Академический Документы
Профессиональный Документы
Культура Документы
Msds Methanol
Загружено:
Dinda DindaАвторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Msds Methanol
Загружено:
Dinda DindaАвторское право:
Доступные форматы
International Journal of Science and Technology Volume 2 No.
10, October, 2013
Performance Characteristics of Argentometric Method of
Cyanide Determination
AttahDaniel B.E1, Ebisike, K1., Adeeyinwo C.E2 , Adetunji A.R.3, Olusunle S.O.O.1 ,Adewoye O.O.4
1
Engineering Materials Development Institute, Akure;
2
Federal University of Technology, Akure;
3
Prototype Equipment Development Institute, Ilesa;
4
African University of Science and Technology, Abuja.
ABSTRACT
The performance characteristics of the argentometric method of cyanide determination was investigated using the calibration
curve method. The assessment offer linearity within the range 1.7x10 -3moles to 3.5x10-3moles with a sensitivity of
0.6millimoles of cyanide per dm3 of 0 .0200 moldm-3 AgNO3. It gave a detection limit of 0.7 millimoles (0.02gCN).The
interference studies within the linear range shows that halides (Cl -, Br -, I -) do not interfere in the analysis when there is
excess of CN - in the sample.
The assessed method was applied to determine cyanide in cassava samples and pack cyaniding compositions and residues.
Keywords: pack cyaniding, calibration curve
1. INTRODUCTION A comparative study was undertaken comparing the
electrochemical method with the colorimetric method [1].
Methods of cyanide determination in samples have been It was observed that in the electrochemical method, a
developed and more are still being developed or existing solution can be assayed for cyanide using an ion selective
methods are being improved upon. These methods include electrode and a double junction glass reference electrode
those applicable for determining cyanide at trace with a suitable cyanide electrode. The potential difference
concentrations especially in water samples. These are could be measured with a pH meter/multimetre available
mainly instrumental methods like the rapid and sensitive in simple laboratories [1]. But interferences due to the
gas chromatographic method in which cyanide ion is presence of Cl -, Br -, I - at high concentrations require the
reacted with Bromine to form CNBr in an acidic medium running of blank measurement for each analysis. The
and extracted into benzene. electrochemical method was found to be simple but
complicated and cannot be used for samples containing
The benzene extract is then injected into a gas less than 20 mg HCN Kg-1 because of the need for blank
chromatograph and the CNBr gives a narrow peak which determination in each analysis. The average deviation of
results in linear calibration plot [1]. Another one is the electrochemical results from the colorimetric method is
fibre optics Flourimetric method. In this method, ascorbic 8.5% [1].
acid is used in a calcein Cu-CN system and gives a
detection limit of 0.2ppb while in a flow injection system A picric kit for the determination of cyanogens in all
cyanide is determined selectively at concentration in the cassava products within the range of 10-800 mg HCN
range 2x10-6 mol/L by injection of 20µl samples at a rate equivalent Kg-1 even in the field has also been developed
of 360 samples per hour. By the use of continuous flow, [3]. A titrimetric method of cyanide quantification using
cyanide could be determined at levels down to 5x10 -7 silver nitrate with the difficulty of the precipitate
mol/L [1]. redissolving was improved upon by adding ammonia
solution in which it is readily soluble and a little
A simplified colorimetric method was developed by potassium iodide as the indicator before titration is
replacing pyridine/barbituric acid as a colour reagent with commenced or by employing diphenyl carbazide as an
isonicotinic/barbituric acid which was found to be more absorption indicator in which the end point is marked by
effective than the former [2]. In this method, cyanide ion the pink colour becoming pale violet i.e. almost colourless
is first oxidized to a cyanogen halide and of the colour [4].
reagent added at room temperature for colour
development and the absorbance taken at 600nm. Analytical techniques available for determination of CN-
in plants and biological fluids include amperometery [13],
voltammetery [15], polarography [20], potentiometery
IJST © 2013– IJST Publications UK. All rights reserved. 735
International Journal of Science and Technology (IJST) – Volume 2 No. 10, October, 2013
[22], piezoelectricity [16], gas chromatography [21], method of cyanide quantification to determining its
visible spectrophotometry [18], mass spectrometry [14], detection limit, precision and sensitivity using the
HPLC [12] and flow injection [11, 19]. calibration curve method. After which it was used to
monitor cyanide in the pack cyaniding of mild steel
The variety of analytical methods reported for CN- in samples.
