Академический Документы
Профессиональный Документы
Культура Документы
Narayana Medical Academy, India.: SR Bipc N40+Lt N40 (Prog-1) Series-1 DATE: 11-04-18 Neet Part Test - 7 Solutions
Загружено:
Ihtisham Ul Haq0 оценок0% нашли этот документ полезным (0 голосов)
152 просмотров2 страницыGyu
Оригинальное название
5_6323320164800528493
Авторское право
© © All Rights Reserved
Доступные форматы
PDF, TXT или читайте онлайн в Scribd
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документGyu
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PDF, TXT или читайте онлайн в Scribd
0 оценок0% нашли этот документ полезным (0 голосов)
152 просмотров2 страницыNarayana Medical Academy, India.: SR Bipc N40+Lt N40 (Prog-1) Series-1 DATE: 11-04-18 Neet Part Test - 7 Solutions
Загружено:
Ihtisham Ul HaqGyu
Авторское право:
© All Rights Reserved
Доступные форматы
Скачайте в формате PDF, TXT или читайте онлайн в Scribd
Вы находитесь на странице: 1из 2
NARAYANA MEDICAL ACADEMY,INDIA.
SR BIPC N40+LT N40 (PROG-1) SERIES-1 DATE: 11-04-18
NEET PART TEST – 7 SOLUTIONS
CHEMISTRY greater will be the liquefaction of
137. Tf K f molality i a gas and greater the adsorption
0 (1.91) 1.86 1 i x 1
146. log log k log p
m n
1.191
i= = 1.0268
1.86 Similar to y = C + mx
But for HI dissociation, 1
slope
n
i = 1 + (2 1)α
148. Vapour
1.027 1 α 1
pressure
α 0.027 molality × no.of ions
α 27% 150. Auto catalyst is Mn+2 Mn+2 is [Ar]
450 3d5
138. Tf Kf molality
11111
wt 1
0 6 1.86
62 4 unpaired electrons = 5
Wt of ethylene glycol = 800 g µ n n 2 B.M
139. A = Pentane, B = Hexane 5(5 2) B.M
PA P0 X
YA 0 A A0 µ = 5.92 B.M
Ptotal PA X A PB X B
P0 P
1 154. Molefraction of solute
440 P0
174
=
1 4 m
440 120 =
1 4 1 4 m 55.5
440 m
= 0.478 0.2
440 480 m 55.5
141. Freezing point 2 m
1 10 m 55.5
molality no.of ions
2m + 111 = 10 m
144. Coagulating power charge 8m = 111
145. greater the critical temperature
Page 1
M = 13.8 m
158. The order of basic character is
K 2O BaO CaO MgO on basis of
electropositivity of metal
159. The E.A of Be is similar to that of
He, because the E.C of Be is similar
to He is 2 He; 1S 2
161. Sorel’s cement is, MgCl2 .5 MgO.xH 2O
172.
H H
H
B 97 B 122
H H
H
175. In Goldschmidt aluminothermic
process, thermite contains 3 parts
of Fe2O3 and 1 part of Al.
B is no more electron deficient
hence BF3 is a weak acid.
B is no more electron deficient
hence BF3 is a weak acid.
176. p p back bonding in BF3
177.
179.
