Академический Документы
Профессиональный Документы
Культура Документы
Corrosion and Fatigue of Heat Treated Martensitic Stainless Steel 1.4542 Used For Geothermal Applications
Загружено:
Global Research and Development ServicesОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Corrosion and Fatigue of Heat Treated Martensitic Stainless Steel 1.4542 Used For Geothermal Applications
Загружено:
Global Research and Development ServicesАвторское право:
Доступные форматы
MATTER: International Journal of Science and Technology
ISSN 2454-5880
Pfennig & Kranzmann, 2019
Volume 5 Issue 1, pp. 138-158
Date of Publication: 19th April, 2019
DOI-https://dx.doi.org/10.20319/mijst.2019.51.138158
This paper can be cited as: Pfennig, A., & Kranzmann, A. (2019). Corrosıon and Fatıgue of Heat Treated
Martensıtıc Staınless Steel 1.4542 used for Geothermal Applıcatıons. MATTER: International Journal of
Science and Technology, 5(1), 138-158.
This work is licensed under the Creative Commons Attribution-Non Commercial 4.0 International
License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc/4.0/ or send a
letter to Creative Commons, PO Box 1866, Mountain View, CA 94042, USA.
CORROSION AND FATIGUE OF HEAT TREATED
MARTENSITIC STAINLESS STEEL 1.4542 USED FOR
GEOTHERMAL APPLICATIONS
Anja Pfennig
HTW-Berlin, University of Applied Sciences, Berlin, Germany
anja.pfennig@htw-berlin.de
Axel Kranzmann
BAM, Federal Institute for Materials Research and Testing Berlin, Berlin, Germany
axel.kranzmann@bam.de
Abstract
During capture and storage technology (CCS) as well as in geothermal energy production steels
need to withstand the corrosive environment such as: heat, pressure, salinity of the aquifer and
CO2-partial pressure. 1.4542 shows unusual corrosion phenomena, but is still sufficiently
resistant in corrosive environments. To better understand its behaviour differently heat treated
coupons of 1.4542 and for comparison X20Cr13 and X46Cr13 were kept in the artificial brine of
the Northern German Basin at T=60 °C. Ambient pressure as well as p=100 bar for 700 h -
8000 h in water saturated supercritical CO2 and CO2-saturated synthetic aquifer environment
was applied. Fatigue tests were performed via push-pull tests with a series of 30 specimens from
150 MPa to 500 MPa (sinusoidal dynamic test loads, R=-1; resonant frequency ~ 30 Hz). FeCO3
and FeOOH are corrosion products also after dynamic corrosion tests. Martensitic
microstructure offers good corrosion resistance in geothermal environment. The S-N-curve
showing no typical fatigue strength and very steep slopes of possible fatigue strength for finite
life. Possible influencing artefacts, such as Al-inclusions could not be correlated to early rupture
Available Online at: http://grdspublishing.org/ 138
MATTER: International Journal of Science and Technology
ISSN 2454-5880
despite specimens containing inclusions at the fracture surface and cross section reached lower
number of cycles. Applied potential proofed to enhance fatigue life tremendously.
Keywords
High Alloyed Steel, Pitting; Corrosion Fatigue, Corrosion, Endurance Limit, CCS, CO2-Storage
1. Introduction
Emission gasses e.g. from combustion processes of power plants are compressed into
deep geological saline aquifers CCS-site (CCS Carbon Capture and Storage (Thomas, 2005; van
den Broek, Hoefnagels, Rubin, Turkenburg, Faaij, 2010)) will result in corrosion of piping and
casing of the CCS engineering site (Nešić, 2007; Hurter, 2008; Zhang, Yang, Sun, Lu, 2008; Mu
and Zhao, 2009; Seiersten, 2001; Cui, Wu, Uhu, Yang, 2006; Pfennig and Kranzmann, 2011).
Injection pipe steels are highly sensitive towards CO2-corrosion regarding alloy composition,
contamination of alloy and media, temperature, CO2 partial pressure, flow conditions and
protective passivating corrosion scales (Zhang, Yang, Sun, Lu, 2008; Mu and Zhao, 2009;
Zhang, Zhao, Juang, 2005; Alhajji and Reda, 1993; Choi and Nešić, 2008; Jiang, Nešić, Huet,
2008; Ahmand, Allam, Abdul Aleem, 2000; Pfennig and Bäßler, 2009; Pfennig and Kranzmann,
2010; Pfennig, Zastrow, Kranzmann, 2013; Nybor, 2005; Carvalho, Joia, Mattos, 2005; Linter
and Burstein, 1999; Wu, Cui, Zhao, Yan, Zhu, Yang, 2004).
Alloying elements (Bülbül and Sun, 2010) and heat treatment (duration, temperature of
initial austenitizing and annealing as well as cooling rate (Hou, Li, Li, Zhang, 2000; Cijović and
Radenković, 2006; Park and Park, 2007)) significantly influence the corrosion behaviour of
steels. In general Ni- and Cr contents (Banaś, Lelek-Borkowska, Mazurkiewicz, Solarski, 2007;
Bilmes, Llorente, Méndez, Gervasi, 2009) improve the corrosion resistance. Retained austenite
improves pitting corrosion resistance (Banaś et al., 2007). Higher austenitizing temperature for
martensitic steels (Zhang et al., 2001; Brown, Parakala, Nešić 2004; Isfahany, Saghafian,
Borhani, 2011) and annealing at higher temperature of lean duplex stainless steels (Bulbül and
Sun, 2010; Hou et al., 2000; Zhang et al., 2001) decrease the susceptibility towards local
corrosion. NaCl solution containing H2S severely degrades Mn-containing carbon steels with
martensitic microstructure compared to ferritic or ferritic-bainitic microstructures (Lucio-Garciaa
et al., 2009) due to reactive grain boundaries in martensitic microstructures.
Locally corroded samples -with pits acting as corrosion catalyzer- usually reveal similar
corrosion products as the slowly growing surface layers (Pfennig and Bäßler, 2009; Pfennig et
Available Online at: http://grdspublishing.org/ 139
MATTER: International Journal of Science and Technology
ISSN 2454-5880
al., 2013). Generally siderite FeCO3 (Nešić, 2007; Han, Yang, Nešić, 2008; Pfennig, Wolthusen,
Zastrow, Kranzmann, 2015) precipitates on steels in contact with CO2-environment because the
solubility of FeCO3 in water is low (pKsp = 10.54 at 25 °C (Brown et al., 2004; Han et al. 2008))
and consequently anodic iron dissolution takes plase. Transient Fe(OH)2 is formed initially (Mu
and Zhao, 2009; Isfahany et al., 2011) which may locally increase the pH in proximity to the
hydroxide film followed by the consecutive formation an internal and external ferrous carbonate
(Han et al., 2008) according to equations 1 to 6 (Pfennig and Bäßler, 2011; Brown et al., 2004):
CO2 (g) + H2O (l) H+ + HCO3-(aq) (1)
cathodic: 2 HCO3- + 2 e- 2 CO32- + H2 (2)
anodic: Fe Fe2+ + 2e- (3)
Fe2+ + CO32- FeCO3 (4)
Fe2+ + 2 HCO3- Fe(HCO3)2 (5)
Fe(HCO3)2 FeCO3 + CO2 + H2O (6)
Corrosion layers and pitting have been reported to cause early failure of materials under
cyclic load (Madduri and Prakash, 2010; Msahiro, 1999; Vignal, Delrue Peultier, Oltra, 2007).