blood may indicate the difficulty of its analysis in that
there is no universally preferred method [17]. Available Standard solutions of potassium cyanide of 0.005M
methods are expensive, laborious, and require technical concentration serially diluted were titrated with 0.0200M
expertise and sophisticated equipment [11]. These silver nitrate solution to determine the detection limit,
equipment are usually difficult to find in simple sensitivity, and the precision of the method using the
laboratories. This work was done to address this difficulty calibration curve method. The calibration curve method
in our laboratory and could address similar challenges in was used in the determination of the detection limit and
other simple laboratory. sensitivity of the silver nitrate titration of cyanide. The
plot of the volume of silver nitrate used in cm3 against
Cassava is known to contain the cyanogenic glucosides- concentration of potassium cyanide in moles is shown in
linamarin and lotaustralin in the ratio 93:7 in the leaves figure 1.
[5]. Cyanogenic glucoside of 1500 mg HCN equivalent
Kg-1 dry weight of fresh root of bitter cassava has been 3. EXTRACTION OF CYANOGENS
reported [6]. Other works have also reported that more
cyanogens are concentrated in the peel of the sweet Cyanogens were extracted from cassava leaves by
cultivar and the leaves containing about 200 mg HCN Kg- blending 50g each of processed cassava leaves with cold
1
[7]. Also, cyanogenic concentration of between 193.3- dilute ortho phosphoric acid (0.1000M) in a household
951.5 mg HCN Kg-1 has been reported [8]. blender and centrifuged. The cold ortho phosphoric acid
prevents the evaporation of cyanide [9] and inhibits the
2. MATERIALS AND METHOD endogenous linamarase from acting on linamarin and
stabilizes the cyanohydrin till hydrolysis [10]. The
Chemicals used for the investigation of the Silver Nitrate extracts were hydrolyzed in boiling tubes using 4.0M
titration method were BDH analytical grade chemicals. H2SO4 in a water bath at 1000C for 55 minutes.Then
While cassava (Manihot esculenta crantz) samples were allowed to cool to room temperature and 3.6M NaOH was
from the Teaching and Research farm of the Federal added for the spontaneous breakdown of cyanohydrin to
University of Technology Akure. release and fix CN - in NaOH.
In this work, the leaves of the bitter cultivar of cassava A portion of the aliquot was titrated with 0.0200M
were processed, analysed and used for pack cyaniding AgNO3 using KI as the indicator and 2M NH4OH added
using the argentometric method of cyanide determination. as solubilizer. The results of the application are shown in
Table 1.
The present study first presents an investigation of the
performance characteristics of the silver nitrate titration
4. RESULTS AND DISCUSSION
Table 1: Concentration of Cyanide ion in different parts of Cassava plant
Cassava Fresh Fresh peels Fresh tuber Fresh whole Dried leaves Dried peels Dried whole
Sample leaves tissue tuber tissue tuber
mgCN in 300±6 268±9.2 342±2.6 266±3.2 160±3.2 140±1.0 232±4.2
50g sample
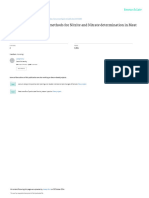
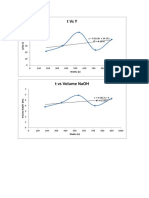
The plot of volume AgNO3(dm3) Vs Concentration of deviation of 0.7millimoles (0.02g).The interference
KCN (in moles) in figure 1 shows linearity from studies within the linear range showed that halides (Cl -,
concentrations of 1.7x10-3 mol dm-3 to 3.5x10-3mol dm-3. Br -, I -) do not interfere with the analysis as in the
This implies that at concentrations below 1.7x10 -3 electrochemical method which require the running of
molodm-3 this method will not be reliable. The method blank measurement for each analysis. Figures 2 to 6 show
showed sensitivity of 0.6millimoles of CN- dm-3 of 0.0200 plots of the interference studies which validate the
moldm-3AgNO3 and detection limit at thrice standard
IJST © 2013– IJST Publications UK. All rights reserved. 736
International Journal of Science and Technology (IJST) – Volume 2 No. 10, October, 2013
simplicity of the method void of the complicity of running blank for each sample analysed.