NaAlO2 3 H 2
Al NaOH H 2O
2
0/0 (w/w) density 10
180. a) M =
M.wt. of solute
98 1.84 10
98
M = 18.4 M
b) M1V1 = M2V2
18.4 V1 5.52 500
V1 = 150 ml
Page 2
Вам также может понравиться
- LivingScience CBSE CompanionДокумент56 страницLivingScience CBSE Companionnjlenovo95% (19)
- Silo WallДокумент5 страницSilo WallMunish GaurОценок пока нет
- Solution Manual for an Introduction to Equilibrium ThermodynamicsОт EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsОценок пока нет
- Temperauture.: Prob. Solution: GivenДокумент9 страницTemperauture.: Prob. Solution: Givenharsh patelОценок пока нет
- ICGSE Chemistry Chapter 1 - The Particulate Nature of MatterДокумент29 страницICGSE Chemistry Chapter 1 - The Particulate Nature of MatterVentus TanОценок пока нет
- Quiz 2 - SolДокумент1 страницаQuiz 2 - SolVanessa100% (2)
- Electronic Fetal MonitoringДокумент4 страницыElectronic Fetal MonitoringMauZungОценок пока нет
- Melancholic PersonalityДокумент5 страницMelancholic PersonalityChris100% (1)
- Bonding and Adhesives in DentistryДокумент39 страницBonding and Adhesives in DentistryZahn ÄrztinОценок пока нет
- 05 - Energy BalanceДокумент28 страниц05 - Energy BalanceNoman AslamОценок пока нет
- Eye Essentials Cataract Assessment Classification and ManagementДокумент245 страницEye Essentials Cataract Assessment Classification and ManagementKyros1972Оценок пока нет
- Ther 3Документ11 страницTher 3albert nicolasОценок пока нет
- DM MCQ 2Документ10 страницDM MCQ 2Irfan AhmedОценок пока нет
- S.No Company Name: Companies NamesДокумент4 страницыS.No Company Name: Companies NamesIhtisham Ul Haq0% (1)
- Biology Lab ReportДокумент5 страницBiology Lab Reportapi-2576094460% (1)
- All 1B CSM PDFДокумент486 страницAll 1B CSM PDFConstanza Cáceres Vidal67% (3)
- Cervical Changes During Menstrual Cycle (Photos)Документ9 страницCervical Changes During Menstrual Cycle (Photos)divyanshu kumarОценок пока нет
- Tut 4 VLE of Pure Fluids - SolutionsДокумент13 страницTut 4 VLE of Pure Fluids - SolutionsAsma NasserОценок пока нет
- Adobe Scan 27-Feb-2023Документ8 страницAdobe Scan 27-Feb-2023Praveenkumar VОценок пока нет
- QuizДокумент21 страницаQuizIhaw HalimОценок пока нет
- TPP Excel BaruДокумент7 страницTPP Excel BaruAtika HapsatiОценок пока нет
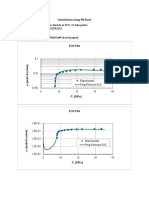
- USA Nozzle 01Документ2 страницыUSA Nozzle 01Justin MercadoОценок пока нет
- R l k r r k r r π π: a a k k b b l lДокумент1 страницаR l k r r k r r π π: a a k k b b l lSameerChauhanОценок пока нет
- 2016 n6 Power Machines April 2016 MemorandumДокумент11 страниц2016 n6 Power Machines April 2016 MemorandumPino PinoОценок пока нет
- Ch2b Tutor ResultantДокумент33 страницыCh2b Tutor ResultantharwezelОценок пока нет
- Four Forces Act at Bolt A As Shown. Determine The Force.: Beer, 5th. Ed. Sample Problem 2.3Документ33 страницыFour Forces Act at Bolt A As Shown. Determine The Force.: Beer, 5th. Ed. Sample Problem 2.3Jeneath TahilОценок пока нет
- Problems IceДокумент2 страницыProblems IceSriramulu JaichandarОценок пока нет
- Temperatures Charts (Heisler Charts) : The Biot Number and Fourier NumberДокумент12 страницTemperatures Charts (Heisler Charts) : The Biot Number and Fourier NumberAhmed Shaaban Soliman HamedОценок пока нет
- Problem Set No. 3 Thermodynamic Analysis - 2016 v2Документ2 страницыProblem Set No. 3 Thermodynamic Analysis - 2016 v2Emrico Luiz PerezОценок пока нет
- Sam 2 SolДокумент8 страницSam 2 SoljeyyelramosОценок пока нет
