Академический Документы
Профессиональный Документы
Культура Документы
Xercise: Tough Subjective Problems
Загружено:
Ashu MishraОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Xercise: Tough Subjective Problems
Загружено:
Ashu MishraАвторское право:
Доступные форматы
Solutions Slot – 3 (Physics) Page # 7
EXERCISE – IV TOUGH SUBJECTIVE PROBLEMS
A–4
T Q T = 4.78 MeV 2
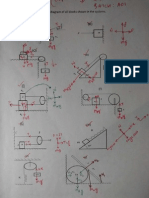
1.
A P1 ' 2 P2 ' 2 P1
A = 226 Q 2m 2m – 2m
p 0 1
226 – 4
4.78 × 106 = Q Q = 4.86 MeV
226 1 1 1 2 1 1 1 2
Q 2 m m P1 ' 2 m – m P1
2. Intial Activity R1 = N1 p 0 0 1
Activity after time t R2 = N2
Now, N2 = N1 e– t P2
Ek Now
Because only one -particle out of 4000 induces 2m
a reaction we can find the number of radon
atoms introduced into the source. mp m
Q K p 1 – K 1 1 1
m
nN m0 0
N' nN1 – 2t nN 2 e t
e
mass of radon m 1
AN' A Ane t .R 2 5. T1/2 =
= nN 2 e t
NA NA NA
Given that A = 222, n = 4000, T = 3.8 days dN
= fraction of body disintegrate in time dt
t = 7.6 days N
0.693
5
e t e 3 .8
6
2.49 , R2 = 1.2 × 10 sec dN
dt
N
m = 3.3 g
3. m = (10.01167 + 1.00894 – mL i – 4.00386) v t
dm dv dv udt
Q = 1.83 MeV or dt or dt
or Q = m × 931 MeV m v 0 0
m = 0.001965 v = u t
mL i = 7.01675 – 0.001965
m L i = 7.01478 a.m.u
Rate of decay N
4. Intially m1 has a monmentum P1 & m2 is at rest 6. N
(P2 = 0) in the lab frame. The masses of the
particular after collision are mp & mo.
Rate of formation
The conservation of momentum given
P1 'P2 ' P1 or P2 ' P1 – P1' ...(1)
Let N be the no of radionucler any time t. Then
net rate of form of nuclei at time t is
y y P1 '
N t
dN dN
– N or dt
dt – N 0
P1 m2 0
x x
m1 O P2=0 N (1 – e – t )
P2 ' Number of nuclei formed in time t = t
After collision & Number of nuclei left after time
Squaring above equation
t= (1 – e – t )
2 A
P2 ' 2 (P1 – P1 ' )2 P P1 ' 2 –2P1.P1 ' = P12 + P1’2
1
energy released till time
{ P1.P1 ' 0} – t
t = E 0 [t – (1 – e )]
394,50 - Rajeev Gandhi Nagar Kota, Ph. No. : 93141-87482, 0744-2209671
IVRS No : 0744-2439051, 52, 53, www.motioniitjee.com, info@motioniitjee.com
Page # 8 Solutions Slot – 3 (Physics)
But only 20% of it is used in rasing the tamprature Now A = A0 e–t
of water A0 = 50 × 12 = 600
A = 320
So 0.2 E 0 [t – (1 – e – t )] Q From above data t = 5196 years
10. Energy from one deacy
where Q = ms 248 244 4
96
cm 94
Pu 2He
Q
= increase in temprature of water = m = 248. 072220 – 244.064100 – 4.002603
ms
– t = 0.005 517
0.2 E 0 [t – (1 – e )]
E = mx931
ms = 5. 136327 Mev.
7. At the time of observation t = t
Total energy
m1 140 A 1 238
1.01 8 92 20
m2 1 A 2 235 = 100 200 100 5.1366327 10
m
Number of atoms N = = (20. 725421) Mev. × 1020
A
Average cufe – 1013 sec.