y - axis GRAPH OF AgNO3 (cm3)
vs CONCENTRATION
Volume of
OF KCN(moles)
AgNO3
(cm3)
5
0 0.02 0.04 0.06 0.08 0.1 0.2 0.4 0.6 0.8 1.0 2.0 4.0 6.0 (X 10 -3 ) x - axis
Concentration of KCN (moles)
Figure 1: Plot of AgNO3 (dm3) Vs Concentration of KCN (moles)
Figure 2: Graph of volume of 0.200M AgNO3 used (cm3) against KCN concentration in moles
Figure 3: Graph of volume of 0.200M AgNO3 used (cm3) against KCN standard concentration
plus 1cm3 0.00483moles BaCl2
IJST © 2013– IJST Publications UK. All rights reserved. 737
International Journal of Science and Technology (IJST) – Volume 2 No. 10, October, 2013
Figure 4: Graph of volume of 0.200M AgNO3 used (cm3) against KCN standard concentration
plus 2cm3 0.00483moles BaCl2
Figure 5: Graph of volume of 0.200M AgNO3 used (cm3) against KCN standard concentration
plus 3cm3 0.00483moles BaCl2
Figure 6: Graph of volume of 0.200M AgNO3 used (cm3) against KCN standard concentration
IJST © 2013– IJST Publications UK. All rights reserved. 738
International Journal of Science and Technology (IJST) – Volume 2 No. 10, October, 2013
plus 4cm3 0.00483moles BaCl2
The results of the cassava samples showed the fresh tuber edition Longman Group London. p 74-75; 258-259
having the highest concentration of cyanide, 342.2 ± and 271-272.
2.6mgCN and the list concentration by the dried peels,
140±1.0mgCN. While the dried pulverized leaves to be [5] Adewusi, S.R.A, Ojumu, T.V. and Falade, O.S.
used in pack cyaniding had cyanide concentration of 160 1999.The effect of processing on total organic acids
±3.2mg/50g. The application of the assessed method was content and mineral availability of simulated
to monitor the usage of cyanide from cassava for pack Cassava-vegetable diets. In Plants Foods for Human
cyaniding of mild steel indicated no presence of cyanide. Nutrition 53: 367-380, Kluver Academic publishers,
The non detection of cyanide in the pack cyaniding Netherlands pp 367 – 370.
residue is suspected to have resulted from either the
cyanide being used up during pack cyaniding or the [6] Rosling, H. 1988.Cassava toxicity and food security.
concentration of cyanide could be at trace level which A review of health effects of Cyanide exposure from
could not be detected using this method. Cassava and ways to prevent these effect, in report to
UNICEF. Printed by Toyck, Kontakt, Uppsala,
5. CONCLUSION Sweden, pp 5-9.
The linear range of this method was found to be between [7] Okafor, N. 1998. An integrated Bio-System for the
1.7x10-3 mol dm-3 and 3.5x10-3mol dm-3. The sensitivity Disposal of Cassava wastes. In Integrated Biosystems
was 0.6millimoles of CN- cm-3 of 0.0200moldm-3AgNO3 in Zero Emissions Applications Proceedings of the
and detection limit at thrice standard deviation was internet conference on Integrated Bio-systems, pp1-6.
0.7millimoles (0.02g).
[8] Agbor-Egbe, T., Albome, L.I. and Triche, S., 2001
There was no interference by halides (Cl -, Br -, I -) Effectiveness of processing Techniques in reducing
requiring the running of blank measurement for each total Cyanogenic during Cassava production of some
analysis. Cassava foods. Book of abstract, 5th International
The simplicity of the method and immunity to halides Scientific meeting of the Cassava Biotechnology
interference within the linear range makes it a reliable Network CBN-V.
method in the absence of modern instrumental method of
analyzing cyanide. [9] Bradbury, H. J. and Holloway D. W., 1988.Cyanide
analysis in Cassava. In Chemistry of Tropical Root
ACKNOWLEDGEMENT crops: Significance for nutrition and Agriculture in
the pacific, Australian Centre for International
The authors express their gratitude to the Raw Materials Research.
and Development Council for the sponsorship for this
project. [10] Bokanga M. 1994. Distribution of Cyanogenic
potential in Cassava germplasm: In Cassava
Safety. Acta Horticulturae 373. pp 117
REFERENCES
[11] Muhammad Avais, Muhammad Sarwar Khan,
[1] MacCarthy, P. and Klusman, W. Ronald and Rice, S
Muhammad Arif Khan, Kamran Ashraf, Amar Nasir,
James, 1987. Water Analysis 321R. In Analytical
Masood Rabbani and Abu Saeed Hashmi, (2011),
Chemistry Vol. 52 No. 12.