Local corrosion on stainless steels is enhanced by
• Chemical corrosion reactions,
• Changes of lattice energy locally within the steel`s surface and
• Mechanical load (Pfennig, Wolf, Böllinghaus, 2016; Pfennig, Wiegand, Wolf, Bork,
2013).
Cracks are initiated and crack propagation is enhanced and accelerated at dual or triple
points of grain/phase boundaries where the grain/phase boundary energy is higher (Madduri and
Prakash, 2010; Vignal et al., 2007). The local lattice mismatch at grain/phase boundaries also
initiates pit formation, selective corrosion and inter granular deterioration resulting crack
formation (Masahiro, 1999).
Parts of this work have been presented at GHGT13 (Pfennig et al., 2015), ICFEE 2017
(Pfennig and Kranzmann, 2017) and ICBEE 2019 (to be published) and we will now give an
update on new findings, summarize all our previous work of the last 10 year and compare results
to heat treated martensitic stainless steels (1.4022 and 1.4043). This is our final review work on
corrosion kinetics of X20Cr13, X46Cr13 and X5CrNiCuNb16-4. Further investigation will
comprise the corrosion mechanism, respectively.
Available Online at: http://grdspublishing.org/ 140
MATTER: International Journal of Science and Technology
ISSN 2454-5880
2. Materials and Methods
Both, immersion tests as well as corrosion fatigue tests were undertaken for the same
steel quality (martensitic AISI 630, X5CrNiCuNb16-4, 1.4542, tensile strength in air: 1078 MPa)
(Table 1)) using synthesized Stuttgart Aquifer (Förster et al., 2006): Ca2+: 1760 mg/L, K2+: 430
mg/L, Mg2+: 1270 mg/L, Na2+: 90,100 mg/L, Cl-: 143,300 mg/L, SO42-: 3600 mg/L, HCO3-: 40
mg/L). For fatigue tests the surfaces were activated via machining to Rz=4.
Table 1: Element distribution of 1.4542 (X5CrNiCuNb16-4, AISI 630), (mass percent)
Element C Si Mn P S Cr Mo Ni Cu Nb
acc ≤ 0.07 ≤ 0.70 ≤ 1.50 ≤ 0.04 ≤ 0.015 15.0 - ≤ 0.60 3.00 – 3.00 – 0.20 –
a
standard 17.0 5.00 5.00 0.45
analysed b 0.03 0.42 0.68 0.018 0.002 15.75 0.11 4.54 3.00 0.242
a) elements in accordance to DIN EN 10088-3 in %
b) measured via spark emission spectrometry
Heat treatment (Table 2), sample preparation, laboratory scale exposure tests using
coupons (8 mm thickness, 20 mm width, 50 mm length) and corrosion fatigue tests as well as
electrochemical in-situ and optical analysis procedures have been widely described in detail in
our previous work (Pfennig and Bäßler, 2009; Wolf, Afanasiev, Böllinghaus, Pfennig, 2016;
Pfennig and Kranzmann, 2017, Pfennig, Heynert, Wolf, Böllinghaus, 2014)
Table 2: Heat Treatment Protocol for X5CrNiCuNb16-4
Heat Treatment Temperature Time Cooling Medium
°C / °C in min
normalizing 850 30 mmdemedium
oil
hardening 1040 30 oil
1040 30 oil
hardening and tempering 1, 550 °C 1040 / 550 30 oil
hardening and tempering 2, 650 °C 1040 / 650 30 oil
1040 / 550 30
hardening and tempering 3, 755 °C 1040 / 755 30 oil
oil
1040 / 650 30
Temperature, pH and electrochemical potential were measured directly within the
oil
corrosion chamber using an Ag/AgCl wire electrode fixed in a Teflon channel to measure the
open-circuit electrochemical potential. Fatigue tests were carried out at 30 – 40 Hz in stress-
strain mode under CCS aquifer environment with additional technical CO2 (flow rate. 9 L/h).
Temperature fluctuation was kept below ± 0.3 K and mean frequency of the cyclic load
accounted to 30-33 Hz.
Available Online at: http://grdspublishing.org/ 141
MATTER: International Journal of Science and Technology
ISSN 2454-5880
3. Results and Discussion
According to DIN 6601 the following equation was used to calculate surface corrosion
rates:
[ ] [ ] [ ]
Corrosion rate: (7)
[ ] [ ] [ ]
Corrosion rates obtained after exposure to the liquid phase are much higher (by a factor
of 3 after 8000 h of exposure) indicating the different reaction kinetics in different atmospheres.
Diffusion in the liquid phase is slower prohibiting the precipitation of a continuous passivating
corrosion layer whereas fast diffusion allows for the formation of a protective corrosion layer
rather quickly.
3.1 Surface Corrosion
Samples exposed to the vapour phase (water saturated CO2) show no dependence on
exposure time or method of heat treatment. Corrosion rates remain nearly constant at
approximately 0.004 mm/year. The carbonate layer is sufficient thick and can act as a diffusion
barrier and reducing mutual diffusion of ions (CO32-- and O2--species) into the steel and Fe-ions
towards the outer surface (Pfennig et al., 2015).
In the liquid phase (CO2-saturated saline aquifer) the corrosion rates increase, then
decrease due to the formation of a passivating layer, and then increase again. This phenomenon
(increase/decrease) is stronger for coupons immersed into the CO2-saturated brine (corrosion
rates around 0.004 to 0.014 mm/year). This may be due to the break-down of the passivating
layer allowing the rather fast mutual diffusion of gaseous CO32-- and O2 -species into the metal
and iron towards the surface where mainly siderite FeCO3 layers are formed (Figure 1) (Pfennig
et al, 2013; Pfennig, Wiegand et al., 2013). Large areas of the corrosion layer may locally detach
lateral to the surface enhancing and accelerating new processes at a fresh surface. Specimens
exposed to CO2-saturated saline aquifer form a carbonate layer because the siderite FeCO3-
solubility in CO2-containing water with rather low pH is low after carbonic acid has formed
(Cvijović and Randković, 2006). These heterogeneous corrosion scales of different magnitude
cover the specimens` surface and pits. Main phases are: siderite FeCO3 and cementite Fe3C.
Also, goethite -FeOOH at 100 bar and mackinawite FeS, akaganeite Fe8O8(OH)8Cl1.34 and
spinel-phases of various compositions are present at ambient pressure (Pfennig et al., 2015;
Pfennig and Kranzmann, 2017).
Available Online at: http://grdspublishing.org/ 142
MATTER: International Journal of Science and Technology
ISSN 2454-5880
Figure 1: Schematic drawing of the precipitation of the corrosion layer on 1.4542 and example
of sample surfaces after 8000 h of exposure to water saturated supercritical CO2
Han et al., 2008 introduced a crack initiation model which was modified by Pfennig et
al., 2016, 2013) applying for all states of heat treatment after exposure to CCS environment at 60
°C.
The three phase boundary during intermission of the CCS-process leads an unusual
corrosion formation characterized by corroded ellipsoidal regions (Pfennig et al., 2017; Pfennig
et al., 2016; Pfennig, Wolthusen, Wolf, Kranzmann, 2014). Siderite FeCO3 and goethite -
FeOOH passivate the remaining surfaces. The leopard structure is a consequence of the
decreasing water solubility in supercritical carbon dioxide (Banaś et al., 2007). As shown in
Figure 1 thin and small water droplets cover the metal surface that then consolidate (Pfennig et
al., 2016). The initial ferrous hydroxide film is mechanically and/or chemically damaged and the
highly porous non-protective ferrous carbonate is exposed directly to the geothermal water.
