Академический Документы
Профессиональный Документы
Культура Документы
CHE171 - Kinetics 1
Загружено:
JannineИсходное описание:
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
CHE171 - Kinetics 1
Загружено:
JannineАвторское право:
Доступные форматы
PROBLEM SET 2.
PART A
1. Liquid A decomposes by first-order kinetics, and in a batch reactor 50% of A is converted in a 5-
minute run. How much longer would it take to reach 75% conversion? Repeat the previous
problem for second-order kinetics.
2. A 10-minute experimental run shows that 75% of liquid reactant is converted to product by a %-
order rate. What would be the fraction converted in a half-hour run?
3. In a homogeneous isothermal liquid polymerization, 20% of the monomer disappears in 34
minutes for initial monomer concentration of 0.04 and also for 0.8 mol/liter. What rate equation
represents the disappearance of the monomer?
4. After 8 minutes in a batch reactor, reactant (CA O = 1 mol/liter) is 80% converted; after 18
minutes, conversion is 90%. Find a rate equation to represent this reaction.
5. Aqueous A reacts to form R (A R) and in the first minute in a batch reactor its concentration
drops from CAo = 2.03 mol/liter to C Af = 1.97mol/liter. Find the rate equation for the reaction if
the kinetics are second order with respect to A.
6. Enzyme E catalyzes the transformation of reactant A to product R as follows:
If we introduce enzyme (CEO = 0.001 mol/liter) and reactant (C Ao = 10 mol/liter) into a batch
reactor and let the reaction proceed, find the time needed for the concentration of reactant to
drop to 0.025 mol/liter. Note that the concentration of enzyme remains unchanged during the
reaction.
7. The reaction of saponification: NaOH + C2H5(CH3COO) → Na(CH3COO) + C2H5OH is second order
and irreversible at low concentrations. In a batch reactor at constant temperature a water
solution is loaded containing NaOH and ethyl acetate, both with initial concentrations 0.1 N.
After 15 minutes, conversion of ethyl acetate is 18%. Calculate the reaction time needed to
obtain a conversion of 25% NaOH when loading the reactor with a solution 0.2 N NaOH and 0.1N
ethyl acetate.
8. Find the conversion after 1 hour in a batch reactor for
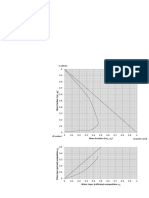
9. Huang and Dauerman (1969) have studied the acetylation of benzyl chloride in dilute solution at
102°C. Using equimolal concentrations of sodium acetate and benzyl chloride (0.757 kmol/m 3),
Table 3-16 lists the reported data on the fraction of benzyl chloride remaining unconverted
versus time. Determine the order of the reaction and the reaction rate constant at this
temperature.
10. The first-order reversible liquid reaction A R takes place in a batch reactor. After 8 minutes,
conversion of A is 33.3% while equilibrium conversion is 66.7%. Find the rate equation for this
reaction. CA0 = 0.5 M, CRO=0
Вам также может понравиться
- Separation Processes IДокумент3 страницыSeparation Processes IAmesh Chiyogami100% (1)
- Tarea Extracción LiquidoДокумент3 страницыTarea Extracción Liquidovanessa ramosОценок пока нет
- Manufacturing of StyreneДокумент1 страницаManufacturing of StyreneNornadiahnadhirah MdNadzriОценок пока нет
- Activation Catalytic EnglishДокумент1 страницаActivation Catalytic EnglishplennyОценок пока нет