‘Modified Picrate Method for Determination of
Cyanide in Blood’, Pak. J. Pharm. Sci., Vol.24, No.2,
[2] Bradbury H.J., Bradbury, G. Meredith and Egan, V.
April 2011, pp.149-153
S. 1994.Comparism of methods of analysis of
Cyanogens in Cassava.In Cassava Society, Acta
[12] Akiyama H, Toida T, Sakai S, Amakura Y, Kondo K,
Horticulturae 375 pp 87-95.
Konishi YS and Matani T (2006). Determination of
cyanide and thiocyanate in Sugihiratake mushroom
[3] Sylvia V.E., Hock, H. Y., and J. Howard Bradbury,
using HPLC method with fluorometric detection. J.
1998, Simple picrate paper kits for determination of
Hlth. Sci., 52: 73-77.
the Cyanogenic potential of Cassava flour: Journal of
the Science of Food and Agriculture; 1978, 76: 39
[13] Park TM, Iwuoha EI and Smyth MR (1997).
Development of a Sol-Gel Enzyme Inhibition-Based
[4] Vogel, I.A, 1961.Determination of Cyanide, in a
Amperometric Biosensor for Cyanide.
textbook of Quantitative Inorganic Analysis
Electroanalysis, 9: 1120-1123.
including Elemental instrumental Analysis, third
IJST © 2013– IJST Publications UK. All rights reserved. 739
International Journal of Science and Technology (IJST) – Volume 2 No. 10, October, 2013
[14] Tracqui A, Raul JS, Geraut A, Berthelon L and Ludes spectrophotometric blood cyanide determination
B (2002). Determination of blood cyanide by HPLC- applicable to emergency toxicology. J. Anal.
MS. J. Anal. Toxicol., 26: 144-148. Toxicol., 18: 173-175
[15] Tatsuta H, Nakamura T and Hinoue T (2001). [19] Recalde-Ruiz DL, Andrés-García E and Díaz-García,
Thermal modulation voltammetric observation of ME (2000). Fluorimetric flow injection and flow-
cyanide ion in the membrane part of an ion-selective through sensing systems for cyanide control in waste
electrode based on a polymer modified with cobalt water. Analyst, 125: 2100-2105
phthalocyanine in acetonitrile. Anal. Sci., 17: 991.
[20] do Nascimento PC, Bohrer D and de Carvalho LM
[16] Gomes MTSR, Silva AAF, Duarte AC and Oliveira (1998). Cyanide determination in biological fluids
JABP (1998). Determination of cyanide in waste using a microdiffusion method with a flow system
waters using a quartz crystal microbalance. Sensors and polarographic detection. Analyst, 123: 1151-
and Actuators B: Chemical., 48: 383-386. 1154
[17] Hughes C, Lehner F, Dirikolu L, Harkins D, Boyles [21] Cardeal ZL, Gallet JP and Pradeau D (1995). Cyanide
JK, McDowell and Tobin T (2003). A simple and assay: statistical comparison of new gas
highly sensitive spectrophotometric method for the chromatographic method versus the classical
determination of cyanide in equine blood. Toxicol. spectrometric method. J. Anal. Toxicol., 19: 31-34.
Mech. Methods, 13: 129-138.
[22] Sequeira M, Hibbert DB and Alexander PW (1999).
