Академический Документы
Профессиональный Документы
Культура Документы
Assumptions 1 This Is A Steady-Flow Process and Thus The Mass Flow Rate of Dry Air Remains Constant During The Entire
Загружено:
OsbaldoSolorzanoHerreraОригинальное название
Авторское право
Доступные форматы
Поделиться этим документом
Поделиться или встроить документ
Этот документ был вам полезен?
Это неприемлемый материал?
Пожаловаться на этот документАвторское право:
Доступные форматы
Assumptions 1 This Is A Steady-Flow Process and Thus The Mass Flow Rate of Dry Air Remains Constant During The Entire
Загружено:
OsbaldoSolorzanoHerreraАвторское право:
Доступные форматы
14-31
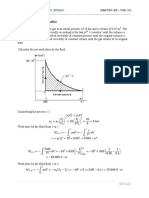
14-73 Saturated humid air at a specified state is heated to a specified temperature. The relative humidity at the exit and the
rate of heat transfer are to be determined.
Assumptions 1 This is a steady-flow process and thus the mass flow rate of dry air remains constant during the entire
process (m a1 m a 2 m a ) . 2 Dry air and water vapor are ideal gases. 3 The kinetic and potential energy changes are
negligible.
Analysis The amount of moisture in the air remains constant ( 1 = 2) as it flows through the heating section since the
process involves no humidification or dehumidification. The inlet state of the air is completely specified, and the total
pressure is 200 kPa. The properties of the air at the inlet and exit states are determined to be
Pv1 1 Pg1 1 Psat @ 15C (1.0)(1.7057 kPa) 1.7057 kPa
h g1 h g @ 15C 2528.3 kJ/kg
Pa1 P1 Pv1 200 1.7057 198.29 kPa Heating
coils
R a T1
v1
Pa1 15C 30C
1 100% RH 2
(0.287 kPa m 3 / kg K)(288 K) 200 kPa AIR
20 m/s
198.29 kPa
0.4168 m 3 / kg dry air
0.622 Pv1 0.622(1.7057 kPa)
1 0.005350 kg H 2 O/kg dry air
P1 Pv1 (200 1.7057) kPa
h1 c p T1 1 h g1 (1.005 kJ/kg C)(15C) + (0.005350)(2528.3 kJ/kg) 28.60 kJ/kg dry air
Pv 2 Pv1 1.7057 kPa
Pg 2 Psat @ 30C 4.2469 kPa
Pv 2 1.7057 kPa
2 0.402 40.2%
Pg 2 4.2469 kPa
h g 2 h g @ 30C 2555.6 kJ/kg
2 1
h2 c p T2 2 h g 2 (1.005 kJ/kg C)(30C) + (0.005350)(2555.6 kJ/kg) 43.82 kJ/kg dry air
Then,
D2 (0.04 m) 2
V1 V1 A1 V1 (20 m/s) 0.02513 m 3 /s
4 4
V1 0.02513 m 3 / s
m a 0.06029 kg/s
v 1 0.4168 m 3 / kg dry air
From the energy balance on air in the heating section,
Q in m a (h2 h1 ) (0.06029 kg/s)(43.82 28.60)kJ/kg 0.918 kW
PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course
preparation. If you are a student using this Manual, you are using it without permission.