- 1,3 Butadiene: (Extraction From Mixed C)Документ2 страницы1,3 Butadiene: (Extraction From Mixed C)Rudy Mamani CuellarОценок пока нет
- Solubility Parameter Cellulose Esters PDFДокумент3 страницыSolubility Parameter Cellulose Esters PDFcaioltbgОценок пока нет
- Distillation 13 22Документ2 страницыDistillation 13 22blueberrytimeОценок пока нет
- Chapter 2 LLE-part 2 - 18nov2020Документ22 страницыChapter 2 LLE-part 2 - 18nov2020CaratsSVTОценок пока нет
- Solubility of Carbon Dioxide in Pentadecane, Hexadecane, and Pentadecane + HexadecaneДокумент3 страницыSolubility of Carbon Dioxide in Pentadecane, Hexadecane, and Pentadecane + Hexadecanerezagholami87Оценок пока нет
- Tài Methyl-Acetate-Plant-DesignДокумент18 страницTài Methyl-Acetate-Plant-DesignLe Anh QuânОценок пока нет
- From The Arrhenius To The Clausius-Clapeyron Equation: Igor NovakДокумент2 страницыFrom The Arrhenius To The Clausius-Clapeyron Equation: Igor NovakJonathanОценок пока нет
- ChE 195 Problem Set No. 2Документ1 страницаChE 195 Problem Set No. 2Jahz ChannelОценок пока нет
- Production of Diethyl EthereДокумент163 страницыProduction of Diethyl Ethereيزيد العزانيОценок пока нет
- Thus, This Term Actually Means A in A Constant-Volume System The Measure of Reaction Rate of Component I BecomesДокумент23 страницыThus, This Term Actually Means A in A Constant-Volume System The Measure of Reaction Rate of Component I Becomesalice Annabelle100% (1)
- Ejercicios de Ingeniería EconómicaДокумент2 страницыEjercicios de Ingeniería EconómicaDiego MoralesОценок пока нет
- Azeotrope AssignmentДокумент2 страницыAzeotrope AssignmentKamran Zeb100% (1)
- MT 304 - Gas Liquid Absorption: CL 333 Chemical Engineering Lab-III (2021)Документ13 страницMT 304 - Gas Liquid Absorption: CL 333 Chemical Engineering Lab-III (2021)Shivansh SinghОценок пока нет
- Tutorial Material & Energy BalanceДокумент4 страницыTutorial Material & Energy BalanceMuiz ZahuriОценок пока нет
- Design of Drying Oil ProjectДокумент7 страницDesign of Drying Oil ProjecttissaanuradhaОценок пока нет
- Production of Dishwashing Liquid Detergent (Base) : Cebu Institute of Technology - UniversityДокумент10 страницProduction of Dishwashing Liquid Detergent (Base) : Cebu Institute of Technology - UniversityNicely EleccionОценок пока нет
- Distillation Assignment PDFДокумент13 страницDistillation Assignment PDFcalliemozartОценок пока нет
- Spheripol Web ArtДокумент2 страницыSpheripol Web ArtnahulaeОценок пока нет
- Assignment 2Документ2 страницыAssignment 2KhairulAzwanizam0% (2)
- LECTURE - 6: Ethylene Derivatives: Ethylene Oxide and Ethanol Amines 6.1 Ethylene OxideДокумент7 страницLECTURE - 6: Ethylene Derivatives: Ethylene Oxide and Ethanol Amines 6.1 Ethylene Oxideمحمود محمدОценок пока нет
- Chemical Reactor Design++ PDFДокумент72 страницыChemical Reactor Design++ PDFKiran Patil0% (1)
- MTBE Unit Expansion-ConversionДокумент13 страницMTBE Unit Expansion-Conversiontunganh1110100% (1)
- Parametri Per L'equazione Di Antoine Ed Altre ProprietàДокумент1 страницаParametri Per L'equazione Di Antoine Ed Altre ProprietàAlberto NovelloОценок пока нет
- Lecture 9Документ4 страницыLecture 9Asif AliОценок пока нет
- Lab 3Документ16 страницLab 3Paen Zulkifli100% (1)
- Example 6.3 Kelompok VДокумент13 страницExample 6.3 Kelompok VMiranda Amiroh SulaimanОценок пока нет