[18] Laforge M, Buneaux F, Houeto H, Bourgeois F, A portable cyanide analyzer using gold wire
Bourdon R and Levillain P (1994). A rapid electrodes. Electroanalysis, 11: 194-198
IJST © 2013– IJST Publications UK. All rights reserved. 740
Вам также может понравиться
- Jurnal Argentometri Kelompok 1Документ7 страницJurnal Argentometri Kelompok 1cut puspitaОценок пока нет
- Keywords:-Spectrophotometry, Nickel, Triton X-100, 5 - (2'Документ9 страницKeywords:-Spectrophotometry, Nickel, Triton X-100, 5 - (2'International Journal of Innovative Science and Research TechnologyОценок пока нет
- Petruci 2018Документ29 страницPetruci 2018Ngọc Ánh LêОценок пока нет
- Exp 206P M.tech2ndДокумент70 страницExp 206P M.tech2nddishika1991Оценок пока нет
- Spectrophotometric Determination of Hydrazine With para - (Dimethylamino) Benzaldehyde in Aqueous Streams of Purex ProcessДокумент4 страницыSpectrophotometric Determination of Hydrazine With para - (Dimethylamino) Benzaldehyde in Aqueous Streams of Purex ProcessSEP-PublisherОценок пока нет
- 1 Selective Determination of ChlorineДокумент7 страниц1 Selective Determination of Chlorinevey bieberОценок пока нет
- Bacteria Potassium SolubilizingДокумент6 страницBacteria Potassium Solubilizing7641217Оценок пока нет
- Gold Nanoparticles-Based Colorimetric Sensor For Cysteine DetectionДокумент9 страницGold Nanoparticles-Based Colorimetric Sensor For Cysteine Detectionbelqis ratuОценок пока нет
- Archer 2003Документ4 страницыArcher 2003Jon Be GoodОценок пока нет
- Peng Et Al. (2019)Документ6 страницPeng Et Al. (2019)Natasha MaharaniОценок пока нет
- A Morphology-Based Ultrasensitive Multicolor Colorimetric Assay For Detection of Blood Glucose by Enzymatic Etching of Plasmonic Gold NanobipyramidsДокумент6 страницA Morphology-Based Ultrasensitive Multicolor Colorimetric Assay For Detection of Blood Glucose by Enzymatic Etching of Plasmonic Gold NanobipyramidsPhat HuynhОценок пока нет
- Extractive Spectrophotometric Determination of Nicergoline Through Ion-Pair Complexation ReactionДокумент7 страницExtractive Spectrophotometric Determination of Nicergoline Through Ion-Pair Complexation ReactionHeidi HughesОценок пока нет
- Derivative Spectrophotometric Determination of Mercury (Ii) Using 3,4-Dihydroxybenzaldehydetiosemicarbazone (DHBTSC) in Presence of Micelle MediumДокумент8 страницDerivative Spectrophotometric Determination of Mercury (Ii) Using 3,4-Dihydroxybenzaldehydetiosemicarbazone (DHBTSC) in Presence of Micelle MediumInternational Organization of Scientific Research (IOSR)Оценок пока нет
- Molecules: HPLC-DAD Determination of Nitrite and Nitrate in Human Saliva Utilizing A Phosphatidylcholine ColumnДокумент15 страницMolecules: HPLC-DAD Determination of Nitrite and Nitrate in Human Saliva Utilizing A Phosphatidylcholine ColumnFajar Ari HidayatОценок пока нет
- L. Voltammetric Determination of CodeineДокумент8 страницL. Voltammetric Determination of CodeineRoxana StanОценок пока нет
- Bruno, CaselliДокумент6 страницBruno, CaselliYury Andrea MartinezОценок пока нет
- Microchemical Journal: Shuyun Bi, Rui Zhao, Yue Yuan, Xu Li, Di ShaoДокумент6 страницMicrochemical Journal: Shuyun Bi, Rui Zhao, Yue Yuan, Xu Li, Di ShaoAna-Maria DucuОценок пока нет
- F4a7 PDFДокумент7 страницF4a7 PDFSarah riantiОценок пока нет
- Citrate-Capped Silver NanoparticlesДокумент10 страницCitrate-Capped Silver NanoparticlesElizabeth PeraltaОценок пока нет
- Tia KloramfenicolДокумент10 страницTia KloramfenicolDEVI SETYA ARIANIОценок пока нет
