major Polyphenol in Green TeaДокумент24 страницыCancer Preventive and Therapeutic Effects of EGCG The Major Polyphenol in Green TeaEmanuela MerticariuОценок пока нет
- SIOP 2005 Education Book PDFДокумент122 страницыSIOP 2005 Education Book PDFHabu John KocuОценок пока нет
- Oxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic StrategiesДокумент41 страницаOxidative Stress and Metabolic Disorders: Pathogenesis and Therapeutic StrategiesnihaaОценок пока нет
- The Biology of Cancer Chapter 08Документ36 страницThe Biology of Cancer Chapter 08BelindaDcostaОценок пока нет
- Cell Cycle Control A System of Interlinking OscillatorsДокумент17 страницCell Cycle Control A System of Interlinking OscillatorsMaría José Triana TamayoОценок пока нет
- Exercise Rejuvenates Quiescent Skeletal Muscle Stem Cells in Old Mice Through Restoration of Cyclin D1Документ27 страницExercise Rejuvenates Quiescent Skeletal Muscle Stem Cells in Old Mice Through Restoration of Cyclin D1Alana HОценок пока нет
- Flex Monoclonal Rabbit Anti-Human Cyclin D1 Ready-to-Use: Clone EP12 (Link)Документ8 страницFlex Monoclonal Rabbit Anti-Human Cyclin D1 Ready-to-Use: Clone EP12 (Link)Nutsa ToduaОценок пока нет
- 2.4.a Dr. Samuel J Haryono Rationale of Using CDK 4:6 Inhibitors in HR Positive ABCДокумент24 страницы2.4.a Dr. Samuel J Haryono Rationale of Using CDK 4:6 Inhibitors in HR Positive ABCtepat rshsОценок пока нет
- Lymphoproliferative DisordersДокумент36 страницLymphoproliferative DisordersBrett FieldsОценок пока нет
- 4 6001055797780414555 PDFДокумент245 страниц4 6001055797780414555 PDFAtomОценок пока нет
- An Evidence-Based Approach To Pediatric MelanonychiaДокумент13 страницAn Evidence-Based Approach To Pediatric MelanonychiaMarice QCОценок пока нет
- Cytotoxic Effects of Ultra-Diluted Remedies On Breast Cancer CellsДокумент9 страницCytotoxic Effects of Ultra-Diluted Remedies On Breast Cancer CellsAPH - Associação Portuguesa de Homeopatia (repositório 2008-15)100% (4)
- Cancer Therapy: PreclinicalДокумент8 страницCancer Therapy: PreclinicalGeorge GeorgakisОценок пока нет
- CV RemyДокумент6 страницCV RemyremythomasОценок пока нет