- (A) Example 8-1. (1) What Are - (2) What Would Have Been...Документ16 страниц(A) Example 8-1. (1) What Are - (2) What Would Have Been...Anonymous Hzdnl0WNОценок пока нет
- Design & Simulation of Nitrobenzene Manufacturing Process: Name of Student: Kasar Khanadal MheДокумент23 страницыDesign & Simulation of Nitrobenzene Manufacturing Process: Name of Student: Kasar Khanadal Mheکبری ادریس رسولОценок пока нет
- 06 Chapter 12 (Compiled) PDFДокумент80 страниц06 Chapter 12 (Compiled) PDFHaziq KhairiОценок пока нет
- Momentum Transfer FiltrationДокумент76 страницMomentum Transfer FiltrationMinjdeDiosОценок пока нет
- S16 CPSDДокумент4 страницыS16 CPSDGohit BhatОценок пока нет
- Fluid Fluid Reaction KineticsДокумент27 страницFluid Fluid Reaction KineticsIlyas AzmanОценок пока нет
- Hexamine 1 PDFДокумент4 страницыHexamine 1 PDFPradhita Ramdani HОценок пока нет
- Experiment Chemical ReactorДокумент4 страницыExperiment Chemical ReactorIboniks Beponpiks DabondatskiОценок пока нет
- Material Balance With Out RXN Example Unit3-RSДокумент129 страницMaterial Balance With Out RXN Example Unit3-RSGodolias WoldemariamОценок пока нет
- Batch ReactorДокумент4 страницыBatch ReactorHarini BugattiveyronОценок пока нет
- Hostalen Brochure PDFДокумент2 страницыHostalen Brochure PDFRebecca LimbardoОценок пока нет
- Ayırma İşlemleri SorularДокумент9 страницAyırma İşlemleri SorularElif Yaren Öztürk0% (1)
- Example 6Документ5 страницExample 6Dewi AnggreniОценок пока нет
- Deep Eutectic Solvents Syntheses, Properties and ApplicationsДокумент39 страницDeep Eutectic Solvents Syntheses, Properties and ApplicationsJulio Cesar Almeida100% (1)
- Acetone BДокумент9 страницAcetone BIrdani IdrisОценок пока нет
- Chapter 2 - Data InterpretationДокумент24 страницыChapter 2 - Data InterpretationPHƯƠNG ĐẶNG YẾNОценок пока нет
- Lab 5 Production of Ethyl ChlorideДокумент19 страницLab 5 Production of Ethyl ChloridelynОценок пока нет
- KR3543-Lecture 2-VLE and Flash Calculation 20192020 - Revised-20190912065536 PDFДокумент66 страницKR3543-Lecture 2-VLE and Flash Calculation 20192020 - Revised-20190912065536 PDFNatasha Mgt JoharОценок пока нет
- Organic Chemistry Laboratory I BSK1402 Lab Report: Name Fathul Aiman Bin Fahmi Matrix No. Sa18094 Section 02 DateДокумент8 страницOrganic Chemistry Laboratory I BSK1402 Lab Report: Name Fathul Aiman Bin Fahmi Matrix No. Sa18094 Section 02 DateCucu AlbertОценок пока нет
- Introduction To Separation Process EngineeringДокумент12 страницIntroduction To Separation Process EngineeringAna K. CalderónОценок пока нет
- Tutorial 3 - Questions Only - PVT Behavior - Ideal Gas - Virial EOSДокумент5 страницTutorial 3 - Questions Only - PVT Behavior - Ideal Gas - Virial EOSMihir Kumar Mech100% (1)
- CRE IdocxДокумент8 страницCRE IdocxParth DesaiОценок пока нет
- AsdfghjklДокумент4 страницыAsdfghjklJV CustodioОценок пока нет
- CHE3044F, 2013: Reactor Design 1: TUTORIAL 5Документ2 страницыCHE3044F, 2013: Reactor Design 1: TUTORIAL 5nmhatityeОценок пока нет
- PS LevenspielДокумент3 страницыPS LevenspielRichelle CharleneОценок пока нет
- Kinetics Probset (LE4)Документ4 страницыKinetics Probset (LE4)Jewls HatudОценок пока нет
- R (DC /DT) 0.2 Mol L C: ASSIGNMENT #2 - Reaction Kinetics 2Документ2 страницыR (DC /DT) 0.2 Mol L C: ASSIGNMENT #2 - Reaction Kinetics 2AndreОценок пока нет