Because these reactions result in a decreasing pH of the surrounding water the ferrous carbonate
film dissolves and the steel depassivates. Then small pits that contain sulphates (FeSO4)
precipitating from the brine are formed and centered on the edges of former droplets (Figure 1).
The former droplet center mainly reveals hematite (Fe2O3) (Han et al., 2008; Pfennig and
Kranzmann, 2017). The flowing corrosive media removes the remaining film causing the pit to
grow wider.
Independent of heat treatment, atmosphere (liquid, supercritical/vapour) or pressure (1
bar, 100 bar) the corrosion rate for X20Cr13 and X46Cr13 as well as X5CrNiCuNb16-4 is below
0.04 mm/year. High pressure in accordance with high CO2 partial pressure reduced corrosion
reactions and the lower corrosion rates after long exposure are likely to blocked capillaries
within corrosion layers of adequate thickness that prevent fast diffusion. Therefore supercritical
CO2 possesses inhibiting function.
Available Online at: http://grdspublishing.org/ 143
MATTER: International Journal of Science and Technology
ISSN 2454-5880
The influence of the corrosion behavior on the heat treatment of steels prior to exposure
has been widely described (Alhajii and Reda, 1993; Pfennig et al., 2013; Bülbül and Sun, 2010;
Zhang et al., 2008; Bron et al, 2004; Pfennig et al., 2014; Pfennig et al., 2017) (Figure 2). All
corrosion rates of the steels investigated in this study increase with exposure time. Whereas in
comparison to X5CrNiCuNb16-4 the corrosion rates of both heat treated martensitic steels
X20Cr13 and X46Cr13 strongly increase by a factor of 3 at ambient pressure there is no increase
at 100 bar (Figure 2) from 4000 h to 8000 h.
With exception of steel specimen hardened and tempered at 700 °C the corrosion rates of
X20Cr13 and X46Cr13 are similar at 100 bar to those measured for the higher alloyed steel
X5CrNiCuNb16-4 (below 0.005 mm/year). After long exposure times all specimen show similar
corrosion rates with exception of steel specimen of X20Cr13/X46Cr13 hardened and tempered at
700/755 °C. Possibly chromium carbides precipitate and deplete the metal matrix so that no
chromium oxide passivation layer may be formed and the steel degrades after long exposure
times.
At 100 bar all graphs are parallel indicating that the heat treatment seems to influence the
corrosion rate less than at ambient pressure. It may be assumed that heat treatment for
X5CrNiCuNb16-4 plays a minor role with chromium content and atmosphere being more
decisive. Hardened or hardened and tempered coupons give lowest corrosion rates at ambient
pressure (Figure 2). Taking into account surface corrosion only, at 100 bar martensitic
microstructure (hardened and tempered at 650 °C for the supercritical phase: < 0.001 mm/year)
is preferred in water saturated supercritical CO2. In CO2 saturated aquifer water, however,
normalized microstructure show good corrosion resistance (ca. 0.004 mm/year) (Pfennig et al.,
2016).
Available Online at: http://grdspublishing.org/ 144
MATTER: International Journal of Science and Technology
ISSN 2454-5880
normalized
normalized
hardened
hardened
hardened & tempered 600 °C
hardened & tempered 550 °C
hardened & tempered 670 °C
hardened & tempered 650 °C
hardened & tempered 700/755 °C
hardened & tempered 755 °C
0.04
0.03
1 bar X20Cr13, liquid
Corrosion rate in mm/y
X5CrNiCuNb16-4
Corrosion rate in mm/y
0.02
0.03
0.02
liquid
0.02
0.02 1 bar
0.01
0.01 0.01
0.00
0.00 -0.01
0 1,000 2,000 3,000 4,000 5,000 6,000 0 2.000 4.000 6.000 8.000
0.04
Corrosion rate in mm/y
1 bar X46Cr13, liquid 0.014
Corrosion rate in mm/y
0.012
0.03
0.010 liquid
0.008
100 bar
0.02
0.006
0.01 0.004
0.002
0.00 0.000
0 1,000 2,000 3,000 4,000 5,000 6,000 0 2,000 4,000 6,000 8,000
0.04
100 bar X46Cr13 and X20Cr13 combined 0.015
supercritical
Corrosion rate in mm/y
Corrosion rate in mm/y
0.03
liquid 0.013 100 bar
0.010
0.02 0.008
0.005
0.01
0.003
0.00 0.000
0 2,000 4,000 6,000 8,000 0 2,000 4,000 6,000 8,000
Exposure time in h Exposure time in h (supercritical)
Figure 2: The role of heat treatment on the corrosion rate of X5CrNiCuNb16-4 at 60 °C exposed
to CO2 saturated saline aquifer water at 1 bar and 100 bar (top (Pfennig et al., 2013; Pfennig
and Kranzmann 2014) and in water saturated supercritical CO2 (bottom) at 100 bar (Pfennig et
al., 2013) in comparison to the corrosion rate of X20Cr13 and X46Cr13 (Pfennig et al., 2013;
Pfennig et al., 2014).
In general corrosion rates for X5CrNiCuNb16-4 are low due to the passivating nature of
the steel with even lower corrosion rates in the supercritical than in the liquid phase. Possible
explanations are the lack of electrolytes (Pfennig et al., 2013; Pfennig et al., 2016) or the
cathodic reactions described in equation (1) and (2). With a higher H2CO3 concentration the
Available Online at: http://grdspublishing.org/ 145
MATTER: International Journal of Science and Technology
ISSN 2454-5880
environment becomes more acidic and reactive compared to the CO2 saturated liquid phase
(Seiersten, 2001; Banaś et al., 2007). For X5CrNiCuNb16-4 all corrosion rates increase at 100
bar in the supercritical phase indicating fast reaction kinetics and depassivation of the steel
surface while the corrosion rates for specimen kept in the aquiver water remain at approximately
0.003 mm/y after 4000h and do not increase further with no regard to heat treatment. Due to
carbide formation the initial passivating layer deteriorates after 1000 h of exposure (Figure 3,
left). Because chromium is used to form carbides surface passivation –dependent on excess free
chromium- is prohibited (Pfennig et al., 2016).
Figure 3: left: SEM micrographs and composition of the ellipsoidal corrosion layer formed on
1.4542 hardened and tempered at 670 °C prior to exposure after 8000 hours of exposure at 60
°C and 100 bar to water saturated supercritical CO2;
3.2 Local Corrosion
Pit intrusion depths generally increase with exposure time (Figure 4). Because pits reveal
similar corrosion products as the surface corrosion layer it is necessary to separate these from
saline precipitations such as CaCO3 as seen in Figure 3, right. The presence of chlorides
enhances local corrosion of the microstructure (Pfennig et al., 2016) as well as a high number of
dislocations, grain boundaries, precipitation at phase boundaries, such as carbides. As a
consequence local lattice mismatch leads to higher local boundary energy (Vignal et al., 2007)
leaving the steel highly susceptible to corrosive attack of the CCS environment.