N1 m1 A 2 140
N m A 1.01 ...(i) Power output
A 2 1
Let N0 be the no. of atoms of both isotopes at
20 .725421 1020 1.6 1019 106
the time of formation the =
1013
N1 N0 e – 1t
– 2t
e ( 2 – 1 ) t ...(ii) = 33.16 W
N 2 N0 e
Equation (i) & (ii) we have len2
11. =
15 3600
140
e ( 2 – 1 ) t Activity of 24
Na after 5 hours
1.01
A = 1 × 10–6 × 3.7×1010
(2 – 1)t = n (140) – n (1.01)
1 cm3 296
4.9305
t 6.04 10 9 yrs x cm3 296 x
0.693 45 – 7.13
And 296 x = 3.7 × 104 × e–ln2/3
10 8 45 7.13
8. Given that Activity = 8.4 sec–1 x = 6 liters
According to Avagadro hypothesis the no. of 25
12. = x e 10
atoms in 2.5 mg. 100
6.02 10 23 1
N 2.5 10 – 3 e 10
230 2
N = 6.54 × 1018 ln 2
=
Now N = 8.4 sec–1 10
8 .4 8.4
N 6.54 1018 t1
= 10 sec.
= 1.28 × 10 sec–1
–18 2
0.6931
T 1.7 1010 year 10
tan g = ln 2
0.693 0.693
9. From t 1/ 2 t = 40 sec.
5730
394,50 - Rajeev Gandhi Nagar Kota, Ph. No. : 93141-87482, 0744-2209671
IVRS No : 0744-2439051, 52, 53, www.motioniitjee.com, info@motioniitjee.com
Вам также может понравиться
- Solution Manual for an Introduction to Equilibrium ThermodynamicsОт EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsОценок пока нет
- Ion Beams for Materials AnalysisОт EverandIon Beams for Materials AnalysisR. Curtis BirdОценок пока нет
- Electronic Devices VlsiДокумент23 страницыElectronic Devices VlsiSubhalaxmi BeheraОценок пока нет
- Isc Physics Prelim 1 - Modern Physics: (Solutions)Документ4 страницыIsc Physics Prelim 1 - Modern Physics: (Solutions)NeelОценок пока нет
- Solution Xi Physics Full Syllabus Paper 01Документ10 страницSolution Xi Physics Full Syllabus Paper 01atharvsawat1Оценок пока нет
- Nuklearna Fizika: Građa JezgraДокумент5 страницNuklearna Fizika: Građa JezgraArijana100% (1)
- AttachmentДокумент1 страницаAttachmentMuhammet CakmakОценок пока нет
- Tutorial 2 Ans PDFДокумент4 страницыTutorial 2 Ans PDFMa SeenivasanОценок пока нет
- Lecture 7. Vacuum Technology: 7-1. Kinetic Theory of GasesДокумент12 страницLecture 7. Vacuum Technology: 7-1. Kinetic Theory of Gases최종윤Оценок пока нет
- Basic Electronics Engineering PDFДокумент22 страницыBasic Electronics Engineering PDFAbhishekОценок пока нет
- 01 State of Matter Gaseous StateДокумент12 страниц01 State of Matter Gaseous StateAkashGauravОценок пока нет
- Quiz 4Документ13 страницQuiz 4Hân BảoОценок пока нет
- Gen PhysicsДокумент3 страницыGen PhysicsMin YeolОценок пока нет
- PCweek8 2015Документ7 страницPCweek8 2015Roy VeseyОценок пока нет
- Thermodynamics Problems1-100Документ5 страницThermodynamics Problems1-100Monique OrugaОценок пока нет
- Chapter 11 - Section A - Mathcad SolutionsДокумент33 страницыChapter 11 - Section A - Mathcad SolutionsKhalid M MohammedОценок пока нет
- Centrifugal Compressor Design.Документ2 страницыCentrifugal Compressor Design.Shoaib AliОценок пока нет
- Ph1FCAT04 OJEAD120C08 100619 SOLUTION PDFДокумент4 страницыPh1FCAT04 OJEAD120C08 100619 SOLUTION PDFmehul pantОценок пока нет
- 351 F 22 Exam EquationsДокумент1 страница351 F 22 Exam EquationsEdaОценок пока нет
- Thermal Engineering PDFДокумент76 страницThermal Engineering PDFKartik KuriОценок пока нет
- Legea Lui Maxwell Pentru Distribuția Moleculelor După Vitezele Lor RelativeДокумент3 страницыLegea Lui Maxwell Pentru Distribuția Moleculelor După Vitezele Lor RelativecristianОценок пока нет
- ) Energy (E He H H : Atomic Nucleus Solutions Level 3Документ9 страниц) Energy (E He H H : Atomic Nucleus Solutions Level 3Munish DograОценок пока нет