- 2 - Prob Batch 11-12 14-22 EnglishДокумент3 страницы2 - Prob Batch 11-12 14-22 EnglishAbiola AjiginniОценок пока нет
- 3-Interpretation & The Use of Rate LawДокумент16 страниц3-Interpretation & The Use of Rate LawTom FlynnОценок пока нет
- Course Syllabus EE105L Editable 2T 2015-16 REV 1Документ4 страницыCourse Syllabus EE105L Editable 2T 2015-16 REV 1JannineОценок пока нет
- CHE134P 1 PrelimsДокумент8 страницCHE134P 1 PrelimsJannineОценок пока нет
- AC Amplitude Measurement: 1 AbstractДокумент2 страницыAC Amplitude Measurement: 1 AbstractJannineОценок пока нет
- $experiment #02 - Serie-Parallel Resistive CircuitsДокумент25 страниц$experiment #02 - Serie-Parallel Resistive CircuitsJannineОценок пока нет
- Diodes and Half-Wave Rectification: 1 AbstractДокумент3 страницыDiodes and Half-Wave Rectification: 1 AbstractJannineОценок пока нет
- Heat EffectsДокумент25 страницHeat EffectsJannineОценок пока нет
- AceticAcid Water EtherДокумент1 страницаAceticAcid Water EtherJannineОценок пока нет
- Series/Parallel Resistive Circuits: 1 Abstract 4 ResultsДокумент2 страницыSeries/Parallel Resistive Circuits: 1 Abstract 4 ResultsJannineОценок пока нет
- DC Power Sources: 1.abstractДокумент2 страницыDC Power Sources: 1.abstractJannineОценок пока нет
- Entropy: Prepared by Engr. Joseph R. Ortenero Mapua Institute of Technology at Laguna Malayan Colleges LagunaДокумент17 страницEntropy: Prepared by Engr. Joseph R. Ortenero Mapua Institute of Technology at Laguna Malayan Colleges LagunaJannineОценок пока нет
- Leaching Reviewer PDFДокумент19 страницLeaching Reviewer PDFJannineОценок пока нет
- Work and HeatДокумент17 страницWork and HeatJannineОценок пока нет
- First LAw of Thermodynamics - Open SYstemДокумент12 страницFirst LAw of Thermodynamics - Open SYstemJannineОценок пока нет
- First Law of Thermodynamics - Closed SystemДокумент22 страницыFirst Law of Thermodynamics - Closed SystemJannineОценок пока нет
- Boardwork - Work and HeatДокумент6 страницBoardwork - Work and HeatJannineОценок пока нет
- Boardwork - Work and HeatДокумент6 страницBoardwork - Work and HeatJannineОценок пока нет
- Boardwork - Closed SystemДокумент12 страницBoardwork - Closed SystemJannineОценок пока нет
- Syllabus On CHE ThermodynamicsДокумент4 страницыSyllabus On CHE ThermodynamicsJannineОценок пока нет
- CHE150 - Nitrogen IndustryДокумент17 страницCHE150 - Nitrogen IndustryJannineОценок пока нет
- PolymathДокумент12 страницPolymathJannineОценок пока нет
- Thermodynamics IДокумент34 страницыThermodynamics IJannineОценок пока нет
- Chapter 1 LevenspielДокумент14 страницChapter 1 LevenspielJannineОценок пока нет
- Prelims Che191 PDFДокумент6 страницPrelims Che191 PDFJannineОценок пока нет
- Aristotles Three Modes of PersuasionДокумент3 страницыAristotles Three Modes of PersuasionJoy Grace DuremdesОценок пока нет
- Effects of Reservoir Heterogeneity in Laminated ReservoirsДокумент3 страницыEffects of Reservoir Heterogeneity in Laminated ReservoirsErik Andres Garcia VillarroelОценок пока нет
- Acer V193 Service ManualДокумент46 страницAcer V193 Service Manualagun92Оценок пока нет
- Json Out InvoiceДокумент2 страницыJson Out Invoicetkpatel529Оценок пока нет
- FNDWRR PDFДокумент5 страницFNDWRR PDFngole ngoleОценок пока нет