Available Online at: http://grdspublishing.org/ 146
MATTER: International Journal of Science and Technology
ISSN 2454-5880
normalizded normalizded
hardened hardened
hardened+tempered 600°C hardened+tempered 600°C
hardened+tempered 670°C hardened+tempered 670°C
hardened+tempered 755°C hardened+tempered 755°C
Number of pits per m²
40,000
Number of pits in m2
1 bar X20Cr13 liquid 100,000 liquid X5CrNiCuNb16-4
30,000 80,000 1 bar
60,000
20,000
40,000
10,000
20,000
0 0
0 2,000 4,000 6,000 8,000 0 2,000 4,000 6,000 8,000
40,000
Number of pits in m2
exposure time in h at ambient pressure (liquid)
1 bar X46Cr13 liquid
Number of pits per m2
30,000
1,000,000 X5CrNiCuNb16-4
liquid
20,000 800,000 100 bar
600,000
10,000
400,000
0 200,000
0 2,000 4,000 6,000 8,000
Number of pits per m²
400,000 0
100 bar X20Cr13 and X46Cr13 0 2,000 4,000 6,000 8,000
300,000 combined, liquid exposure time in h at 100 bar (liquid)
Number of pits per m²
200,000
1,000,000 X5CrNiCuNb16-4
100,000 800,000 supercritical
600,000
100 bar
0
0 2,000 4,000 6,000 8,000 400,000
Exposure time in h 200,000
0
0 2,000 4,000 6,000 8,000
Exposure time in h (supercritical)
Figure 4: The role of heat treatment on the local corrosion of X5CrNiCuNb16-4 at 60 °C
exposed to CO2 saturated saline aquifer water at 1 bar and 100 bar (top partly taken from
Pfennig et al., 2016 and in water saturated supercritical CO2 (bottom) at 100 bar (Pfennig et al.,
2013).Results are compared to the local corrosion of X20Cr13 at 60 °C, partly taken from
Pfennig et al., 2013 and Pfennig et al., 2014
Overall the penetration depth is independent of the heat treatment, showing lower
intrusion depth (8-25 μm) for X20Cr13 and X46Cr13 after normalizing and
hardening+tempering at 600 °C (Maximum intrusion depths ca. 300 μm for hardened X20Cr13
and a little lower for X46Cr13). For X5CrNiCuNb16-4 hardening+tempering from 670 °C to 755
°C offer low penetrations depths (10μm) (Pfennig et al., 2014) (10-250 µm average pit depths
after exposure at 100 bar and 60 °C).
Figure 4 (Pfennig et al., 2013; Pfennig et al., 2016) normalizes comparable results
obtained for X20Cr13 and X46Cr13 revealing smallest number of pits after 6000 h for hardening
Available Online at: http://grdspublishing.org/ 147
MATTER: International Journal of Science and Technology
ISSN 2454-5880
and tempering 600°C-670°C. Unusual low numbers of pits are likely because pits consolidate to
shallow pits. At ambient pressure the number of pits are similar (<40,000 per m2) for X20Cr13,
X46Cr13 and X5CrNiCuNb16-4. At 100 bar X5CrNiCuNb16-4 shows a high number of pits per
m2 in both atmospheres (Figure 6) (Pfennig et al, 2014). In general the number of pits formed in
the supercritical and liquid phase is the same with exception for samples heat treated and
hardened and tempered at 670 °C. There is no significant dependence of local corrosion
phenomena on the heat treatment prior to exposure. Although X5CrNiCuNb16-4 is known as
corrosion resistant in CCS environment it exhibits a significantly high number of pits at ambient
pressure and 100 bar.
3.3 Corrosion Fatigue
The distinct corrosion fatigue behavior was described earlier by Pfennig et al. 2017,
Pfennig et al., 2016 and Wolf et al., 2016). But, because these results were very unusual the
fatigue tests were redone with a different charge of samples and newly artificially produced
laboratory aquifer water (Figure 5). These results have been presented at ICBEE 2018.
Earlier results showed that the S-N-curve did not show expected fatigue strength and
non-linear very steep slopes of possible fatigue strength for finite life (Wöhler-exponent of k =
3.59, scatter range TN=1:34, very small coefficient of correlation r2 = 0.33), max. number of
cycles (10 x 107) at Sa=150 MPa) (Figure 6, bottom). Although the scatter range is much smaller,
the slope of the fatigue limit line is still very steep indicating fast degradation of the material and
resulting in an even lower max. number of cycles (10x107) at Sa=130 MPa). The corrosion
fatigue strength of X5CrNiCuNb16-4 is 60% lower than the endurance limit measured in air (620
MPa) (Pfennig et al., 2016), with steeper slope of the fatigue limit line as a function of increasing
number of cycles (Pfennig and Kranzmann, 2017; Wolf et al., 2016).
Available Online at: http://grdspublishing.org/ 148
MATTER: International Journal of Science and Technology
ISSN 2454-5880
350
Cycles
300
Runouts
N10
N50
Stress amplitude Sa in MPa
250
N90
200
X5 CrNiCuNb 16-4
150 R1, f 33 Hz, kt 1
North German Basin
T 96 °C, Dcrit = 12.5 mm
push/pull
isolated, polish
100
104 105 106 107
Cycles N
0,1 1 10 100
Test Duration in h
Figure 5: S-N-curve and crack formation on 1.4542 exposed to saline aquifer water and
technical CO2. For comparison results from former experiments are given (Wolf et al., 2016)
Available Online at: http://grdspublishing.org/ 149
MATTER: International Journal of Science and Technology
ISSN 2454-5880
No correlation was found regarding artificial aquifer water (Pfennig and Kranzmann,
2017) stress amplitude and number of failures, with increasing stress not inevitably leading to a
lower number of cycles as expected. However, Aluminium was measured and heterogeneously
distributed non-metallic inclusions were detected in the martensitic structure embedded with δ-
ferrite (Figure 6, bottom) for samples with presumably short number of cycles to failure (Pfennig
et al., 2016). This finding is appointed to this particular charge of the alloy, because in general it
could not clearly be stated that Aluminum found within the microstructure leads to lower number
of cycles and unpredictable early failure (Pfennig et al., 2017). X5CrNiCuNb16-4 reveals
multiple cracks that are easily backtracked to pits as source of crack initiation. Inclusions,
Aluminum and corrosion layer do not significantly influence the endurance limit – neither during
the first fatigue testing nor the second. In general, early failure could not be related to
Stress Amplitude,
Impurities within the Microstructure
Precipitation and Thickness of the Corrosion Layer
Number of Striations.
As a consequence of the mechanical load the local boundary energy within the
microstructure of the steel is increased due to local lattice mismatch (Pfennig et al, 2013)
resulting in enhanced local corrosion as a result of:
chlorides (Unigovski et al., 2009)
Increased Dislocation Number
Grain Boundaries
Precipitation Phase Boundaries and Carbides.
Available Online at: http://grdspublishing.org/ 150
MATTER: International Journal of Science and Technology
ISSN 2454-5880
Figure 6: Surface Image of Corrosion Layer on Fatigue Specimen Revealing Striations (Bottom)
and Microstructure of X5CrNiCuNb16-4
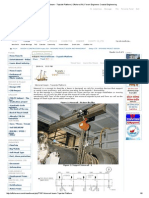
The application of a cathodic potential proofed to enhance the corrosion fatigue life
expectancy by a factor of 20 to 70. The potentials USHE = –400 to –150 mV only covered
cathodic domains (Figure 7). Depending on the potential applied the deterioration was slower
(low potentials) or faster (high potentials). A decreasing potential leads to the increase of the
alloy’s corrosion fatigue life expectancy i.e. to an increase of number of cycles to failure. Runout
specimens were loaded within the potentials USHE = –150 to –400 mV (preset threshold of 107
cycles, no mechanical failure). Note, a cathodic potential discharges hydrogen from the
specimen’s surface, enhances embrittlement of the alloy and may then lead to unpredicted and
earlier failure.