- Marina Icomst2013.Org T e Book Papers Poster S7B 19 PDFДокумент4 страницыMarina Icomst2013.Org T e Book Papers Poster S7B 19 PDFdofladonquixote0Оценок пока нет
- A Spectrophotometric Method For The Determination of Nitrite and NitrateДокумент11 страницA Spectrophotometric Method For The Determination of Nitrite and Nitratepatrica_23velezОценок пока нет
- A New System For The Spectrophotometric Determination of Arsenic in Environmental and Biological SamplesДокумент5 страницA New System For The Spectrophotometric Determination of Arsenic in Environmental and Biological Samplesmuhammad hadisulhanОценок пока нет
- النانو في الموبايلДокумент5 страницالنانو في الموبايلmarwaneman1998Оценок пока нет
- Kmno4 Apha 4500Документ4 страницыKmno4 Apha 4500Daryusman0% (1)
- Solvent Free Green Synthesis of 5-Arylidine Barbituric Acid Derivatives Catalyzed by Copper Oxide NanoparticlesДокумент6 страницSolvent Free Green Synthesis of 5-Arylidine Barbituric Acid Derivatives Catalyzed by Copper Oxide NanoparticlesbaskhemОценок пока нет
- Maulidina Pasha - 01211840000103 RESUME ISoC 2020Документ3 страницыMaulidina Pasha - 01211840000103 RESUME ISoC 2020Maulidina PashaОценок пока нет
- Spectrophotometric Determination of ArseДокумент4 страницыSpectrophotometric Determination of ArseTalhaОценок пока нет
- Penentuan Kadar Ion Magnesium Dalam Air Dengan Metoda Pengendapan SelektifДокумент7 страницPenentuan Kadar Ion Magnesium Dalam Air Dengan Metoda Pengendapan SelektifBudi Santoso AsmaliОценок пока нет
- Colorimetric Determination KДокумент4 страницыColorimetric Determination KHendi abdillah BadjoОценок пока нет
- English FarmasiДокумент16 страницEnglish FarmasiWidya Gusti PradiniОценок пока нет
- 8271-Article Text-29026-1-10-20130629 PDFДокумент4 страницы8271-Article Text-29026-1-10-20130629 PDFPankaj DahalОценок пока нет
- Determination of Bendamustine Hydrochloride in Pure and Dosage Forms by Ion-Associative Complex FormationДокумент6 страницDetermination of Bendamustine Hydrochloride in Pure and Dosage Forms by Ion-Associative Complex FormationHeidi HughesОценок пока нет
- W6 PDFДокумент2 страницыW6 PDFHardi AhmedОценок пока нет
- Lu 2020Документ7 страницLu 2020Azad H AlshatteriОценок пока нет
- Bradbury 1999Документ9 страницBradbury 1999asdsffggeettrgbfbfbftggrg ergrtertererefrerrОценок пока нет
- (Materials Science-Poland) Nanosized MoO3 As A Reusable Heterogeneous Catalyst For The Synthesis of 26-Bis (Benzylidene) CyclohexanonesДокумент6 страниц(Materials Science-Poland) Nanosized MoO3 As A Reusable Heterogeneous Catalyst For The Synthesis of 26-Bis (Benzylidene) CyclohexanonesMina ArshadОценок пока нет
- Journal of Radioanalytical and Nuclear CДокумент9 страницJournal of Radioanalytical and Nuclear Cnauman18Оценок пока нет
- Breuer 09Документ6 страницBreuer 09Jesus ParedesОценок пока нет
- 1 s2.0 S003991400600631X MainДокумент6 страниц1 s2.0 S003991400600631X MaincutdianОценок пока нет
- CP and CNP - SizeДокумент27 страницCP and CNP - SizemayamaruguerraОценок пока нет
- Alternative Do MeasurementДокумент3 страницыAlternative Do Measurementfendi setyoОценок пока нет
- Arab 2021Документ7 страницArab 2021AbdurRehman QamarОценок пока нет
- ANDINI FIKARDA - 102316030 - PAPER - Bu Laksmi EditednewДокумент22 страницыANDINI FIKARDA - 102316030 - PAPER - Bu Laksmi Editednewbarbara edisonОценок пока нет
- Diazotasi 2Документ4 страницыDiazotasi 2Iinthand BEncii DyОценок пока нет
- Gas-Liquid Separator Integrated To HG-QFAAS Method For Determination of Tin at Trace Levels in The Water SamplesДокумент11 страницGas-Liquid Separator Integrated To HG-QFAAS Method For Determination of Tin at Trace Levels in The Water SamplesGanjar OfficialОценок пока нет