- Lecture 12. Life in the low Reynolds-number world: Zhanchun Tu (涂展春)Документ51 страницаLecture 12. Life in the low Reynolds-number world: Zhanchun Tu (涂展春)Devil ioОценок пока нет
- The Last Digit of 2nCn and Sigma..Документ8 страницThe Last Digit of 2nCn and Sigma..api-26401608Оценок пока нет
- KinjutsuДокумент21 страницаKinjutsuLocus Jhun MichaelОценок пока нет
- PHYS1001 Test 1 2017 SEM-1 SolutionsДокумент21 страницаPHYS1001 Test 1 2017 SEM-1 SolutionsAngad MangatОценок пока нет
- Cambridge International AS & A Level: PHYSICS 9702/41Документ28 страницCambridge International AS & A Level: PHYSICS 9702/41Tino KambaniОценок пока нет
- JEE Main 2017 Paper With SolutionДокумент25 страницJEE Main 2017 Paper With SolutionSreetejaNadellaОценок пока нет
- Jee (Main) - 2017 Test Paper With Solution (Held On Sunday 02 APRIL, 2017)Документ26 страницJee (Main) - 2017 Test Paper With Solution (Held On Sunday 02 APRIL, 2017)Shubham Anil ShahareОценок пока нет
- SOLUTIONS & ANSWERS FOR AIEEE-2011 (11-05-2011) Version - B: Part A Physics 1. Ans: 2Документ8 страницSOLUTIONS & ANSWERS FOR AIEEE-2011 (11-05-2011) Version - B: Part A Physics 1. Ans: 2Lokesh KumarОценок пока нет
- SPM 2021 Formula ListДокумент2 страницыSPM 2021 Formula ListAshley FooОценок пока нет
- Answer Test1 Phase III 15 03 2022 Main P1Документ11 страницAnswer Test1 Phase III 15 03 2022 Main P1ik62299Оценок пока нет
- Optics Tutorial 1 SolutionsДокумент5 страницOptics Tutorial 1 SolutionsKhushi AgrawalОценок пока нет
- Idc401 Theo Bio A2Документ15 страницIdc401 Theo Bio A2ms20101Оценок пока нет
- UT - 08 Advanced Paper - 1 Practice Paper Solution - Physics & MathsДокумент10 страницUT - 08 Advanced Paper - 1 Practice Paper Solution - Physics & Mathsshreshthagupta2111Оценок пока нет
- Physics - Solution Set For Homework Book Chapter - 15 KTG & ThermodynamicsДокумент4 страницыPhysics - Solution Set For Homework Book Chapter - 15 KTG & ThermodynamicsKishorОценок пока нет
- Homework 6 SolutionДокумент8 страницHomework 6 SolutionjohnОценок пока нет
- Scroll of Seals 1Документ25 страницScroll of Seals 1Anthony MacalindongОценок пока нет
- Analisa Struktur PortalДокумент21 страницаAnalisa Struktur PortalFRANSISKUS KELVIN SIMANTOОценок пока нет
- Analisa Struktur PortalДокумент21 страницаAnalisa Struktur PortalFRANSISKUS KELVIN SIMANTOОценок пока нет
- 2015 Physics Trial Exam Solutions PDFДокумент3 страницы2015 Physics Trial Exam Solutions PDForhanaliuОценок пока нет
- Fiitjee All India Test Series: Concept Recapitulation Test - IvДокумент14 страницFiitjee All India Test Series: Concept Recapitulation Test - IvItsecret NameОценок пока нет
- " For A Radioactive Source, The Decay Rate Is Directly Proportional To The Number of Radioactive Nuclei N Present in The SourceДокумент18 страниц" For A Radioactive Source, The Decay Rate Is Directly Proportional To The Number of Radioactive Nuclei N Present in The SourceSiow Shung ChurnОценок пока нет
- 10.37 Spring 2007 Homework 1 Due Wednesday, Feb. 14Документ6 страниц10.37 Spring 2007 Homework 1 Due Wednesday, Feb. 14ÅdnAn MehmOodОценок пока нет
- Exercise Sheet 9 - May 14th, 2018: MATH-F302 - Probabilit Es IIДокумент2 страницыExercise Sheet 9 - May 14th, 2018: MATH-F302 - Probabilit Es IIAlcalinaPhysicsОценок пока нет