Available Online at: http://grdspublishing.org/ 151
MATTER: International Journal of Science and Technology
ISSN 2454-5880
Figure 7: CF life expectancy of X5CrNiCuNb16-4 with applied potential; isolated test set up,
polished surface finish, push/pull load
4. Conclusion
The corrosion process and corrosion fatigue behavior of martensitic stainless steel 1.4542
was investigated to predict reliability of the steel in geothermal environment. At 100 bar as well
as at ambient pressure and 60 °C hardening and tempering at low temperatures (600 to 670 °C)
for 1.4022, 1.4043 and 1.4542 result in lowest corrosion rates and reveal good resistance against
local corrosion.
Surface corrosion resistance is better in supercritical CO2, whereas pit corrosion
resistance is better in CO2 saturated brine. Non-uniform corrosion scales with differences in
thickness cover the specimens` surface are mainly composed of: siderite FeCO3 and carbides,
mainly Fe3C. Generally, ellipsoidal surface corrosion regions appear after exposure of >4000 h
revealing hematite Fe2O3 and iron sulfate FeSO4, FeCO3 and FeOOH – the latter also after
Available Online at: http://grdspublishing.org/ 152
MATTER: International Journal of Science and Technology
ISSN 2454-5880
corrosion fatigue tests. CCS environment cuts the endurance limit of 1.4542 measured in air in
half. No significant correlation could be found between inclusions, discontinuous phase
distribution, and Aluminum content within the base alloy and early rupture. Inclusions at the
fracture surface and its cross section, pit and intercrystalline corrosion are source of crack
formation lowering the number of cycles to failure.
Pitting corrosion phenomena, which most likely lead to a notch-based initiation region
propagating fatigue crack growth, can be successfully depressed applying an appropriate
cathodic potential. During geothermal application the fatigue life expectancy can therefore be
enhanced to a great proportion by cathodic protection.
Acknowledgements
This work was supported by the FNK (Fachkonferenz für wissenschaftlichliche
Nachwuchskräfte) of the Applied University of Berlin, HTW and by IMPACT (EU-Project
EFRE 20072013 2/21). The authors would like to thank Andre Gröber, Rainer Wiegand, Roman
Simkin and Roman Afanasiev for their worthy contribution to this paper.
References
Ahmad Z., Allam I.M., Abdul Aleem B.J. (2000). Effect of environmental factors on the
atmospheric corrosion of mild steel in aggressive sea coastal environment, Anti
Corrosion Methods and Materials, 47, 215-225
https://doi.org/10.1108/00035590010344312
Alhajji J.N. and M.R. Reda M.R. (1993). The effect of alloying elements on the electrochemical
corrosion of low residual carbon steels instagnant CO2-saturated brine, Corrosion
Science, 34 (11) 1899-1911 https://doi.org/10.1016/0010-938X(93)90026-D
Banaś, J., Lelek-Borkowska, U., Mazurkiewicz B., Solarski W. (2007). Effect of CO2 and H2S
on the composition and stability of passive film on iron alloy in geothermal water.
Electrochimica Acta, 52, 5704–5714. https://doi.org/10.1016/j.electacta.2007.01.086
Bilmes P.D., Llorente C.L., Méndez C.M., Gervasi C.A. (2009). Microstructure, heat treatment
and pitting corrosion of 13CrNiMo plate and weld metals, Corrosion Science, 51 (4),
876-882 https://doi.org/10.1016/j.corsci.2009.01.018
Brown B., Parakala S. R., Nešić S. (2004). CO2 corrosion in the presence of trace amounts of
H2S, Corrosion, paper no. 04736, 1-28
Available Online at: http://grdspublishing.org/ 153
MATTER: International Journal of Science and Technology
ISSN 2454-5880
Bülbül Ş., Sun Y. (2010). Corrosion behaviours of high Cr-Ni cast steels in the HCl solution.
Journal of Alloys and Compounds, 598, 143-147
https://doi.org/10.1016/j.jallcom.2010.02.203
Carvalho D.S, Joia C.J.B, Mattos O.R. (2005). Corrosion rate of iron and iron-chromium alloys
in CO2-medium. Corrosion Science, 47, 2974-2986.
https://doi.org/10.1016/j.corsci.2005.05.052
Choi, Y.-S. and Nešić, S. (2008). Corrosion behaviour of carbon steel in supercritical CO2-water
environments. No. 09256 NACE Corrosion Conf. & Expo, New Orleans, Louisiana,
USA, March 16th – 20th.
Cui, Z.D., Wu, S.L., Zhu, S.L., Yang, X.J. (2006). Study on corrosion properties of pipelines in
simulated produced water saturated with supercritical CO2. Applied Surface Science,
252, 2368-2374. https://doi.org/10.1016/j.apsusc.2005.04.008
Cvijović Z. and Radenković G. (2006). Microstructure and pitting corrosion resistance of
annealed duplex stainless steel. Corrosion Science, 48,3887-3906
https://doi.org/10.1016/j.corsci.2006.04.003
Förster, A et al. (2010) Reservoir characterization of a CO2 storage aquifer: The Upper Triassic
Stuttgart Formation in the Northeast German Basin. Mar. Pet. Geol., 27, 2156–2172
https://doi.org/10.1016/j.marpetgeo.2010.07.010
Förster, A., Norden, B. Zinck-Jørgensen, K. Frykman, P. Kulenkampff, J. Spangenberg, E.
Erzinger, J. Zimmer, M. Kopp, J. Borm, G. Juhlin, C. Cosma, C. Hurter, S. (2006),
Baseline characterization of the CO2SINK geological storage site at Ketzin, Germany:
Environmental Geosciences, 13 (3), 145-161 https://doi.org/10.1306/eg.02080605016
Han J., Yang Y., Nešić S., Brown B. N (2008)., Roles of passivation and galvanic effects in
localized CO2 corrosion of mild steel, Paper No. 08332, NACE Corrosion 2008, New
Orleans, Louisiana, USA, March 16th – 20th
Hou B., Li Y., Li Y., Zhang J. (2000). Effect of alloy elements on the anti-corrosion properties of
low alloy steel, Bull. Mater. Sci, 23 (3), 189-192
https://doi.org/10.1023/A:1004722603945
https://doi.org/10.1023/A:1004832305979 https://doi.org/10.1023/A:1004718508280 http
s://doi.org/10.1023/A:1004755014553
Available Online at: http://grdspublishing.org/ 154
MATTER: International Journal of Science and Technology
ISSN 2454-5880
Hurter S. (2008). Impact of Mutual Solubility of H2O and CO2 on Injection Operations for
Geological Storage of CO2, International Conference of the Properties of Water and
Steem ICPWS, Berlin, September 8-11
Isfahany A. N., Saghafian H., Borhani G. (2011). The effect of heat treatment on mechanical
properties and corrosion behaviour of AISI420 martensitic stainless steel, Journal of
Alloys and Compounds, 509, 3931-3936 https://doi.org/10.1016/j.jallcom.2010.12.174
Jiang X., Nešić S., Huet F. (2008). The Effect of Electrode Size on Electrochemical Noise
Measurements and the Role of Chloride on Localized CO2 Corrosion of Mild Steel, Paper
No. 09575, NACE Corrosion 2008 Conference and Expo, New Orleans, Louisiana, USA,
March 16th – 20th
Kraus SW. and Nolze G. (1996). POWDER CELL – a program for the representation and
manipulation of crystal structures and calculation of the resulting X-ray powder patterns,
J. Appl. Cryst., 29, 301-303 https://doi.org/10.1107/S0021889895014920
Linter B.R., Burstein G.T. (1999). Reactions of pipeline steels in carbon dioxide solutions,
Corrosion Science, 41,117-139 https://doi.org/10.1016/S0010-938X(98)00104-8
Lucio-Garciaa M.A., Gonzalez-Rodrigueza J.G., Casalesc M., Martinezc L., Chacon-Navaa J.G.,
Neri-Floresa M.A. and Martinez-Villafañea A. (2009). Effect of heat treatment on H2S
corrosion of a micro-alloyed C–Mn steel. Corrosion Science, 51, 2380-2386
https://doi.org/10.1016/j.corsci.2009.06.022
Madduri, C. and Prakash R. V. (2010). Corrosion Fatigue Crack Growth Studies in Ni-Cr-Mn
steels, International Journal of Mechanical and Materials Engineerin,g 1, 20-25
Masahiro Seo, Electrochemical Society. Corrosion Division (2009). Passivity and localized
corrosion: an International Symposium in Honour of Professor Norio Sato, initiation and
stability of localized corrosion processes on stein steels. The Electrochemical Society,
483-490
Mu L.J., Zhao W.Z. (2010). Investigation on carbon dioxide corrosion behavior of HP13Cr110
stainless steel in simulated stratum water. Corrosion Science, 52, 82-89.