- 2500 LB D 1.25in D 2.25 In: Mechanical Engineering DepartmentДокумент5 страниц2500 LB D 1.25in D 2.25 In: Mechanical Engineering DepartmentPHILIPANTHONY MASILANGОценок пока нет
- Qdoc - Tips Theory of Elasticity by Sadhu SinghДокумент7 страницQdoc - Tips Theory of Elasticity by Sadhu Singhking royalОценок пока нет
- Tut 6 Ideal and Nonideal Solutions - SolutionsДокумент19 страницTut 6 Ideal and Nonideal Solutions - SolutionsAsma NasserОценок пока нет
- ps2 sp11 Key 0Документ6 страницps2 sp11 Key 0Crizaldo MempinОценок пока нет
- Temp Raina VRPДокумент1 страницаTemp Raina VRPDevdattaОценок пока нет
- NEET Solutions Important QuestionsДокумент24 страницыNEET Solutions Important Questionsdaksh892006Оценок пока нет
- Problems Set 1Документ7 страницProblems Set 1Mirtunjay KumarОценок пока нет
- Chemical EngineersДокумент37 страницChemical Engineersjessejames.supangОценок пока нет
- Steam Cycle With Reheat: P 10 Bar T 500 Deg CДокумент3 страницыSteam Cycle With Reheat: P 10 Bar T 500 Deg CsurafelshimelesОценок пока нет
- Reactor ModelДокумент32 страницыReactor ModelMohaiminОценок пока нет
- Problem 5 10.213: Mol 5 - 1605 Mol M M 024914 - 0 40 V V NДокумент6 страницProblem 5 10.213: Mol 5 - 1605 Mol M M 024914 - 0 40 V V NAyuÒ'ĮsyОценок пока нет
- Exercise 6: Dew Point and Bubble Point Calculation: Assignment 1Документ2 страницыExercise 6: Dew Point and Bubble Point Calculation: Assignment 1Junaid0% (1)
- PH O P Total P H O: A) Molal SaturationДокумент5 страницPH O P Total P H O: A) Molal SaturationMiraОценок пока нет
- Physical ChemistryДокумент2 страницыPhysical ChemistryJohn paul Stem11Оценок пока нет
- Study Area 8M Minimum Thickness of SlabДокумент8 страницStudy Area 8M Minimum Thickness of Slabdiego lopezОценок пока нет
- HomeworkДокумент7 страницHomeworkTlhologeloОценок пока нет
- hHAO 6,489 M: COO J81O +080)Документ3 страницыhHAO 6,489 M: COO J81O +080)Verito AleОценок пока нет
- Engineer of Chemistry: P MG A That D M V P DVG A V Ah P DHG P 1.00 10 KG M S P 3.00 10 M P P 3928 AtmДокумент5 страницEngineer of Chemistry: P MG A That D M V P DVG A V Ah P DHG P 1.00 10 KG M S P 3.00 10 M P P 3928 AtmMinh Tri TranОценок пока нет
- hws355 4Документ5 страницhws355 4S DОценок пока нет
- Good TestДокумент4 страницыGood TestYash MittalОценок пока нет
- Building Design Lesson 4Документ2 страницыBuilding Design Lesson 4Joshua Melegrito PeraltaОценок пока нет
- Che61 AT5Документ8 страницChe61 AT5Michael Alex MabaoОценок пока нет
- Pyrhonen Squirrel Cage Motor Calculation Mathcad13 PDFДокумент35 страницPyrhonen Squirrel Cage Motor Calculation Mathcad13 PDFluis900000Оценок пока нет
- Chmt3044a Assignment 1Документ6 страницChmt3044a Assignment 1britneybaetsangmogaleОценок пока нет
- AfdhalДокумент11 страницAfdhalRiky Mario YuluciОценок пока нет
- Solutions of ExercisesДокумент87 страницSolutions of ExercisesDianiTz MendOzaОценок пока нет
- HumidificationДокумент4 страницыHumidificationAj SayoОценок пока нет
- Polyethylene Plant:: The Reactions Are As FollowsДокумент17 страницPolyethylene Plant:: The Reactions Are As FollowsSANIKA TALATHIОценок пока нет
- F - Persiapan PAT Juni 2023 Kelas 11 - Tutorial 02 - Utk SMAN1 - JawabanДокумент3 страницыF - Persiapan PAT Juni 2023 Kelas 11 - Tutorial 02 - Utk SMAN1 - JawabanPutra RamadhanaОценок пока нет
- Assignment#2 Reservoir Fluid DR - Hiwa Fall22.Документ7 страницAssignment#2 Reservoir Fluid DR - Hiwa Fall22.Mirei Salam MohamadОценок пока нет