Вам также может понравиться
- ME470 Gas-Vapor Mixtures HW Solutions Inst: Shoeleh Di Julio Chapter 14, Solution 27Документ8 страницME470 Gas-Vapor Mixtures HW Solutions Inst: Shoeleh Di Julio Chapter 14, Solution 27S Kiong TingОценок пока нет
- UltimoДокумент1 страницаUltimoanon_501722255Оценок пока нет
- Cooling and DehumidificationДокумент1 страницаCooling and Dehumidificationt_rajith1179Оценок пока нет
- ME 113 S09 HW2 SolutionДокумент3 страницыME 113 S09 HW2 SolutionallyhawОценок пока нет
- Applied Thermodynamics Exam 2018 Wirh SolutionsДокумент9 страницApplied Thermodynamics Exam 2018 Wirh SolutionsFarouk BassaОценок пока нет
- Chapter 8 - Tut-2Документ24 страницыChapter 8 - Tut-2Raghav ChhaparwalОценок пока нет
- A PistonДокумент3 страницыA PistonSamuel NapitupuluОценок пока нет
- 2W04 Assignment 5 - 2023 Solutions PDFДокумент4 страницы2W04 Assignment 5 - 2023 Solutions PDFas asОценок пока нет
- Chapter 9 Examples&SolutionДокумент42 страницыChapter 9 Examples&SolutionSami ullahОценок пока нет
- Wet Air Properties Calculation Sheet: at Low PressureДокумент2 страницыWet Air Properties Calculation Sheet: at Low Pressurezsmith0% (1)
- FM Sol Chap12-131Документ41 страницаFM Sol Chap12-131CEMOОценок пока нет
- Thermo ExcerciseДокумент28 страницThermo ExcerciseAizel Jeong Aizel JeongОценок пока нет
- Assignment 1 Answer's SchemeДокумент27 страницAssignment 1 Answer's Schemedangoy raulОценок пока нет
- Valdez, Shenny RoseДокумент3 страницыValdez, Shenny Roseyeng botzОценок пока нет
- Thermo 5th Chap17 P096Документ19 страницThermo 5th Chap17 P096UTA - Std - Elvin ChantreОценок пока нет
- BSGS Sample Problems 2 - BB CollabДокумент21 страницаBSGS Sample Problems 2 - BB CollabNeo GarceraОценок пока нет
- Tme 213 Classwork SolutionsДокумент15 страницTme 213 Classwork SolutionsEnenamahОценок пока нет
- 12th PhysucsvipДокумент3 страницы12th Physucsvipphysics a2Оценок пока нет
- Sample Problem ThermoДокумент25 страницSample Problem ThermoJonnah Faye Mojares0% (1)
- Assumptions Properties The Properties of Nitrogen at Room Temperature Are R 0.2968 Kpa.MДокумент1 страницаAssumptions Properties The Properties of Nitrogen at Room Temperature Are R 0.2968 Kpa.MFelipeEscobarОценок пока нет
- 3Документ10 страниц3Ariel Carlos De LeonОценок пока нет
- Exercise 5.3 - Spray Dryer I (2020)Документ36 страницExercise 5.3 - Spray Dryer I (2020)Antonio MoncayoОценок пока нет
- Thermodynamics: Practice Problems For Property EvaluationДокумент9 страницThermodynamics: Practice Problems For Property EvaluationSaurav kumar silОценок пока нет
- K 1.4 (Table A-2)Документ1 страницаK 1.4 (Table A-2)Mohamad FaruqОценок пока нет
- hw#3 SolutionsДокумент7 страницhw#3 SolutionsZumaflyОценок пока нет
- Psychrometry and Wetted-Surface Heat TransferДокумент16 страницPsychrometry and Wetted-Surface Heat TransferLaurence Lee AdventoОценок пока нет
- Pipe Solved ProbsetДокумент115 страницPipe Solved ProbsetRemae Garci100% (1)
- Pen UltimoДокумент1 страницаPen Ultimoanon_501722255Оценок пока нет
- Revision SolutionДокумент19 страницRevision SolutionHassan Abo NagaОценок пока нет
- Hvac Lab 3Документ24 страницыHvac Lab 3Crystian Kobee EmpeynadoОценок пока нет
- Tutorial 9v18sol PDFДокумент4 страницыTutorial 9v18sol PDFSohayb GattousОценок пока нет
- Steady Flow Energy EquationДокумент15 страницSteady Flow Energy EquationDaniel MwangangiОценок пока нет
- Tutorial 3 - Question 3Документ1 страницаTutorial 3 - Question 3DiablofireZAОценок пока нет
- Solution: Chapter 2 Irreversibility and AvailabilityДокумент13 страницSolution: Chapter 2 Irreversibility and AvailabilityChaiyuth ArmyforceОценок пока нет