- Civil Engineering Hydraulics Essential Theory With Worked Examples, 4th EditionДокумент172 страницыCivil Engineering Hydraulics Essential Theory With Worked Examples, 4th EditionPamungkas T.Оценок пока нет
- DC PandeyДокумент137 страницDC Pandeyanshuman.panda.odmОценок пока нет
- AITS 2324 OT I JEEA TD Paper 1 OFFLINE SolДокумент20 страницAITS 2324 OT I JEEA TD Paper 1 OFFLINE SolAshish SharmaОценок пока нет
- IB Physics Data BookletДокумент16 страницIB Physics Data Bookletzoe campbellОценок пока нет
- Euler PDFДокумент4 страницыEuler PDFWabii AddunyaaОценок пока нет
- Power Cycles 1 - 1 PDFДокумент6 страницPower Cycles 1 - 1 PDFclarkmaxОценок пока нет
- Solutions 5 XДокумент3 страницыSolutions 5 XRoy VeseyОценок пока нет
- Hints & Solutions: L Cos 2 2 Sin - Cos M 2 Cos - SinДокумент9 страницHints & Solutions: L Cos 2 2 Sin - Cos M 2 Cos - SinswarupОценок пока нет
- Potential EnergyДокумент10 страницPotential Energyjohn soniОценок пока нет
- QB Sol Relative VelocityДокумент9 страницQB Sol Relative Velocityagrawalayush040Оценок пока нет
- Ktgradiation PDFДокумент8 страницKtgradiation PDFBharat Bapu DukaleОценок пока нет
- Largest Area of Space Is at The Center Where There Are 23 TubesДокумент2 страницыLargest Area of Space Is at The Center Where There Are 23 TubesRenzel ReyesОценок пока нет
- Kerala Engg 2016 Paper1 SolutionДокумент7 страницKerala Engg 2016 Paper1 SolutionkalloliОценок пока нет
- Solutions Manual Internal Combustion Engines: Applied Thermosciences ch08Документ17 страницSolutions Manual Internal Combustion Engines: Applied Thermosciences ch08swastik jenaОценок пока нет
- Velocity 1Документ29 страницVelocity 1Ahmad AshourОценок пока нет
- Electromagnetic WaveДокумент48 страницElectromagnetic Wavemrs azizi100% (2)
- Radiocarbon Dating of Charcoal Samples From Rakhigarhi, Haryana, India Using Accelerator Mass SpectrometerДокумент2 страницыRadiocarbon Dating of Charcoal Samples From Rakhigarhi, Haryana, India Using Accelerator Mass SpectrometerManoj KumarОценок пока нет
- Electromagnetic WavesДокумент16 страницElectromagnetic WavesManoj KumarОценок пока нет
- JEE - Preparation - Mathematical Tools in PhysicsДокумент72 страницыJEE - Preparation - Mathematical Tools in Physicsparashargunjan50% (2)
- Physics 2018 PDFДокумент7 страницPhysics 2018 PDFManoj KumarОценок пока нет
- LensesДокумент23 страницыLensesLaili LeliОценок пока нет
- 5 Wave Motion & SoundДокумент32 страницы5 Wave Motion & Soundhamza00715Оценок пока нет
- Exer 1Документ3 страницыExer 1Manoj KumarОценок пока нет
- Physics 2018 PDFДокумент7 страницPhysics 2018 PDFManoj KumarОценок пока нет
- Exercise 1Документ8 страницExercise 1Manoj KumarОценок пока нет
- CPP - Laws of MotionДокумент14 страницCPP - Laws of MotionSJAIN12Оценок пока нет
- RotaionalДокумент19 страницRotaionalSujay BhattacharyaОценок пока нет
- Newton's Laws of Motion - From AsifДокумент23 страницыNewton's Laws of Motion - From Asifaqeeel777100% (3)
- RotaionalДокумент19 страницRotaionalSujay BhattacharyaОценок пока нет
- Installation and Maintenance Information: Turbine Powered StartersДокумент28 страницInstallation and Maintenance Information: Turbine Powered StartersNajim Ahmed BulbulОценок пока нет
- A Primer On Spray Drying Chemical Engineering Nov09Документ7 страницA Primer On Spray Drying Chemical Engineering Nov09Hikmah Triana HadiОценок пока нет
- Catalogo TiboxДокумент5 страницCatalogo Tiboxfabiola100% (1)