- Cryptography: Presented by Blances SanchezДокумент91 страницаCryptography: Presented by Blances SanchezLian PicartОценок пока нет
- Bird Et Al (2005)Документ11 страницBird Et Al (2005)Ewan MurrayОценок пока нет
- The Influence of Social Media On Crowd Behavior and The Operational EnvironmentДокумент78 страницThe Influence of Social Media On Crowd Behavior and The Operational EnvironmentangryTXОценок пока нет
- Computer Application in BusinessДокумент3 страницыComputer Application in BusinessChegg UserОценок пока нет
- University of Tehran Faculty of New Science & Technology Master's Thesis Proposal DefenseДокумент46 страницUniversity of Tehran Faculty of New Science & Technology Master's Thesis Proposal DefenseSoheilDarvishMotavalliОценок пока нет
- Planer MachineДокумент37 страницPlaner Machinemechfame89% (9)
- Contribucion de Las FE A La AutorregulacionДокумент24 страницыContribucion de Las FE A La AutorregulacionAmanda Riedemann CarrilloОценок пока нет
- 2ND Quarter MTB - Mle Lesson Plan December 2, 2022Документ4 страницы2ND Quarter MTB - Mle Lesson Plan December 2, 2022sherry ann corderoОценок пока нет
- Canon Ir8070 Error Codes List PDFДокумент18 страницCanon Ir8070 Error Codes List PDFGirish KumarОценок пока нет
- Problem Solving Essay Outline - ProcrastinationДокумент2 страницыProblem Solving Essay Outline - ProcrastinationAniqaОценок пока нет
- Sapient Paper On 11Th April, 2008Документ12 страницSapient Paper On 11Th April, 2008rayorizОценок пока нет
- Additional Instructions For Mailing Your Package: Drop Off LocatorДокумент2 страницыAdditional Instructions For Mailing Your Package: Drop Off LocatorEthanОценок пока нет
- Research ProposalДокумент3 страницыResearch Proposaljan ray aribuaboОценок пока нет
- Survey of Mechanical Working: A. IntroductionДокумент4 страницыSurvey of Mechanical Working: A. IntroductionAsif AhmedОценок пока нет
- Variables That Affect Teachers' Attitudes Towards Disability and Inclusive Education in Mumbai, IndiaДокумент12 страницVariables That Affect Teachers' Attitudes Towards Disability and Inclusive Education in Mumbai, IndiaSumaiya HossainОценок пока нет
- Executive Leadership: Artificial Intelligence Primer For 2021Документ10 страницExecutive Leadership: Artificial Intelligence Primer For 2021ranga.raman100% (1)
- NF EN 10028-3-EnglishДокумент17 страницNF EN 10028-3-Englishhakan gecerОценок пока нет
- 2015 Cdi Final CoachingДокумент24 страницы2015 Cdi Final CoachingJehu Lipata Mensurado100% (1)
- Chapter 2 - Analysis of Steam Power Plant CycleДокумент61 страницаChapter 2 - Analysis of Steam Power Plant Cyclerrhoshack100% (1)
- 2 ID FansДокумент43 страницы2 ID Fansshubham vermaОценок пока нет
- Have A One Track Mind: Lorna WingДокумент4 страницыHave A One Track Mind: Lorna WingsophilauОценок пока нет
- Cpu 2000 App ListДокумент11 страницCpu 2000 App ListKaiser IqbalОценок пока нет
- 5 Ijcms-V3i2-2012-02Документ6 страниц5 Ijcms-V3i2-2012-02saiОценок пока нет
- ZXR10 8900 Series: Hardware Installation ManualДокумент109 страницZXR10 8900 Series: Hardware Installation ManualErnestoLopezGonzalezОценок пока нет
- Sully: The Untold Story Behind the Miracle on the HudsonОт EverandSully: The Untold Story Behind the Miracle on the HudsonРейтинг: 4 из 5 звезд4/5 (103)