- Determination of Cyanogenic Glucosides in Cassava Products Sold in Okada, Edo State, NigeriaДокумент5 страницDetermination of Cyanogenic Glucosides in Cassava Products Sold in Okada, Edo State, NigeriaAnonymous GYqBjXoОценок пока нет
- Experiment 3Документ10 страницExperiment 3Nisha Rata KarusanОценок пока нет
- Sensors and Actuators B: Chemical: Yanli Zhou, Hui Dong, Lantao Liu, Miaomiao Li, Kaixia Xiao, Maotian XuДокумент6 страницSensors and Actuators B: Chemical: Yanli Zhou, Hui Dong, Lantao Liu, Miaomiao Li, Kaixia Xiao, Maotian Xubelqis ratuОценок пока нет
- Measurement of Radionuclides in Imported Coffee Consumed in Basra Southern of Iraq and Estimation of Its Annual Effective DoseДокумент5 страницMeasurement of Radionuclides in Imported Coffee Consumed in Basra Southern of Iraq and Estimation of Its Annual Effective DoseShivam SinghОценок пока нет
- 17Документ7 страниц17api-3828788Оценок пока нет
- Determination of Mercury in Water by Hand Held Portable Atomic Fluorescence Spectrometry PDFДокумент4 страницыDetermination of Mercury in Water by Hand Held Portable Atomic Fluorescence Spectrometry PDFPL CarpenteroОценок пока нет
- Adsorption of Indigo Blue Dye and Other Toxic MetalsДокумент8 страницAdsorption of Indigo Blue Dye and Other Toxic MetalsCristel_de_Jesus_284Оценок пока нет
- Journal of Chromatography AДокумент5 страницJournal of Chromatography ACristina Martín JiménezОценок пока нет
- 127.1 E6 Formal ReportДокумент7 страниц127.1 E6 Formal ReportAlma PabilaneОценок пока нет
- NinhydrinДокумент6 страницNinhydriniabureid7460Оценок пока нет
- Comparison of Different Methods For NitrДокумент3 страницыComparison of Different Methods For NitrasdfghjklОценок пока нет
- Colorimetric and Visual Determination of Acrylamide Via Acrylamide-Mediated Polymerization of Acrylamide-Functionalized Gold NanoparticlesДокумент9 страницColorimetric and Visual Determination of Acrylamide Via Acrylamide-Mediated Polymerization of Acrylamide-Functionalized Gold NanoparticlesMohamad ZulkarnaenОценок пока нет
- Analysis of Organoaluminium and Organozinc Compounds: International Series of Monographs in Analytical ChemistryОт EverandAnalysis of Organoaluminium and Organozinc Compounds: International Series of Monographs in Analytical ChemistryОценок пока нет
- Grafik Objek 5 KKДокумент2 страницыGrafik Objek 5 KKDinda DindaОценок пока нет
- Yasscond2016 PDFДокумент5 страницYasscond2016 PDFDinda DindaОценок пока нет
- Yasscond2016 PDFДокумент5 страницYasscond2016 PDFDinda DindaОценок пока нет
- Lab On Paper: Iodometric Titration On A Printed Card: Nicholas M. Myers, Emalee N. Kernisan, and Marya LiebermanДокумент7 страницLab On Paper: Iodometric Titration On A Printed Card: Nicholas M. Myers, Emalee N. Kernisan, and Marya LiebermanDinda DindaОценок пока нет
- Estimation of Chloride Hardness in Drinking Water in University Ofeducation Vehari Campus Vehari Punjab Pakistan 2150 3494 1000179Документ3 страницыEstimation of Chloride Hardness in Drinking Water in University Ofeducation Vehari Campus Vehari Punjab Pakistan 2150 3494 1000179Dinda DindaОценок пока нет
- Objek 1Документ6 страницObjek 1Dinda DindaОценок пока нет
- Chiba International, IncДокумент15 страницChiba International, IncMiklós SzerdahelyiОценок пока нет
- InfltiДокумент13 страницInfltiLEKH021Оценок пока нет
- S L Dixon Fluid Mechanics and Thermodynamics of TurbomachineryДокумент4 страницыS L Dixon Fluid Mechanics and Thermodynamics of Turbomachinerykuma alemayehuОценок пока нет