- KJ/KG 1.95 /s M 1000 KJ/KG 1 2 M/S) (80 M/s 50 2 V V KeДокумент1 страницаKJ/KG 1.95 /s M 1000 KJ/KG 1 2 M/S) (80 M/s 50 2 V V KeSAAD AL.SHAHRANIОценок пока нет
- Latihan Soal PengeringanДокумент4 страницыLatihan Soal PengeringanGobel MaxОценок пока нет
- Baynte Pesos Study Guide in Powerpoint: To AccompanyДокумент38 страницBaynte Pesos Study Guide in Powerpoint: To AccompanyRenzmore GalvanОценок пока нет
- Gas-Vapor Mixtures and Air-Conditioning Study Guide in PowerpointДокумент38 страницGas-Vapor Mixtures and Air-Conditioning Study Guide in PowerpointRenzmore GalvanОценок пока нет
- Question #1 (13 Marks) : A KX MG P P PДокумент10 страницQuestion #1 (13 Marks) : A KX MG P P PFelix DawnОценок пока нет
- Thermodynamics - 1 Midterm SolutionДокумент10 страницThermodynamics - 1 Midterm SolutionEarl Maxie Lagdamin ErederaОценок пока нет
- Fluid Mechanics Cengel (Solutions Manual) Chap12-001Документ34 страницыFluid Mechanics Cengel (Solutions Manual) Chap12-001NURUL SYUHADA BT ISMAIL HAJAR50% (2)
- 2300 HW 9 SolДокумент4 страницы2300 HW 9 SolMonishaОценок пока нет
- Reversible adiabatic processesДокумент17 страницReversible adiabatic processesmehmet hassanОценок пока нет
- H 23.5 KJ/KG Dry AirДокумент1 страницаH 23.5 KJ/KG Dry AirChe AguilarОценок пока нет
- Ps CsДокумент15 страницPs CsChristopher GalasОценок пока нет
- Chapter 8 - Tut-4Документ15 страницChapter 8 - Tut-4Raghav ChhaparwalОценок пока нет
- Solutions To Chapter 2 Problems: V 0.002 M V 0.2 MДокумент13 страницSolutions To Chapter 2 Problems: V 0.002 M V 0.2 MJoanne DelosreyesОценок пока нет
- Additional Psychrometric NotesДокумент14 страницAdditional Psychrometric Notesmakondo.yhОценок пока нет
- Steam Gas Turbine 2 Assignment (18%)Документ12 страницSteam Gas Turbine 2 Assignment (18%)Khairul HishamОценок пока нет
- Gas Laws ExplainedДокумент10 страницGas Laws ExplainedM Rizki MaulanaОценок пока нет
- Mass Flow Air (KG/S) Mass Flow Water (KG/S) Biodiesel Flow (KG/S) Wnet (KWH)Документ8 страницMass Flow Air (KG/S) Mass Flow Water (KG/S) Biodiesel Flow (KG/S) Wnet (KWH)Michelle PinzonОценок пока нет
- Refrigeration Moran Shapiro Solution ManualДокумент10 страницRefrigeration Moran Shapiro Solution ManualNovaCastilloОценок пока нет
- Chapter 4 PDFДокумент3 страницыChapter 4 PDFSusana PérezОценок пока нет
- Chapter 4 PDFДокумент3 страницыChapter 4 PDFMarvin LabajoОценок пока нет
- Chapter 4Документ3 страницыChapter 4nick thompsonОценок пока нет
- Chapter One 1Документ18 страницChapter One 1ahmadalsaiahОценок пока нет
- 2nd Law Analysis For A Control VolumeДокумент13 страниц2nd Law Analysis For A Control VolumeSergey ShkapovОценок пока нет
- Fundamental Numerical Values Made SimpleДокумент64 страницыFundamental Numerical Values Made SimpleFaiz Daud100% (1)
- TermoeléctricaДокумент1 страницаTermoeléctricaOsbaldoSolorzanoHerreraОценок пока нет
- Assumptions 1 This Is A Steady-Flow Process and Thus The Mass Flow Rate of Dry Air Remains Constant During The EntireДокумент1 страницаAssumptions 1 This Is A Steady-Flow Process and Thus The Mass Flow Rate of Dry Air Remains Constant During The EntireOsbaldoSolorzanoHerreraОценок пока нет
- Type Number Key: VFD 007 El 43 AДокумент5 страницType Number Key: VFD 007 El 43 AOsbaldoSolorzanoHerreraОценок пока нет
- Ingles Exam Unit 3Документ1 страницаIngles Exam Unit 3OsbaldoSolorzanoHerreraОценок пока нет
- Delta Vfdel Quickstart PDFДокумент25 страницDelta Vfdel Quickstart PDFHardikОценок пока нет
- PROBLEM 2.61: SolutionДокумент5 страницPROBLEM 2.61: SolutionDanie RoyОценок пока нет