https://doi.org/10.1016/j.corsci.2009.08.056
Nešić, S. (2007). Key issues related to modelling of internal corrosion of oil and gas pipelines –
A review. Corrosion Science, 49, 4308–4338.
https://doi.org/10.1016/j.corsci.2007.06.006
Available Online at: http://grdspublishing.org/ 155
MATTER: International Journal of Science and Technology
ISSN 2454-5880
Nyborg R. (2005). Controlling Internal Corrosion in Oil and Gas Pipelines, Business Briefing:
Exploration & Production: The Oil & Gas Review, 2, 70-74
Park J.-Y., Park Y.-S. (2007). The effects of heat-treatment parameters on corrosion resistance
and phase transformation of 14Cr-3Mo martensitic stainless steel, Materials Science and
Engineering A, 449-451, 1131-1134 https://doi.org/10.1016/j.msea.2006.03.134
Pfennig A., Bäßler R. (2009). Effect of CO2 on the stability of steels with 1% and 13% Cr in
saline water, Corrosion Science, 51 (4), 931-940
https://doi.org/10.1016/j.corsci.2009.01.025
Pfennig A., Heynert K., Wolf M., Böllinghaus T. (2014). First in-situ Electrochemical
Measurement During Fatigue Testing of Injection Pipe Steels to Determine the
Reliability of a Saline Aquifer Water CCS-site in the Northern German Basin. Energy
Procedia, 63, 5773-5786.
https://doi.org/10.1016/j.egypro.2014.11.610 https://doi.org/10.1016/j.egypro.2014.11.60
9
Pfennig A., Kranzmann A. (2010). The role of pit corrosion in engineering the carbon storage
site Ketzin, Germany, WIT Transactions on Ecology and the Environment, 126, 109-118
https://doi.org/10.2495/AIR100101
Pfennig A., Kranzmann A. (2017). Potential of martensitic stainless steel X5CrNiCuNb 16-4 as
pipe steel in corrosive CCS environment, International Conference on Future
Environment and Energy ICFEE 2017, Penang, Malaysia, January 8th-10th 2017
https://doi.org/10.18178/ijesd.2017.8.7.998
Pfennig A., Wiegand R., Wolf M., Bork C.-P. (2013). Corrosion and corrosion fatigue of AISI
420C (X46Cr13) at 60 °C in CO2-saturated artificial geothermal brine, Corrosion
Science, 68, 134–143 https://doi.org/10.1016/j.corsci.2012.11.005
Pfennig A., Wolf M., Böllinghaus Th. (2016). Corrosion Fatigue of X46Cr13 in CCS
Environment, in Energy Technology 2016: Carbon Dioxide Management and Other
Technologies, 1, 49-56
Pfennig A., Wolf M., Gröber A., Böllinghaus T., Kranzmann A. (2016). Corrosion fatigue of
1.4542 exposed to a laboratory saline aquifer water CCS-environment, 13th International
Conference on Greenhouse Gas Control Technologies, GHGT-13, 14th -18th November
2016, Lausanne, Switzerland.
Pfennig A., Wolthusen H., Zastrow P., Kranzmann A. (2015). Evaluation of heat treatment
Available Online at: http://grdspublishing.org/ 156
MATTER: International Journal of Science and Technology
ISSN 2454-5880
performance of potential pipe steels in CCS-environment, Carbon Dioxide Management
and Other Technologies, 1, 15-22
https://doi.org/10.1002/9781119093220.ch2 https://doi.org/10.1007/978-3-319-48220-
0_2
Pfennig A., Zastrow P., Kranzmann A. (2013). Influence of heat treatment on the corrosion
behaviour of stainless steels during CO2-sequestration into saline aquifer, International
Journal of Green House Gas Control, 15, 213–224
https://doi.org/10.1016/j.ijggc.2013.02.016
Pfennig, A. Kranzmann A. (2011). Reliability of pipe steels with different amounts of C and Cr
during onshore carbon dioxid injection. International Journal of Greenhouse Gas Control,
5, 757–769. https://doi.org/10.1016/j.ijggc.2011.03.006
Pfennig, A., Wiegand, R., Wolf, M., Bork, C.-P. (2013).Corrosion and corrosion fatigue of AISI
420C (X46Cr13) at 60 °C in CO2-saturated artificial geothermal brine, Corrosion
Science 68 (2013) 134–143. https://doi.org/10.1016/j.corsci.2012.11.005
Pfennig, A., Wolthusen, H., Kranzmann, A. (2017). Unusual corrosion behavior of 1.4542
exposed a laboratory saline aquifer water CCS-environment. Energy Procedia, 114, 5229-
5240.
https://doi.org/10.1016/j.egypro.2017.03.1678 https://doi.org/10.1016/j.egypro.2017.03.1
679
Pfennig, A., Wolthusen, H., Wolf, M., Kranzmann, A. (2014). Effect of heat treatment of
injection pipe steels on the reliability of a saline aquifer water CCS-site in the Northern
German Basin Energy Procedia, 63, 5762-5772.
Seiersten M. (2001), Material selection for separation, transportation and disposal of CO2,
Corrosion paper no. 01042.
Thomas D.C. (2005). Carbon Dioxide Capture for Storage in Deep Geologic Formations –
Results from CO2 Capture Project, Volume 1: Capture and Separation of Carbon Dioxide
form Combustion Sources, CO2 Capture Project, Elsevier Ltd UK, ISBN 0080445748.
Unigovski, Ya.B., Lothongkum, G., Gutman, E.M., Alush, D., Cohen, R. (2009).Low-cycle
fatigue behaviour of 316L-type stainless steel in chloride solutions, Corrosion Science,
51, 3014-3120. https://doi.org/10.1016/j.corsci.2009.08.035
Van den Broek M., Hoefnagels R., Rubin E., Turkenburg W., Faaij A. (2010). Effects of
technological learning on future cost and performance of power plants with CO2 capture,
Available Online at: http://grdspublishing.org/ 157
MATTER: International Journal of Science and Technology
ISSN 2454-5880
in Projects Costs of Generating Electricity. Progress in Energy and Combustion
Science,177-187
Vignal V., Delrue O., Peultier J., Oltra R. (2007). Critical Factors in Localized Corrosion 5: A
Symposium in Honour of Hugh Isaacs, Local mechanical-electrochemical behavior of
duplex stainless steels. The Electrochemical Society, 102-104
Wei, L., Pang, X., Liu, C., Gao, K. (2015). Formation mechanism and protective property of
corrosion product scale on X70 steel under supercritical CO2 environment. Corrosion
Science, 100, 404–420. https://doi.org/10.1016/j.corsci.2015.08.016
Wolf M., Afanasiev R., Böllinghaus T., Pfennig A. (2016). Investigation of Corrosion Fatigue of
Duplex Steel X2CrNiMoN22 5 3 Exposed to a Geothermal Environment under Different
Electrochemical Conditions and Load Types, 13th International Conference on
Greenhouse Gas Control Technologies, GHGT-13, 14th -18th November 2016,
Lausanne, Switzerland.