- Example 1Документ8 страницExample 1jgolloberОценок пока нет
- Oilproperties.Xls: Γ Api +131 - 5 Ρ Γ R Γ Γ AДокумент3 страницыOilproperties.Xls: Γ Api +131 - 5 Ρ Γ R Γ Γ ATifano KhristiyantoОценок пока нет
- Physical Chemistry 1Документ5 страницPhysical Chemistry 1Sandipan SahaОценок пока нет
- Австри 2011 ХариултДокумент12 страницАвстри 2011 ХариултGerel BayrmagnaiОценок пока нет
- Chemistry 311 Physical ChemistryДокумент3 страницыChemistry 311 Physical ChemistryJosef CatiggayОценок пока нет
- Ikhsan Firdaus - B - UTS - TermodinamikaДокумент2 страницыIkhsan Firdaus - B - UTS - TermodinamikaikhsanОценок пока нет
- Solid Electro Kinetics SolutionДокумент4 страницыSolid Electro Kinetics Solutionjoydeep17590Оценок пока нет
- Problem - 01 - OilpropertiesДокумент3 страницыProblem - 01 - OilpropertiesMuhammad AbubakarОценок пока нет
- System: Naphthalene + Carbon Dioxide At 35°C, 13 Data Points Experimental Data File: Nfcd35Sr.Da3 For Α = 4.451; Β = 5.79 Plots (Sample Calculation In Mathcad In Next Pages)Документ4 страницыSystem: Naphthalene + Carbon Dioxide At 35°C, 13 Data Points Experimental Data File: Nfcd35Sr.Da3 For Α = 4.451; Β = 5.79 Plots (Sample Calculation In Mathcad In Next Pages)Fredy Colpas CastilloОценок пока нет
- Physical Electronics: Handbook of Vacuum PhysicsОт EverandPhysical Electronics: Handbook of Vacuum PhysicsA. H. BeckОценок пока нет
- Web Scraping PortfolioДокумент30 страницWeb Scraping PortfolioIhtisham Ul HaqОценок пока нет
- Bohr RadiusДокумент2 страницыBohr RadiusIhtisham Ul HaqОценок пока нет
- Bond Energy PDFДокумент2 страницыBond Energy PDFIhtisham Ul Haq100% (1)
- Chemical Equilibrium PDFДокумент2 страницыChemical Equilibrium PDFIhtisham Ul HaqОценок пока нет
- Alkane, Alkene and AlkyneДокумент6 страницAlkane, Alkene and AlkyneIhtisham Ul HaqОценок пока нет
- Hints and Tricks in ChemistryДокумент2 страницыHints and Tricks in ChemistryIhtisham Ul HaqОценок пока нет
- Hints and Tricks in Chemistry: " Fields and Its Study"Документ2 страницыHints and Tricks in Chemistry: " Fields and Its Study"Ihtisham Ul HaqОценок пока нет
- Combusion PDFДокумент2 страницыCombusion PDFIhtisham Ul HaqОценок пока нет
- Combusion PDFДокумент2 страницыCombusion PDFIhtisham Ul HaqОценок пока нет
- Order of Reaction 2 PartДокумент2 страницыOrder of Reaction 2 PartIhtisham Ul HaqОценок пока нет
- Hints and Tricks in Chemistry: Important Points From HomeostasisДокумент3 страницыHints and Tricks in Chemistry: Important Points From HomeostasisIhtisham Ul HaqОценок пока нет
- Mcqs On NodesДокумент3 страницыMcqs On NodesIhtisham Ul Haq50% (2)
- Biology Portion of Etea Medical Test 2019Документ21 страницаBiology Portion of Etea Medical Test 2019Ihtisham Ul Haq75% (4)
- Deposit Slip For Affiliated Institutions-StudentsДокумент1 страницаDeposit Slip For Affiliated Institutions-StudentsNAI DUNYAОценок пока нет
- Narayana Medical Academy, India: A K KXДокумент4 страницыNarayana Medical Academy, India: A K KXIhtisham Ul HaqОценок пока нет
- Khyber Medical University Peshawar: First Professional BdsДокумент3 страницыKhyber Medical University Peshawar: First Professional BdsIhtisham Ul HaqОценок пока нет
- BotanyДокумент3 страницыBotanyIhtisham Ul HaqОценок пока нет
- Clearance CertificateДокумент1 страницаClearance CertificateIhtisham Ul HaqОценок пока нет
- SST Bio-Che Answer Key 25 08 2019 PDFДокумент3 страницыSST Bio-Che Answer Key 25 08 2019 PDFIhtisham Ul HaqОценок пока нет