- 7 3Документ1 страница7 3OsbaldoSolorzanoHerreraОценок пока нет
- Sling PsychrometerДокумент3 страницыSling PsychrometerJohn Martin A CastroОценок пока нет
- Ahu DesignДокумент17 страницAhu DesignMohamed Aboobucker Mohamed IrfanОценок пока нет
- TTCI Heat TransferДокумент14 страницTTCI Heat TransferRerrysta YolandaОценок пока нет
- Air Handling Unit Controls Temperature, Humidity, AirflowДокумент23 страницыAir Handling Unit Controls Temperature, Humidity, Airflowyousuff0% (1)
- Engg10k1 Ss Geo The Atmosphere IntroductionДокумент5 страницEngg10k1 Ss Geo The Atmosphere IntroductionCrizel WinkelОценок пока нет
- Phys 23 T2 The Constant Volume Air ThermometerДокумент4 страницыPhys 23 T2 The Constant Volume Air ThermometerShashwat ChakrabortiОценок пока нет
- Smoke Control Calculation - KFDДокумент4 страницыSmoke Control Calculation - KFDpratheesh67% (3)
- Cooling Loads Classified by Kinds of Heat PDFДокумент5 страницCooling Loads Classified by Kinds of Heat PDFKanaga Raj0% (1)
- Air PDFДокумент25 страницAir PDFAustin ColesОценок пока нет
- Conversion of Units in Volume and TemperatureДокумент9 страницConversion of Units in Volume and TemperatureDave GaniolaОценок пока нет
- Technical YCWMДокумент8 страницTechnical YCWMnairam2003Оценок пока нет
- Effective Thermal Design of Cooling TowersДокумент11 страницEffective Thermal Design of Cooling Towershamid vahedil larijaniОценок пока нет
- AIR CONDITIONING LOAD ESTIMATEДокумент3 страницыAIR CONDITIONING LOAD ESTIMATEbenyОценок пока нет
- Thermowell PDFДокумент2 страницыThermowell PDFalisuseОценок пока нет
- Calibrate Infrared Thermometer with Ice BathДокумент4 страницыCalibrate Infrared Thermometer with Ice BathbhaleshОценок пока нет
- Indoor Ice-Rink Dehumidification: Application Note 13Документ4 страницыIndoor Ice-Rink Dehumidification: Application Note 13Việt Đặng XuânОценок пока нет
- Jean Marie Tjibaou Cultural Center Analysis1 PDFДокумент1 страницаJean Marie Tjibaou Cultural Center Analysis1 PDFankithaОценок пока нет
- A2121-31 Sars Brooklyn Hvac Installation Block C Boq 2021-06-30Документ10 страницA2121-31 Sars Brooklyn Hvac Installation Block C Boq 2021-06-30Andile NtuliОценок пока нет
- Air Conditioning AmplifierДокумент5 страницAir Conditioning AmplifierCarlos Ediver Arias RestrepoОценок пока нет
- Ashrae Meteo (Islamabad Climate Data)Документ1 страницаAshrae Meteo (Islamabad Climate Data)Imran Aziz100% (1)
- 08ts Ta26 070103Документ2 страницы08ts Ta26 070103Ashraf Adel Nashed ZakiОценок пока нет
- Heating and Cooling Load Calculations for Composite LabДокумент20 страницHeating and Cooling Load Calculations for Composite LabEhtisham Tanvir100% (1)
- 11-An - Industrial Dehumidifier SizingДокумент4 страницы11-An - Industrial Dehumidifier Sizingthilangac100% (1)
- Condensation in Switchgears and Anti Condensation Heater PDFДокумент2 страницыCondensation in Switchgears and Anti Condensation Heater PDFKok WaiОценок пока нет
- ME183 Lectures 1LE Problem Solving Pages 21-30Документ10 страницME183 Lectures 1LE Problem Solving Pages 21-30Paul RodgersОценок пока нет
- Threlkeld Chap 3 AnswersДокумент38 страницThrelkeld Chap 3 AnswersJaycob Clavel100% (2)
- Air Handling Unit: ComponentsДокумент5 страницAir Handling Unit: ComponentsHeber MarinОценок пока нет
- DIN12880 Memmert enДокумент2 страницыDIN12880 Memmert enLUIS SANTIAGOОценок пока нет
- Engineering Unit Conversion PDFДокумент5 страницEngineering Unit Conversion PDFBarcepandoОценок пока нет
- Dew Point Compressed Air Application Note B210991EN B LOW v1Документ4 страницыDew Point Compressed Air Application Note B210991EN B LOW v1edgardОценок пока нет