Wu S.L, Cui Z.D., Zhao G.X., Yan M.L., Zhu S.L., Yang X.J. (2004). EIS study of the surface
film on the surface of carbon steel form supercritical carbon dioxide corrosion. Applied
Surface Science, 228, 17-25. https://doi.org/10.1016/j.apsusc.2003.12.025
Zhang H., Zhao Y.L, Jiang Z.D. (2005). Effects of temperature on the corrosion behaviour of
13Cr martensitic stainless steel during exposure to CO2 and Cl- environment, Material
Letters, 59, 3370-3374 https://doi.org/10.1016/j.matlet.2005.06.002
Zhang L., Yang J., Sun J.S., Lu M. (2008). Effect of pressure on wet H2S/CO2 corrosion of
pipeline steel, No. 09565, NACE Corrosion 2008 Conference and Expo, New Orleans,
Louisiana, USA, March 16th – 20th
Zhang L., Zhang W., Jiang Y., Deng B., Sun D., Li J. (2008). Influence of annealing treatment
on the corrosion resistance of lean duplex stainless steel, NACE Corrosion 2008
Conference and Expo, New Orleans, Louisiana, USA, March 16th – 20th
Available Online at: http://grdspublishing.org/ 158
Вам также может понравиться
- Resolving A Realistic Middle Ground Towards The Sustainability Transition in The Global Political and Economic Landscape: A Comprehensive Review On Green CapitalismДокумент29 страницResolving A Realistic Middle Ground Towards The Sustainability Transition in The Global Political and Economic Landscape: A Comprehensive Review On Green CapitalismGlobal Research and Development ServicesОценок пока нет
- The Ethical Perception On Utang Na Loob As Basis of Voter's ChoiceДокумент18 страницThe Ethical Perception On Utang Na Loob As Basis of Voter's ChoiceGlobal Research and Development Services100% (1)
- Analyzing The Relationship Between Industrial Policy and Entrepreneurial EcosystemДокумент13 страницAnalyzing The Relationship Between Industrial Policy and Entrepreneurial EcosystemGlobal Research and Development ServicesОценок пока нет
- Examine The Effects of Goods and Services Tax (GST) and Its Compliance: Reference To Sierra LeoneДокумент18 страницExamine The Effects of Goods and Services Tax (GST) and Its Compliance: Reference To Sierra LeoneGlobal Research and Development ServicesОценок пока нет
- Interpretation and Justification of Rules For Managers in Business Organizations: A Professional Ethics PerspectiveДокумент10 страницInterpretation and Justification of Rules For Managers in Business Organizations: A Professional Ethics PerspectiveGlobal Research and Development ServicesОценок пока нет
- Teacher Educators' Perceptions of Utilizing Mobile Apps For English Learning in Secondary EducationДокумент28 страницTeacher Educators' Perceptions of Utilizing Mobile Apps For English Learning in Secondary EducationGlobal Research and Development ServicesОценок пока нет
- Perceived Self-Efficacy and Academic Performance of Stem Senior High School StudentsДокумент13 страницPerceived Self-Efficacy and Academic Performance of Stem Senior High School StudentsGlobal Research and Development ServicesОценок пока нет
- Implementation of Biorhythm Graph With Trigonometric Functions Using PythonДокумент8 страницImplementation of Biorhythm Graph With Trigonometric Functions Using PythonGlobal Research and Development ServicesОценок пока нет
- Potential Factors Influence Failure of The Village-Owned Business: The Entity Village Autonomy of Indonesian Government's ProgramДокумент12 страницPotential Factors Influence Failure of The Village-Owned Business: The Entity Village Autonomy of Indonesian Government's ProgramGlobal Research and Development ServicesОценок пока нет
- Challenges in The Implementation of Schoolbased Management of Developing Schools: Basis For A Compliance FrameworkДокумент14 страницChallenges in The Implementation of Schoolbased Management of Developing Schools: Basis For A Compliance FrameworkGlobal Research and Development ServicesОценок пока нет
- Effect of Metacognitive Intervention Strategies in Enhancing Resilience Among Pre-Service TeachersДокумент12 страницEffect of Metacognitive Intervention Strategies in Enhancing Resilience Among Pre-Service TeachersGlobal Research and Development ServicesОценок пока нет
- Design and Implementation of An Arabic Braille Self-Learning WebsiteДокумент17 страницDesign and Implementation of An Arabic Braille Self-Learning WebsiteGlobal Research and Development ServicesОценок пока нет
- Loss Analysis in Bread Production Process Using Material Flow Cost Accounting TechniqueДокумент16 страницLoss Analysis in Bread Production Process Using Material Flow Cost Accounting TechniqueGlobal Research and Development ServicesОценок пока нет
- Influence of Iot On Smart LogisticsДокумент12 страницInfluence of Iot On Smart LogisticsGlobal Research and Development ServicesОценок пока нет
- Using Collaborative Web-Based Maps To Promote Geography Teaching and LearningДокумент13 страницUsing Collaborative Web-Based Maps To Promote Geography Teaching and LearningGlobal Research and Development ServicesОценок пока нет
- Refining The English Syntax Course For Undergraduate English Language Students With Digital TechnologiesДокумент19 страницRefining The English Syntax Course For Undergraduate English Language Students With Digital TechnologiesGlobal Research and Development ServicesОценок пока нет
- Bank-Specific and Macroeconomic Determinants of Banks Liquidity in Southeast AsiaДокумент18 страницBank-Specific and Macroeconomic Determinants of Banks Liquidity in Southeast AsiaGlobal Research and Development ServicesОценок пока нет
- The Development of Nursing Care Model in Patients With Total Knee Replacement Reconstructive SurgeryДокумент17 страницThe Development of Nursing Care Model in Patients With Total Knee Replacement Reconstructive SurgeryGlobal Research and Development ServicesОценок пока нет
- How Bi-Lingual Fathers Support Their Children To Learn Languages at HomeДокумент35 страницHow Bi-Lingual Fathers Support Their Children To Learn Languages at HomeGlobal Research and Development ServicesОценок пока нет
- Study of Urban Heat Island in Yogyakarta City Using Local Climate Zone ApproachДокумент17 страницStudy of Urban Heat Island in Yogyakarta City Using Local Climate Zone ApproachGlobal Research and Development ServicesОценок пока нет
- Development of A Topical Gel Containing A Dipeptidyl Peptidase-4 Inhibitor For Wound Healing ApplicationsДокумент15 страницDevelopment of A Topical Gel Containing A Dipeptidyl Peptidase-4 Inhibitor For Wound Healing ApplicationsGlobal Research and Development ServicesОценок пока нет
- "Higher, Faster, Stronger": Championing Classroom TeachingДокумент13 страниц"Higher, Faster, Stronger": Championing Classroom TeachingGlobal Research and Development ServicesОценок пока нет
- Work-Related Problems and Performance Level of Senior High School TeachersДокумент22 страницыWork-Related Problems and Performance Level of Senior High School TeachersGlobal Research and Development ServicesОценок пока нет
- Global E-Learning and Experiential Opportunities For Programs and StudentsДокумент12 страницGlobal E-Learning and Experiential Opportunities For Programs and StudentsGlobal Research and Development ServicesОценок пока нет
- Investigating The Effects of Social Media On The Well-Being of TeenagersДокумент18 страницInvestigating The Effects of Social Media On The Well-Being of TeenagersGlobal Research and Development ServicesОценок пока нет