- The End of Craving: Recovering the Lost Wisdom of Eating WellОт EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellРейтинг: 4.5 из 5 звезд4.5/5 (83)
- The Fabric of Civilization: How Textiles Made the WorldОт EverandThe Fabric of Civilization: How Textiles Made the WorldРейтинг: 4.5 из 5 звезд4.5/5 (58)
- Hero Found: The Greatest POW Escape of the Vietnam WarОт EverandHero Found: The Greatest POW Escape of the Vietnam WarРейтинг: 4 из 5 звезд4/5 (19)
- The Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyОт EverandThe Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyОценок пока нет
- Dirt to Soil: One Family’s Journey into Regenerative AgricultureОт EverandDirt to Soil: One Family’s Journey into Regenerative AgricultureРейтинг: 5 из 5 звезд5/5 (125)
- The Future of Geography: How the Competition in Space Will Change Our WorldОт EverandThe Future of Geography: How the Competition in Space Will Change Our WorldРейтинг: 4 из 5 звезд4/5 (6)
- Process Plant Equipment: Operation, Control, and ReliabilityОт EverandProcess Plant Equipment: Operation, Control, and ReliabilityРейтинг: 5 из 5 звезд5/5 (1)
- The Technology Trap: Capital, Labor, and Power in the Age of AutomationОт EverandThe Technology Trap: Capital, Labor, and Power in the Age of AutomationРейтинг: 4.5 из 5 звезд4.5/5 (46)
- When the Heavens Went on Sale: The Misfits and Geniuses Racing to Put Space Within ReachОт EverandWhen the Heavens Went on Sale: The Misfits and Geniuses Racing to Put Space Within ReachРейтинг: 3.5 из 5 звезд3.5/5 (6)
- Permaculture for the Rest of Us: Abundant Living on Less than an AcreОт EverandPermaculture for the Rest of Us: Abundant Living on Less than an AcreРейтинг: 4.5 из 5 звезд4.5/5 (33)
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaОт EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaОценок пока нет
- Four Battlegrounds: Power in the Age of Artificial IntelligenceОт EverandFour Battlegrounds: Power in the Age of Artificial IntelligenceРейтинг: 5 из 5 звезд5/5 (5)
- Faster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestОт EverandFaster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestРейтинг: 4 из 5 звезд4/5 (28)
- Pale Blue Dot: A Vision of the Human Future in SpaceОт EverandPale Blue Dot: A Vision of the Human Future in SpaceРейтинг: 4.5 из 5 звезд4.5/5 (588)
- How to Build a Car: The Autobiography of the World’s Greatest Formula 1 DesignerОт EverandHow to Build a Car: The Autobiography of the World’s Greatest Formula 1 DesignerРейтинг: 4.5 из 5 звезд4.5/5 (54)
- ChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindОт EverandChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindОценок пока нет
- The Book of the Moon: A Guide to Our Closest NeighborОт EverandThe Book of the Moon: A Guide to Our Closest NeighborРейтинг: 4.5 из 5 звезд4.5/5 (11)
- From Darwin to Derrida: Selfish Genes, Social Selves, and the Meanings of LifeОт EverandFrom Darwin to Derrida: Selfish Genes, Social Selves, and the Meanings of LifeРейтинг: 4 из 5 звезд4/5 (2)
- Packing for Mars: The Curious Science of Life in the VoidОт EverandPacking for Mars: The Curious Science of Life in the VoidРейтинг: 4 из 5 звезд4/5 (1396)
- This Is What It Sounds Like: What the Music You Love Says About YouОт EverandThis Is What It Sounds Like: What the Music You Love Says About YouРейтинг: 4 из 5 звезд4/5 (33)