- A) Alien Species I) Himalayas B) Sacred Groove II) Lantana C) National Park III) Ashtamudi Lake D) Biodiversity Hot Spot IV) Periyar V) SargujaДокумент3 страницыA) Alien Species I) Himalayas B) Sacred Groove II) Lantana C) National Park III) Ashtamudi Lake D) Biodiversity Hot Spot IV) Periyar V) SargujaIhtisham Ul HaqОценок пока нет
- Geography: Test SeriesДокумент9 страницGeography: Test SeriesIhtisham Ul HaqОценок пока нет
- 1.physics 24-4-18Документ6 страниц1.physics 24-4-18VimalОценок пока нет
- ChemistryДокумент5 страницChemistryIhtisham Ul HaqОценок пока нет
- Topic:: Chemistry Lecture NotesДокумент10 страницTopic:: Chemistry Lecture NotesIhtisham Ul HaqОценок пока нет
- 4 6030510885359846382Документ12 страниц4 6030510885359846382Ihtisham Ul HaqОценок пока нет
- Geography: Test SeriesДокумент11 страницGeography: Test SeriesIhtisham Ul HaqОценок пока нет
- Essay: Test SeriesДокумент21 страницаEssay: Test SeriesIhtisham Ul HaqОценок пока нет
- Thai Book Lesson From Genius SchoolДокумент85 страницThai Book Lesson From Genius SchoolAlexis L.D (Riker94)Оценок пока нет
- 18-MCE-49 Lab Session 01Документ5 страниц18-MCE-49 Lab Session 01Waqar IbrahimОценок пока нет
- Ideal Discharge Elderly PatientДокумент3 страницыIdeal Discharge Elderly PatientFelicia Risca RyandiniОценок пока нет
- Air MassesДокумент22 страницыAir MassesPrince MpofuОценок пока нет
- Inversor Abb 3 8kwДокумент2 страницыInversor Abb 3 8kwapi-290643326Оценок пока нет
- Canfield FairДокумент3 страницыCanfield Fairapi-546463844Оценок пока нет
- InfertilityДокумент8 страницInfertilityrivannyОценок пока нет
- Mcdes 1Документ7 страницMcdes 1JerdОценок пока нет
- ResДокумент21 страницаResMarian EvangelioОценок пока нет
- Leon County Sheriff'S Office Daily Booking Report 4-Jan-2022 Page 1 of 3Документ3 страницыLeon County Sheriff'S Office Daily Booking Report 4-Jan-2022 Page 1 of 3WCTV Digital TeamОценок пока нет
- Giardiasis PDFДокумент14 страницGiardiasis PDFSaad Motawéa0% (1)
- CASE 1. Non-Cash Assets Are Sold For P 580,000Документ3 страницыCASE 1. Non-Cash Assets Are Sold For P 580,000Riza Mae AlceОценок пока нет
- Texas Steering and Insurance DirectionДокумент2 страницыTexas Steering and Insurance DirectionDonnie WeltyОценок пока нет
- Iso 2281 1990Документ8 страницIso 2281 1990jesus torresОценок пока нет
- Sand Casting OverviewДокумент166 страницSand Casting Overviewsamurai7_77Оценок пока нет
- Minimum Number of Thermocouples-Local PWHTДокумент5 страницMinimum Number of Thermocouples-Local PWHTPradip Goswami100% (1)
- Material Specification: Mechanical Property RequirementsДокумент2 страницыMaterial Specification: Mechanical Property RequirementsNguyễn Tấn HảiОценок пока нет
- Epidemiology of Injury in Powerlifting: Retrospective ResultsДокумент2 страницыEpidemiology of Injury in Powerlifting: Retrospective ResultsJavier Estelles MuñozОценок пока нет
- App Guide EntelliGuard - G 09 - 2020 AplicacionДокумент100 страницApp Guide EntelliGuard - G 09 - 2020 AplicacionjeorginagОценок пока нет
- NHT Series High-Throughput Diffusion PumpsДокумент12 страницNHT Series High-Throughput Diffusion PumpsJosé Mauricio Bonilla TobónОценок пока нет
- 4 - Mixing Equipments Used in Flocculation and CoagulationДокумент27 страниц4 - Mixing Equipments Used in Flocculation and Coagulationhadeer osmanОценок пока нет
- TextДокумент3 страницыTextKristineОценок пока нет