- The Relationship of Teaching Clarity and Student Academic Motivation in Online ClassesДокумент9 страницThe Relationship of Teaching Clarity and Student Academic Motivation in Online ClassesGlobal Research and Development ServicesОценок пока нет
- The Hijiri Calendar and Its Conformance With The Gregorain Calendar by Mathematical CalculationsДокумент19 страницThe Hijiri Calendar and Its Conformance With The Gregorain Calendar by Mathematical CalculationsGlobal Research and Development ServicesОценок пока нет
- Thai and Non-Thai Instructors' Perspectives On Peer Feedback Activities in English Oral PresentationsДокумент22 страницыThai and Non-Thai Instructors' Perspectives On Peer Feedback Activities in English Oral PresentationsGlobal Research and Development ServicesОценок пока нет
- Review of Vibration-Based Surface & Terrain Classification For Wheel-Based Robot in Palm Oil PlantationДокумент14 страницReview of Vibration-Based Surface & Terrain Classification For Wheel-Based Robot in Palm Oil PlantationGlobal Research and Development ServicesОценок пока нет
- Building A Scale of Competence To Apply Interdisciplinary Integrated Knowledge Into Practice For StudentДокумент10 страницBuilding A Scale of Competence To Apply Interdisciplinary Integrated Knowledge Into Practice For StudentGlobal Research and Development ServicesОценок пока нет
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeОт EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeРейтинг: 4 из 5 звезд4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceОт EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceРейтинг: 4 из 5 звезд4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)От EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Рейтинг: 4 из 5 звезд4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingОт EverandThe Little Book of Hygge: Danish Secrets to Happy LivingРейтинг: 3.5 из 5 звезд3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerОт EverandThe Emperor of All Maladies: A Biography of CancerРейтинг: 4.5 из 5 звезд4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItОт EverandNever Split the Difference: Negotiating As If Your Life Depended On ItРейтинг: 4.5 из 5 звезд4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyОт EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyРейтинг: 3.5 из 5 звезд3.5/5 (2259)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureОт EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureРейтинг: 4.5 из 5 звезд4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryОт EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryРейтинг: 3.5 из 5 звезд3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnОт EverandTeam of Rivals: The Political Genius of Abraham LincolnРейтинг: 4.5 из 5 звезд4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaОт EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaРейтинг: 4.5 из 5 звезд4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersОт EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersРейтинг: 4.5 из 5 звезд4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaОт EverandThe Unwinding: An Inner History of the New AmericaРейтинг: 4 из 5 звезд4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreОт EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreРейтинг: 4 из 5 звезд4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)От EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Рейтинг: 4.5 из 5 звезд4.5/5 (121)
- Acknowledgemt: at The Outset of This Project, I Would Like To Express My Deep Gratitude To Mr. RajnishДокумент6 страницAcknowledgemt: at The Outset of This Project, I Would Like To Express My Deep Gratitude To Mr. RajnishPraveen SehgalОценок пока нет
- Bojan ResumeДокумент2 страницыBojan ResumebokiОценок пока нет
- Event in Java PDFДокумент2 страницыEvent in Java PDFMariaОценок пока нет
- Promoting Change Through Paradoxical Therapy - Gerald Weeks PDFДокумент575 страницPromoting Change Through Paradoxical Therapy - Gerald Weeks PDFmarina_apsi100% (2)
- Páginas DesdeTeach Yourself The Basics of Aspen Plus by Ralph Schefflan 2011Документ2 страницыPáginas DesdeTeach Yourself The Basics of Aspen Plus by Ralph Schefflan 2011AlanAlcazarОценок пока нет
- Quantitative Analysis For Management Ch06Документ119 страницQuantitative Analysis For Management Ch06Qonita NazhifaОценок пока нет
- Meanings Symbols and Local Wisdomin The Pinakang Dance Movements Costumesand Accessories of The Kimaragang in SabahДокумент6 страницMeanings Symbols and Local Wisdomin The Pinakang Dance Movements Costumesand Accessories of The Kimaragang in SabahFredBTC007Оценок пока нет
- Fundamentals of ProbabilityДокумент58 страницFundamentals of Probabilityhien05Оценок пока нет
- EEE Syllabus 22 June 2013Документ19 страницEEE Syllabus 22 June 20131manoj1Оценок пока нет
- 3.5 Standard Operations Research Models: Mathematical Modify ModelДокумент2 страницы3.5 Standard Operations Research Models: Mathematical Modify ModelAnonymous IjhB0kuFОценок пока нет
- Presentation Template IR For ManagementДокумент13 страницPresentation Template IR For ManagementYosia SuhermanОценок пока нет
- Course Structure Course: Bio101 Essentials of Biology, Lab (4 CR) PrerequisiteДокумент2 страницыCourse Structure Course: Bio101 Essentials of Biology, Lab (4 CR) PrerequisiteAaron ChongОценок пока нет
- Final BriefДокумент4 страницыFinal BriefPranav Pradyumna Gurulinga murthyОценок пока нет
- Obturation of Root Canal LectureДокумент8 страницObturation of Root Canal LectureOsama AsadiОценок пока нет
- Elester 1500 PR E 1 in EnglishДокумент69 страницElester 1500 PR E 1 in Englishjaved shaikh chaandОценок пока нет
- SICK DT35 Setup InstructionsДокумент6 страницSICK DT35 Setup InstructionsTalicni TomОценок пока нет
- Practice Problems 2012Документ5 страницPractice Problems 2012Anonymous Fj3YPHОценок пока нет
- Monorail Beam - Topside Platform - OffshoreДокумент6 страницMonorail Beam - Topside Platform - OffshoreBolarinwaОценок пока нет
- YDH FEED VerificationДокумент10 страницYDH FEED VerificationbillОценок пока нет
- Chapter 4 Genes Evolution and BehaviourДокумент13 страницChapter 4 Genes Evolution and BehaviourAlex LiОценок пока нет
- GLSL Specification 1.40.08.fullДокумент111 страницGLSL Specification 1.40.08.fullmushakkОценок пока нет
- Layout Strategies: © 2008 Prentice Hall, Inc. 9 - 1Документ17 страницLayout Strategies: © 2008 Prentice Hall, Inc. 9 - 1jeams vidalОценок пока нет
- Amateur Photographer - May 28, 2016Документ84 страницыAmateur Photographer - May 28, 2016Lee100% (1)
- Practice CompletaДокумент3 страницыPractice CompletaEmanuel G.Оценок пока нет
- Nishant Srivastav IPR Full PaperДокумент16 страницNishant Srivastav IPR Full PaperDIVYANSHI PHOTO STATEОценок пока нет
- Sl500 User Manual NewДокумент40 страницSl500 User Manual NewAmrutha SudhakaranОценок пока нет
- Interaction Diagrams Components: ObjectДокумент4 страницыInteraction Diagrams Components: ObjectrekhathiyagarajanОценок пока нет
- Comparison of USCS and AASHTOДокумент2 страницыComparison of USCS and AASHTOkitefly100% (2)
- Advantages and Disadvantages of Competit PDFДокумент2 страницыAdvantages and Disadvantages of Competit PDFDindamilenia HardiyasantiОценок пока нет
- Statistics PDFДокумент17 страницStatistics PDFSauravОценок пока нет