- 3.re Situation in Suez Canal - M.V EVER GIVEN SUCCESSFULLY REFLOATEDДокумент9 страниц3.re Situation in Suez Canal - M.V EVER GIVEN SUCCESSFULLY REFLOATEDaungyinmoeОценок пока нет
- Wave Load Calculation in Transitional Water (Prototype)Документ1 страницаWave Load Calculation in Transitional Water (Prototype)pradewoОценок пока нет
- Understand and Troubleshoot Virtualized Domain Controller in Windows Server 8 BetaДокумент168 страницUnderstand and Troubleshoot Virtualized Domain Controller in Windows Server 8 BetaChiTownITОценок пока нет
- QC of Continuous Flight Auger PilesДокумент1 страницаQC of Continuous Flight Auger Pilesnischal_babuОценок пока нет
- StoichiotryДокумент57 страницStoichiotryJezriel Theana SisonОценок пока нет
- FC-M6100 SM-BB52: DEORE CranksetДокумент1 страницаFC-M6100 SM-BB52: DEORE CranksetDon JonesОценок пока нет
- 5e17f Toshiba Satellite l40 Compal La9862p Compal La9862p r10 Laptop SchematicsДокумент46 страниц5e17f Toshiba Satellite l40 Compal La9862p Compal La9862p r10 Laptop Schematicsamier jrs100% (1)
- Pre Requisites For Project ImplementationДокумент3 страницыPre Requisites For Project ImplementationTage NobinОценок пока нет
- Drag of Conical Nose at Supersonic Speeds (Arthur Saw, EURECA 2013)Документ2 страницыDrag of Conical Nose at Supersonic Speeds (Arthur Saw, EURECA 2013)Arthur Saw Sher-QenОценок пока нет
- Pds Microstran LTR en LRДокумент2 страницыPds Microstran LTR en LRthaoОценок пока нет
- KMH 432 - Estuzem - Week 8 - Part IIДокумент26 страницKMH 432 - Estuzem - Week 8 - Part IIGizem ÇetinerОценок пока нет
- Mining and Earthmoving: Estimating Production Off-the-Job Grade Resistance Total Resistance TractionДокумент4 страницыMining and Earthmoving: Estimating Production Off-the-Job Grade Resistance Total Resistance Tractionali alilouОценок пока нет
- Joraform JK Series Operating PrinciplesДокумент6 страницJoraform JK Series Operating Principlesapi-236782993Оценок пока нет
- The Principles of Pulp Washing - PdfaДокумент9 страницThe Principles of Pulp Washing - Pdfashabi049Оценок пока нет
- Simulasi Pengendalian Level Steam DrumДокумент15 страницSimulasi Pengendalian Level Steam DrumSatria dinusaОценок пока нет
- LCD Monitor DC T201WA 20070521 185801 Service Manual T201Wa V02Документ59 страницLCD Monitor DC T201WA 20070521 185801 Service Manual T201Wa V02cdcdanielОценок пока нет
- FRA5310 TechДокумент2 страницыFRA5310 TechBash MatОценок пока нет
- Toyota 80 SeriesДокумент5 страницToyota 80 Seriesaagi_dОценок пока нет
- Shrinkage Strip Method Statment and Ther PDFДокумент4 страницыShrinkage Strip Method Statment and Ther PDFhakim2020Оценок пока нет
- Schematic 1 - : CMDB-B01.00-9b-L9-1 NTS CMDB-B01.00-9a-L9-1Документ1 страницаSchematic 1 - : CMDB-B01.00-9b-L9-1 NTS CMDB-B01.00-9a-L9-1Michael Camit EsoОценок пока нет
- GPU Programming in MATLABДокумент6 страницGPU Programming in MATLABkhaardОценок пока нет
- Roof Manual p10Документ1 страницаRoof Manual p10AllistairОценок пока нет
- What We Offer.: RemunerationДокумент8 страницWhat We Offer.: Remunerationsurabhi mandalОценок пока нет
- 90205-1031DEB F Series MaintenanceInspectionДокумент31 страница90205-1031DEB F Series MaintenanceInspectionIsaac CarmonaОценок пока нет
- Activity9 PDFДокумент5 страницActivity9 PDFSmitОценок пока нет
- E5263 - M4A87TD EVO PDFДокумент76 страницE5263 - M4A87TD EVO PDFLeandro Henrique AgostinhoОценок пока нет