- Sci5 q3 Module3 NoanswerkeyДокумент22 страницыSci5 q3 Module3 NoanswerkeyRebishara CapobresОценок пока нет
- Design and Implementation of Hotel Management SystemДокумент36 страницDesign and Implementation of Hotel Management Systemaziz primbetov100% (2)
- Cpar Final Written Exam 1Документ3 страницыCpar Final Written Exam 1Jeden RubiaОценок пока нет
- Industrial Training ReportДокумент19 страницIndustrial Training ReportKapil Prajapati33% (3)
- France 10-Day ItineraryДокумент3 страницыFrance 10-Day ItineraryYou goabroadОценок пока нет
- Amplifier Frequency ResponseДокумент28 страницAmplifier Frequency ResponseBenj MendozaОценок пока нет
- Urban LifestyleДокумент27 страницUrban LifestyleNindy AslindaОценок пока нет
- Calculating Measures of Position Quartiles Deciles and Percentiles of Ungrouped DataДокумент43 страницыCalculating Measures of Position Quartiles Deciles and Percentiles of Ungrouped DataRea Ann ManaloОценок пока нет
- Module No.3 Prepare Architectual Job Requirements Architectural Working DrawingДокумент23 страницыModule No.3 Prepare Architectual Job Requirements Architectural Working DrawingJay S. On100% (1)
- FoodhallДокумент3 страницыFoodhallswopnilrohatgiОценок пока нет
- Consumer PresentationДокумент30 страницConsumer PresentationShafiqur Rahman KhanОценок пока нет
- Checklist Code ReviewДокумент2 страницыChecklist Code ReviewTrang Đỗ Thu100% (1)
- The Community Reinvestment Act in The Age of Fintech and Bank CompetitionДокумент28 страницThe Community Reinvestment Act in The Age of Fintech and Bank CompetitionHyder AliОценок пока нет
- Guide Rail Bracket AssemblyДокумент1 страницаGuide Rail Bracket AssemblyPrasanth VarrierОценок пока нет
- 04 - Crystallogaphy III Miller Indices-Faces-Forms-EditedДокумент63 страницы04 - Crystallogaphy III Miller Indices-Faces-Forms-EditedMaisha MujibОценок пока нет
- Cavitation in Francis PDFДокумент373 страницыCavitation in Francis PDFAlberto AliagaОценок пока нет
- SITXWHS001 - Participate in Safe Work Practices Student GuideДокумент42 страницыSITXWHS001 - Participate in Safe Work Practices Student GuideMarianne FernandoОценок пока нет
- Man of The House Faq: About MothДокумент2 страницыMan of The House Faq: About MothPrapya BarmanОценок пока нет
- CP R80.10 Installation and Upgrade GuideДокумент246 страницCP R80.10 Installation and Upgrade GuideAlejandro OrtìzОценок пока нет
- Applied Social Research A Tool For The Human Services 9th Edition Monette Test Bank 1Документ36 страницApplied Social Research A Tool For The Human Services 9th Edition Monette Test Bank 1wesleyvasquezmeoapcjtrb100% (25)
- Homework 1 Tarea 1Документ11 страницHomework 1 Tarea 1Anette Wendy Quipo Kancha100% (1)
- E34-1 Battery Charging and Dishcharging BoardДокумент23 страницыE34-1 Battery Charging and Dishcharging BoardGanesa MurthyОценок пока нет
- Dhulikhel RBB PDFДокумент45 страницDhulikhel RBB PDFnepalayasahitya0% (1)
- CHECK - Chapter 11 TCD AnswersДокумент6 страницCHECK - Chapter 11 TCD AnswersbonolomphaОценок пока нет
- Haematology Test Name Results Biological Reference Interval Units Specimen Test Method CBC - Complete Blood CountДокумент8 страницHaematology Test Name Results Biological Reference Interval Units Specimen Test Method CBC - Complete Blood CountArun DheekshahОценок пока нет
- Problem Solving Questions: Solutions (Including Comments)Документ25 страницProblem Solving Questions: Solutions (Including Comments)Narendrn KanaesonОценок пока нет
- Mechanical Power FormulaДокумент9 страницMechanical Power FormulaEzeBorjesОценок пока нет